Hong
Kong Med J 2020 Dec;26(6):510–9 | Epub 16 Dec 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Medication-related problems in older people:
how to optimise medication management
CW Wong, FHKAM (Medicine), FHKCP
Department of Medicine and Geriatrics, Caritas Medical Centre, Kowloon, Hong Kong
Corresponding author: Dr CW Wong (wong.chitwai@gmail.com)
Abstract
Older patients are at risk of medication-related
problems because of age-related physiological
changes and multiple medications taken for multiple
co-morbidities. The resultant polypharmacy is
frequently associated with inappropriate medication
use, which in turn contributes to a range of adverse
consequences, including geriatric syndromes (eg,
falls, cognitive decline, urinary incontinence)
and hospitalisation. In addition, medication non-adherence
or discrepancies between the medications
prescribed and those actually taken by patients,
either intentional or unintentional, are prevalent and
can lead to treatment failure. A large proportion of
adverse drug events are preventable, and medication
errors occur most commonly at the stages of
prescribing and subsequent monitoring. There are
a number of strategies to address these issues with
the aim of ensuring safe prescribing. Furthermore, deprescribing with withdrawal of medications that
are inappropriate or of minimal value for patients is
increasingly emphasised for optimising medication
management. In general, optimisation of medication
management should be patient-centred, considering
individual circumstances and preferences to
determine the treatment goals or priorities for
individual patients, and a multidisciplinary approach
is recommended.
Introduction
With advances in medicine, new drugs are developed
and approved every year for particular diseases and
conditions. In addition, evidence-based clinical
practice guidelines are being developed to assist
clinicians’ decisions about the best care for patients
with specific diseases or clinical conditions for which
medications are frequently recommended. The urge
for increased prescribing gives rise to medication-related
problems, which are particularly problematic
in elderly patients because of their tendency to have
multiple co-morbidities and age-related changes
in pharmacokinetics and pharmacodynamics. The
resultant polypharmacy seems to be unavoidable
nowadays, and it is often associated with potentially
inappropriate medications (PIM) and adverse drug
events. Furthermore, medication adherence is
insufficient in elderly patients. All of these factors
predispose patients to adverse health outcomes,
which deviate from the original intention of
therapeutic benefit but produce morbidity and
mortality with hospitalisation1 2 and increase the
social health cost.
Appropriate medication management with
reduction of polypharmacy, reduction of use of
inappropriate medications, and improvement of
medication adherence is relevant to optimisation
of older patients’ care. Because older people are
highly heterogeneous in terms of co-morbidities,
functional state, and psychosocial aspects, clinical and medication management should be
individualised. The principle of prescribing to older
people should weigh the clinical benefits against the
risks of medication therapies or overprescribing to
address the patients’ individual care goals, current
functioning level, social support, life expectancy,
values, and preference.
This article starts with case scenarios in each
section to illustrate and discuss the medication-related
problems of polypharmacy/inappropriate
medications, adverse drug events and prescribing
cascade, medication non-adherence, and post-discharge
medication discrepancy. These are followed
by patient-centred measures that recommend
prioritisation of treatment, deprescribing, and use of
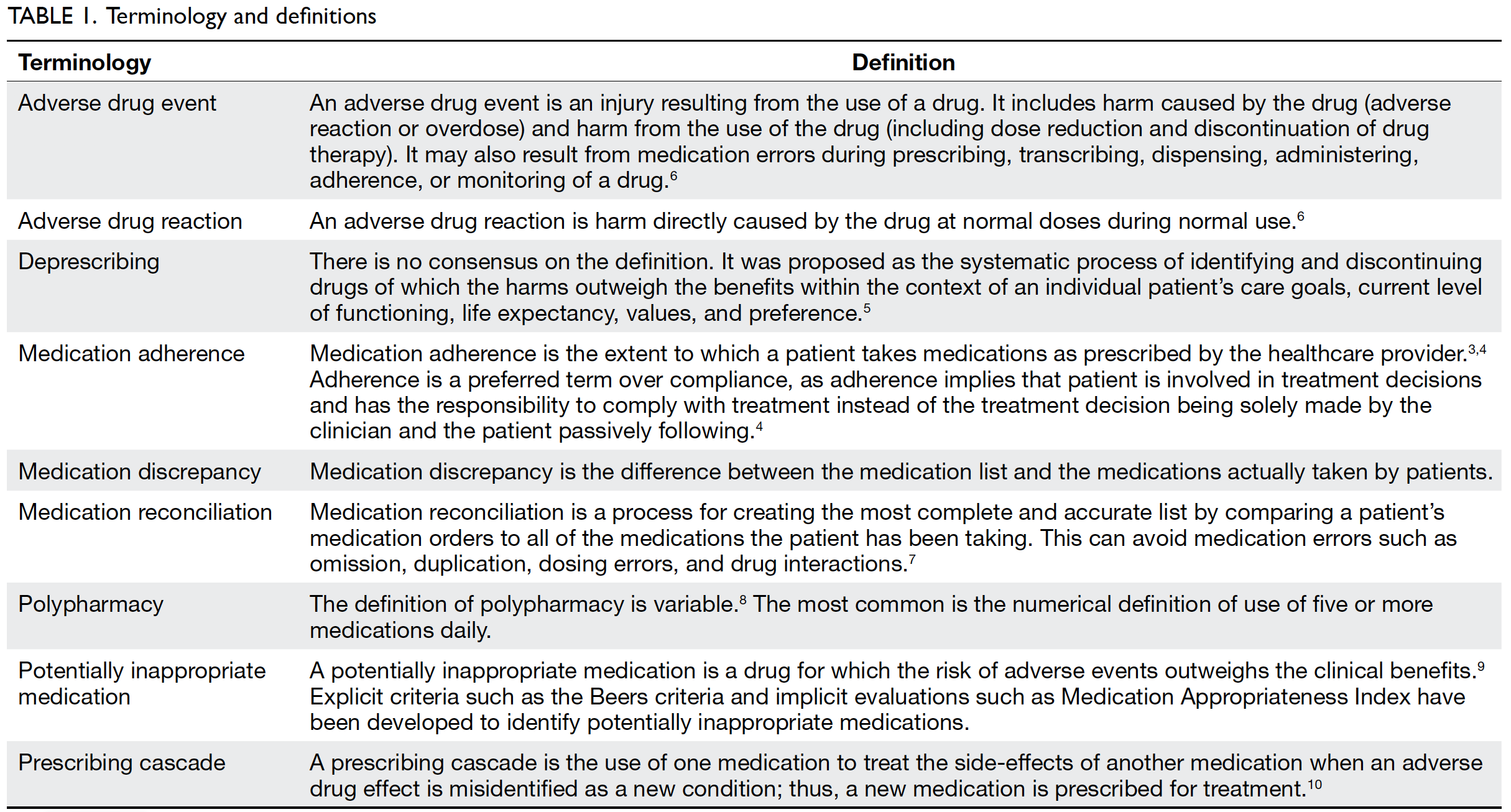
a multidisciplinary approach. Table 1 3 4 5 6 7 8 9 10 summarises
the terms mentioned in this article with definitions.
Polypharmacy and potentially
inappropriate medications
Case scenario 1
An 80-year-old woman had hypertension, diabetes
mellitus, and ischaemic heart disease. She had
17 medications on her prescription list: aspirin,
famotidine, amlodipine, methyldopa, enalapril,
gliclazide, metformin, simvastatin, enalapril,
isosorbide, frusemide, potassium chloride, and
as-needed drugs (betahistine, chlorphenamine,
cocillana, benadryl expectorant, and zopiclone). She strongly refused medications tapering during
follow-up at an out-patient clinic because of fear
of worsening blood pressure control, feet oedema,
dizziness, coughing, and insomnia. She was recently
admitted because of dizziness and fall, found to have
low blood pressure of 108/50 mm Hg and a heart
rate of 90 beats per minute, mild dehydration with
hyperkalaemia (urea 7.8 mmol/L, creatinine 60 μmol/L,
sodium 144 mmol/L, potassium 4.8 mmol/L) and
vitamin B12 deficiency (100 pmol/L).
Case scenario 2
A 75-year-old man had benign prostatic hyperplasia
and was taking terazosin. He developed herpes zoster
with postherpetic neuralgia, and amitriptyline was
prescribed. He then developed retention of urine.
Multiple co-morbidities are often prevalent
among older people. In Hong Kong, older patients
attending specialist out-patient clinics had an
average of four to five co-morbidities.11 With new
drugs and clinical guidelines being developed for
each disease, the number of medications taken by
individual patients is increasing. The mean number of
medications prescribed per patient were six and nine
in out-patient and in-patient settings, respectively.11 12 13
Using the concurrent use of five or more daily
medications to define polypharmacy, as many as
77.8% of older patients in Hong Kong had the burden
of polypharmacy, which was more than double the
corresponding proportion of 32% from two decades
ago,12 14 and 11% took 10 or more medications.11
Polypharmacy is positively associated with PIM
and subsequent adverse drug events, drug–drug
interactions, and drug–disease interactions.11 14 15 16
Potentially inappropriate medications are not solely
attributable to inappropriate prescribing but are
also related to changes in the benefits and risks of
a medication with time: medications can become
inappropriate for a given patient even if they were
previously appropriate. Approximately 30% to 59%
of patients in Hong Kong have been found to be
taking at least one inappropriate medication.11 12 13
The most common causes of inappropriateness
were medications without clear indications, lack of
effectiveness, incorrect dosage, difficult instructions,
unacceptable duration of therapy, and high cost in
comparison to alternatives of equal efficacy and
safety.13 Very often, it is difficult to measure the direct influence of inappropriate medications
on the patient and healthcare system. The non-specific
symptoms and geriatric syndromes caused
by inappropriate medications include fatigue,
dizziness, falls, urinary incontinence, and functional
or cognitive decline. These might be disregarded as
being related to ageing, making adverse drug events
difficult to recognise.15 16 Nonetheless, inappropriate
medications would potentially increase adverse
drug events or reactions with increased morbidity,
hospitalisation, and healthcare cost.17 18 19
Screening for potentially inappropriate
medications
Since polypharmacy are difficult to avoid nowadays,
the practical way to deal with is to enhance
appropriate use of medications with reduction
of PIM. Criteria and evaluation processes have
been developed to screen for PIM in older people.
Most are explicit criteria based on trial evidence,
systematic reviews, expert panel suggestions, and
consensus evaluation with the aims of improving
medication appropriateness and minimising adverse
drug events and subsequent hospitalisation. The
most widely used explicit prescribing criteria are
the Beers criteria and the Screening Tool of Older
Person potentially inappropriate Prescriptions
(STOPP)/Screening Tool to Alert doctors to the
Right Treatment (START) criteria.
The Beers criteria were designed for use in
people aged 65 years or older in ambulatory, acute,
and institutionalised settings but not for those
receiving hospice or palliative care.20 The Beers
criteria have undergone review and been updated
regularly since first being published in 1991. The
most updated version, the Beers 2019 criteria,21 lists
PIMs to be avoided in five categories: (1) Drugs and
drug classes to avoid; (2) Drugs and drug classes to
avoid in certain diseases or syndromes; (3) Drugs to
be used with caution; (4) Drug–drug interactions
that should be avoided; and (5) Drugs to be avoided
or dosage reduced in cases with kidney disease. Each
criterion stated is supplemented by the rationale for
the recommendation, level of evidence, and strength
of the recommendation.
The STOPP/START criteria provide
prescribing guidance tailored for older people
aged 65 years or older.22 Like the Beers criteria, the
STOPP criteria comprise a list of PIMs. In addition, a list of potential prescribing omissions
(START criteria) alert clinicians about appropriate
and indicated prescribing. Instead of listing the
offending drugs, the STOPP/START criteria outline
clinical circumstances with each criterion to
address the drug or drug class that is deemed to be
inappropriate or drugs that should be considered, so
they are considered to be more relevant in clinical
practice. The STOPP/START criteria have also been updated to maintain their clinical relevance: the
latest edition (version 2) was published in 2015.23
These explicit criteria provide only evidence-based
references but do not address individual
patients’ circumstances, such as co-morbidities and
preference. Therefore, they cannot replace clinical
judgement about patient-centred decisions but can
alert clinicians to potential instances of inappropriate
medication use.
Implicit evaluation, in contrast, is judgement-based
and focused on patients rather than drugs or
diseases. The Medication Appropriateness Index
is one set of implicit tool and is frequently used in
research.24 It includes 10 items to determine the
appropriateness of a given medication: indication,
effectiveness, correct dosage, practical direction,
drug–drug interactions, drug–disease interactions,
duplication, acceptable duration, and expense. “Yes”
or “No” is applied for each item in which “Yes” scores
0 whereas “No” scores from 1 to 3 depending on its
importance in the assessment of the appropriateness
of a given drug with indication and effectiveness are
given most weigh. A total score is then generated
with higher scores indicating more inappropriate
medications. Although these evaluation processes
are patient-centred and address the entire medication
regimen, their applicability is limited by the fact that
they are time-consuming and dependent on the
clinician’s knowledge and experience.
Remarks
Scenario 1 illustrated polypharmacy with the adverse
outcomes of dizziness and fall. The inappropriate
medications prescribed were those with no clear
indications or questionable effectiveness, or for
the treatment of a drug adverse reaction, such
as frusemide (STOPP criteria) for feet oedema,
which was possibly related to the adverse effects
of amlodipine; benadryl and cocillana for cough,
which were possibly related to the adverse effects of
enalapril; and betahistine for dizziness, which was
possibly related to methyldopa (STOPP and Beers
criteria) and vitamin B12 deficiency as a result of long-term
metformin intake. Medications were adjusted
by replacing enalapril with losartan, replacing
methyldopa and amlodipine with metoprolol
for blood pressure control and ischaemic heart
disease (START criteria), and adding a vitamin B12
supplement. The patient was tapered off frusemide,
potassium chloride, and all as-needed medications.
In scenario 2, prescribing amitriptyline (which
has strong anticholinergic properties) to an elderly
man with benign prostatic hypertrophy is considered
to be inappropriate (Beers and STOPP criteria), as
it could precipitate urinary retention, and there are
alternatives with fewer anticholinergic effects. For
example, gabapentin can be chosen for postherpetic
neuralgia.
Adverse drug events and
prescribing cascade
Case scenario 3
A 70-year-old man had behavioural psychological
symptoms of dementia and was prescribed
memantine, sertraline, quetiapine, and lorazepam.
Because of fall with back pain, paracetamol and
tramadol were prescribed. He developed nausea and
vomiting, and then metoclopramide was given. Later,
he developed fever, restlessness, and limb rigidity.
Serotonin syndrome as a result of concomitant use
of sertraline, tramadol, and metoclopramide was
suspected.
Adverse drug events are common in clinical
practice. Large-scale studies have found overall
rates of adverse drug events of 50.1 per 1000
person-years in ambulatory older people and 1.89
per 100 person-months in institutionalised elderly
residents.25 26 The most commonly implicated agents
were cardiovascular drugs in the ambulatory setting
and antipsychotics in the institutional setting, which
might be related to the frequent use of these drugs in
those corresponding settings. Accordingly, the most
common types of adverse events were electrolyte/renal, gastrointestinal tract, and haemorrhage events
in ambulatory patients, whereas neuropsychiatric
events (oversedation, confusion, hallucinations,
delirium) predominated in the institutional setting.
Up to 51% of the observed adverse drug events were
preventable, and serious, life-threatening and fatal
events were more likely to be preventable than were
less severe events.25 26 The errors associated with those
preventable events most commonly occurred at the
stages of prescribing and monitoring. Prescribing
errors included wrong dosage, significant drug
interactions, and wrong choice of drugs (eg, using
drugs with significant anticholinergic effects instead
of safer alternatives). Monitoring errors refer to
inadequate laboratory monitoring, delayed response
or failure to respond to signs and symptoms, and/or
laboratory evidence of toxicity.
Adverse drug events or reactions may
precipitate a prescribing cascade. Prescribing cascade
occurs when adverse drug events are mistaken as a
new medical condition and leads to addition of new
drugs for treatment.10 It places patients at risk of
developing additional adverse drug events because
of the potentially unnecessary treatment. Adverse
consequences of prescribing cascade include
polypharmacy and its associated adverse event as
in Case 1 (see Case 1 remarks), and exacerbation of
the harmful effects of adverse drug reactions as in
Case 3, serotonin syndrome resulted from the use of
serotonin reuptake inhibitors together with tramadol
and metoclopramide. In addition to the drugs
involved in Cases 1 and 3 (amlodipine, angiotensin-converting
enzyme inhibitor, serotonin reuptake inhibitor, and tramadol), other common drugs
implicated in prescribing cascade are cholinesterase
inhibitors which cause urinary incontinence with
subsequent oxybutynin added, non-steroidal anti-inflammatory
drugs which cause or exacerbate
hypertension with antihypertensive agent added, and
antipsychotic agents which cause extrapyramidal
sign with levodopa or anticholinergic added.27 They
are largely preventable provided that clinicians are
aware of this during the prescribing process.
Preventing adverse drug events and
prescribing cascade
Provided that a significant proportion of adverse drug
events and common sources of error are preventable,
adverse drug events are amenable to prevention
strategies. Computerised order entry is widely used
nowadays to alert prescribers about the drug dosage,
need for dose adjustment according to renal function,
potential drug interactions, or allergic reactions,
and this significantly reduces medication errors.28
However, this cannot replace clinical judgements
about relevant indications, correct drug choices, and
simplification of medication regimens. Regular review
to obtain an updated medication list is good practice,
especially when a new prescription or change in
prescription is instituted. Whenever a new symptom
occurs, assessment to rule out adverse effects from
currently taken medications to prevent a ‘prescribing
cascade’, or adding drugs to treat other drugs’ adverse
effects, should be considered. Non-pharmacological
therapies could be effective alternatives to replace
some psychoactive medications.29 The development
of a systematic approach based on patient-centred
care to facilitate decision-making about prescribing
certain medications to frail, elderly patients (eg,
anticoagulants) and subsequent monitoring
is anticipated. Furthermore, enhancement of
surveillance and reporting systems for adverse drug
events with subsequent analysis and correction of the
underlying systematic faults could achieve significant
error reduction.30
Remarks
In scenario 3, the series of adverse drug events
and the serious medical consequence of serotonin
syndrome was preventable if the prescribing cascade
had been broken by minimising medications for the
behavioural psychological symptoms of dementia,
such as stopping quetiapine or lorazepam for fall
risk at the initial stage or stopping tramadol to treat
the adverse drug reaction of nausea and vomiting
instead of giving metoclopramide at a later stage.
Medication adherence
Case scenario 4
A 76-year-old man had paroxysmal supraventricular
tachycardia, for which he had been prescribed sotalol, and iatrogenic Cushing syndrome, for which he was
on hydrocortisone replacement. He was admitted for
a supraventricular tachycardia attack. He admitted
that he did not take sotalol because of fatigue and low
heart rate and took hydrocortisone only occasionally
because of facial puffiness.
Case scenario 5
A 79-year-old woman lived alone and was referred to a
community nurse for medication management. Many
drug stocks were found in her home. She was suspected
to have cognitive impairment on initial assessment.
Poor medication adherence is common in
clinical practice, with a 50% adherence rate observed
among patients with chronic conditions.3 Poor
medication adherence is even more problematic
in elderly people because they may have decreased
functionality and cognitive impairment. Because of
the differences in measurement methods used and
the settings of the studied populations, the prevalence
of medication non-adherence among elderly patients
has varied widely in local studies (9%-54%).31 32 33
Medication non-adherence leads to drug waste and
treatment failure, with resultant hospitalisation
and increased healthcare cost. It may also prompt
inappropriate increases in drug dosage, addition
of or changes to more potent drugs, and increased
risk of adverse drug events if it is unrecognised and
regarded as poor response to treatment.
There are many ways to assess medication
adherence, such as measuring medication or their
metabolite levels in blood, using questionnaires or
self-reports, and pill counting. Nonetheless, the
simplest and most direct method is asking the patient
nonjudgmentally about how often they miss doses
and encouraging them to talk about their difficulties
with medication management.4 Barriers to poor
medication adherence are multifactorial and can
generally be categorised into prescriber-related (eg,
poor communication or relationship with patients,
lack of time for patient education), patient-related (eg,
poor knowledge, fear of adverse reactions, depression,
diminished physical or mental capacity), medication-related
(eg, polypharmacy, complexity of medication
regimen), and poor social support.3 34 35 Common
factors related to poor medication adherence in studies
have included adverse drug events,32 33 35 complicated
drug regimen,32 33 35 36 recent changes in medication
regimen,37 and multiple morbidities or self-perceived
poor health.31 35 36 Because both cognitive impairment
and depression are prevalent among elderly patients,
and these conditions can impede functionality and
thus medication management, these two conditions
should be looked for in older patients with poor
medication adherence.31 35 36
Improving medication adherence
As poor medication adherence is often multifactorial, a multidimensional approach is required. In general,
such an approach includes patient education,
enhancing clinician–patient communication,
improving medication regimens, and facilitating
social support. Providing education to the patient and
their family members or caregivers on medication-and
disease-related information, indications and
adverse reactions of the medications prescribed, and
how to handle the regimen is effective for improving
adherence.38 Besides, enhancing communication by
listening to the patient or caregiver’s concerns and
formulating a compromised treatment plan can
encourage adherence.4 Furthermore, encouraging
the patient to participate in disease management,
such as self-monitoring of blood pressure or blood
glucose, can also enhance adherence.4 Simplification
and regular review of the medication regimen should
be emphasised.31 38 Older patients with suspicion
of cognitive impairment or depression should be
assessed for management. Family members of
patients with cognitive impairment, mood disorders,
or decreased functionality are encouraged to assist
in medication management.31 32 For those with poor
family support, social support from, eg, a community
nursing service to assist in packing medications
with use of medication boxes or charts can also be
helpful.32 38 Reinforcement of the above strategies
and assessment of adherence should be performed
continuously to maintain adherence.
Remarks
In scenario 4, adverse drug reactions resulted
in poor medication adherence. After education
and explanation about his medical condition and
medication indications, and after his concerns were
addressed, the patient agreed to take hydrocortisone
and resumed low-dose sotalol with regular review at
follow-up clinic.
In scenario 5, cognitive impairment impeded
proper drug management. Patients with poor social
support, poor disease control, and many drug stocks
at home should have a high index of suspicion.
The drug regimen was simplified to once/day, and
the patient was referred for cognitive assessment,
management, and social support.
Post-hospital discharge
medication discrepancies
Case scenario 6
An 80-year-old woman complained of dizzy spells,
and she had a blood pressure of 87/39 mm Hg and a
pulse of 50 beats per minute at follow-up clinic. She
had recently been hospitalised for congestive heart
failure, and her medications were adjusted (atenolol
was replaced by carvedilol, and ramipril and
frusemide were added). However, she did not notice
that the medications had been adjusted and took all of the previous medications together with the newly
prescribed medications.
Sometimes, it is not the patient’s intention not
to follow the medication instructions, but they may
not notice or may misunderstand the changes in
their medication regimen. Such errors often occur at
the time of hospital discharge, as hospital admission
typically results in adjustments to medication
regimens. Discrepancies between the medications
listed on discharge instructions and the medications
actually taken by patients were found in up to half
of patients following hospital discharge.39 40 Older
patients are particularly at risk, with fewer than
10% of community-dwelling older patients adhering
completely to their discharge medication lists in one
study.41 Medication discrepancies include addition/duplication or omission of medications and changes
to dosing or frequency. This might endanger patients’
health because of adverse drug events or suboptimal
disease control. One study found that 14% of older
patients with medication discrepancies were re-hospitalised
at 30 days after hospital discharge.42
The discharge process is often criticised for
its contribution to medication discrepancy. Poor
clinician–patient communication, the lack of or
incomplete review of medication regimen, or failure
to inform the patient about medication changes
upon discharge are often blamed as causes.43
In addition, inaccurate discharge medication
instructions, such as duplication, omission,
incorrect dosage or frequency of medications, and
unclear prescribing instructions are commonly
encountered.40 42 44 Discharge medication lists that
are not integrated with medications the patient took
from other specialties before admission may cause
confusion as to whether the patient should continue
to take those prior medications or adjust their
dosages after discharge. Patients at high risk of post-discharge
medication discrepancy include those
with depression,39 impaired cognitive function or
low medication literacy (ie, difficulty understanding
medication-related information),39 40 and those
who receive multiple medications or complicated
regimens.41 42
Minimising post-discharge medication
discrepancy
Improvement of the hospital discharge process
is the first and most important step to improve
medication adherence and reduce preventable
post-hospitalisation complications. Medications
reconciliation to construct an integrated discharge
medication list, which combines medications
adjusted during hospitalisation and those from
other specialties, is recommended.45 In addition,
reminders to the involved clinicians from other
departments about the changes to medications
previously prescribed by them are suggested. Counselling and education for patients and their
families or caregivers about the new regimen of
medications with focus on medication changes
from pre-admission and high-risk medications (eg,
warfarin and insulin) could promote adherence and
medication safety after discharge.46 Post-discharge
surveillance by phone contact with patients to
address post-discharge problems or answer questions
has been found to be beneficial.45 Arrangements
for early follow-up can help with monitoring post-hospitalisation
medication adherence, adverse
effects, and prescribing mistakes.
Remarks
Medication incidents similar to those in scenario 6
could be avoided with better communication
highlighting the adjusted medications upon discharge.
Prioritisation of disease
management
Case scenario 7
A 60-year-old man had metastatic lung carcinoma
and opted for palliative care. Other medical history
included diabetes mellitus, hypertension, and
cerebrovascular disease. He had cachexia, pain,
dyspnoea, and poor oral intake. Medications included
morphine, metoclopramide, senokot, enalapril,
metformin, gliclazide, simvastatin, aspirin, and
pantoprazole.
Patient-centred care has increasingly
emphasised addressing the needs of individual
patients to maintain their quality of life and
functioning.45 The goal of care should be
individualised, taking into account the disease’s
impact on both the patient’s short- and long-term
health, the patient’s circumstances, and their
preference. Patient-centred care identifies the
patient’s clinically dominant condition to determine
the priority of care. This can guide medication use
towards the area most important to the patient and
reduce or stop medications that are less meaningful
to their health status. In scenario 6, a patient with
multiple co-morbidities had been diagnosed
with metastatic carcinoma and had a limited life
expectancy. Both the patient and their family opted for
palliative care (goal of care), and their main concerns
were pain and shortness of breath (clinical dominant
condition). Proper control of these distressing
symptoms should be the priority. In addition to
non-pharmacological therapy, medications should
be adjusted for symptomatic relief. Medications for
other chronic conditions, such as diabetes mellitus
and cerebrovascular disease, should be minimised, as
the potential benefits would likely not be observed,
but there would be increased drug interactions and
adverse events (eg, hypoglycaemia, poor appetite,
bleeding).
Deprescribing
Case scenario 8
A 90-year-old woman with right middle cerebral
artery infarction was discharged to a residential
home. She was bedbound, non-communicable,
and totally dependent. Her medications included
warfarin, amlodipine, simvastatin, lansoprazole,
donepezil, haloperidol, and quetiapine.
Deprescribing is increasingly considered as a
part of good clinical practice. Through the process
of medication review to withdraw medications that
are no longer appropriate or to taper medications
to the minimum effective dosage, the benefits and
risks of medications can be balanced according to
the patient’s current health status. Deprescribing is
particularly relevant to patients with the burden of
polypharmacy or changing clinical conditions. The
evidentiary base for deprescribing in older people
is growing. Systematic reviews have demonstrated
that carefully selecting patients to undergo planned
medications withdrawal had no detrimental effects in
a substantial proportion of older people.47 48 49 Common
drug classes studied in medication withdrawal trials
are antihypertensive agents, benzodiazepines, and
psychotropic agents. The benefits of discontinuation
of these medications is not limited to polypharmacy
reduction but also include reduced fall risk and
improved cognition and psychomotor function.47 48 49
Although deprescribing is generally regarded
as feasible and safe, fear of rebound or return of
symptoms and exacerbation of underlying conditions
are the major barriers to prescribing. There are
practical guides and algorithms to assist with the
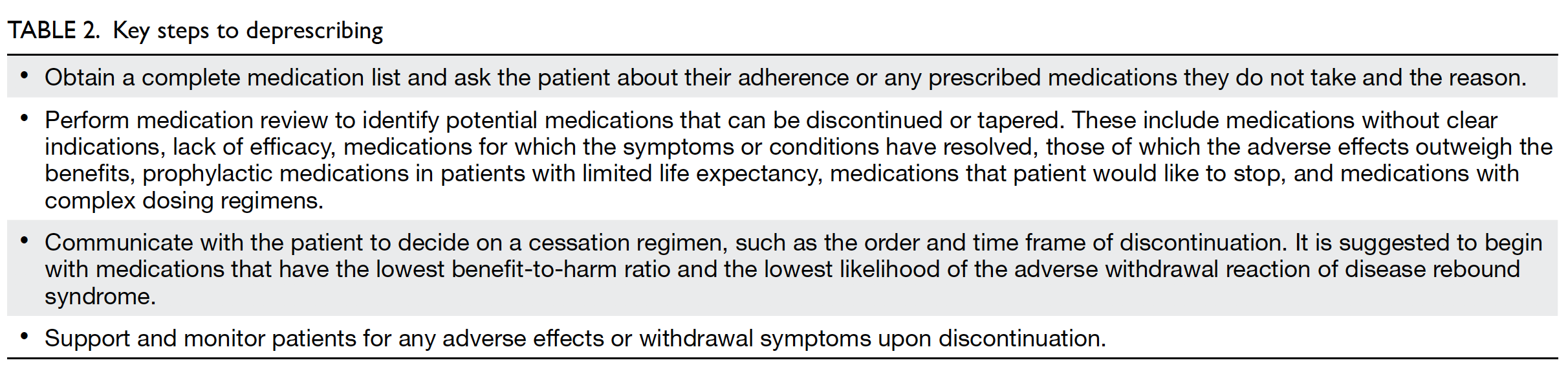
deprescribing process.5 50 51 All highlight that it is a
supervised process. Table 2 shows the key steps to
deprescribing.
Besides the general approach to deprescribing,
there is medication class-based guidance on how to
taper particular medications. There are evidence-based
guidelines that provide the reasons for and
benefits of deprescribing an identified medication,
recommendations on how to taper or stop, the
period and symptoms to monitor, non-medication
approaches for management of symptoms, and instructions if symptoms relapse.52 53 The medication
classes considered as high priority for deprescribing
in older patients are those with high prevalence of
use or overtreatment, significant adverse effects,
other effective treatment options available, or those
that are easy to stop. These medication classes
include antipsychotics, statins, benzodiazepines,
proton pump inhibitors, nonsteroidal anti-inflammatory
drugs, furosemide, antihypertensive
agents, antihyperglycaemic agents, cholinesterase
inhibitors, and memantine.51 52 53 54
Patients or caregivers who are reluctant or
disagree with medications cessation are common.
Such attitudes negatively influence the success of
deprescribing. The reasons against medications
cessation include patients’ perceptions that the
medications are necessary or beneficial and their
fears of worsening clinical conditions or withdrawal
effects, especially if they had previous bad
experiences with medication cessation.55 Another
barrier is the limited consultation time and lack
of support from clinicians.55 A stepwise approach
with time given for shared decision-making about
deprescribing is reasonable.51 Such an approach
starts with patient or caregiver education on the
purpose of deprescribing, including exploration of
and addressing their concerns. Then, options should
be provided with respect to medication tapering,
non-pharmacological interventions, and monitoring
for adverse withdrawal effects, and reassurance
should be given that the discontinued medications
can be restarted if needed. A multidisciplinary
approach with the clinician being supported by other
healthcare professionals can relieve time pressure
during consultations. Patients with depression and
anxiety disorders may need treatment for their
psychiatric conditions before they can participate in
deprescribing.
Remarks
The patient in scenario 8 was non-communicable and
totally dependent. Anticoagulants and simvastatin,
which no longer provided cardiovascular/cerebrovascular benefits, and donepezil, which no longer provided cognitive benefits, were tapered off.
Haldol and quetiapine were also tapered off because
of the patient’s lack of disturbing behaviour.
Multidisciplinary team approach
A multidisciplinary team approach is recommended
to optimise medication management and treatment
decisions, minimise adverse drug effects, enhance
medication safety, and promote medication
adherence.45 56
Clinician
The clinician plays a central role in prescribing
and the subsequent medication-related problems.
The following points are recommended to improve
clinicians’ medication management for older
patients:
Pharmacist
The role of the pharmacist in the healthcare system is
expanding from dispensing service to direct patient
care. Pharmacists can help with medication-related
problems in different settings.13 57 58 59 Polypharmacy
and inappropriate medications: the pharmacist can
check the appropriateness of medications to make
recommendations for clinicians; Non-adherence:
the pharmacist can check the patient’s medication
adherence or discrepancy, provide counselling,
identify the patient’s difficulties, and communicate
with clinicians to modify the medication regimen.
Pharmacists provide both in-patient and out-patient
service, the latter in the form of pharmacist-led
drug compliance clinics, and their services further
extend to the community through public-private
partnerships.
Although the results of studies on the efficacy
of clinical pharmacy service are equivocal,58 pharmacists’ positive impact cannot be denied, as
those equivocal results are probably related to the
complexity of pharmacists’ interventions and the lack
of an evaluation standard in studies. Nonetheless, a
recent local study on a pharmacist-led medication
review programme for hospitalised elderly patients
that included medication reconciliation, medication
review, and medication counselling showed
significantly reduced numbers of inappropriate
medications and unplanned hospital admissions.13
Another local study also demonstrated a positive
impact of pharmacists on identifying, resolving, and
preventing medication-related problems.59
Nurse
Besides administering medications, nurses are also
involved in medication care for older patients in the
following ways:
Conclusion
Numerous studies have shown that medication-related
problems (eg, polypharmacy, inappropriate
medication, adverse drug events, medication non-adherence,
and medication discrepancy) are common
in older patients. Strategies or interventions such
as screening tools for inappropriate medications,
deprescribing, and multidisciplinary approaches
have been introduced to optimise medication
management. However, helping patients to take
medications safely and effectively is still challenging.
Very often, we are aware of the problem, but it is
difficult to alter or deal with, as there are multiple
barriers (eg, pressure from patients/caregivers, short
consultation time). Thus, in addition to continuous
education, reminding clinicians about appropriate
prescribing, regular review, and medication regimen
adjustment, public education to promote the
rational use of medications is important. Continuing to review and address the effects of deficiencies in
the healthcare system on medication safety could
lead to a reduction in medication-related problems
with time.
Author contributions
The author contributed to the concept or design of the study,
acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. The author had
full access to the data, contributed to the study, approved the
final version for publication, and takes responsibility for its
accuracy and integrity.
Conflicts of interest
The author has disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding
agency in the public, commercial, or not-for-profit sectors.
References
1. Lazarou J, Pomeranz BH, Coret RN. Incidence of adverse
drug reactions in hospitalized patients: a meta-analysis of
prospective studies. JAMA 1998;279:1200-5. Crossref
2. Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR,
Pirmohamed M. Adverse drug reactions in hospital in-patients:
prospective analysis of 3695 patient-episodes.
PLoS One 2009;4:e4439. Crossref
3. World Health Organization. Adherence to
long term therapies: evidence for action. 2003.
Available from: https://apps.who.int/iris/bitstream/handle/10665/42682/9241545992.pdf. Accessed 23 Nov
2020.
4. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487-97. Crossref
5. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate
polypharmacy: the process of deprescribing. JAMA Intern
Med 2015;175:827-34. Crossref
6. VA Centre for Medication Safety, VHA Pharmacy
Benefits Management Strategic Healthcare Group,
Medical Advisory Panel. Adverse drug events, adverse
drug reactions, medications error. Frequently asked
questions. November 2006. Available from: https://www.pbm.va.gov/PBM/vacenterformedicationsafety/tools/AdverseDrugReaction.pdf. Accessed 23 Nov 2020.
7. The Joint Commission. National patient safety goals
effective July 2020 for the ambulatory health care program.
Available from: https://www.jointcommission.org/-/media/tjc/documents/standards/national-patient-safety-goals/2020/npsg_chapter_ahc_jul2020.pdf. Accessed 23
Nov 2020.
8. Masnoon N, Shakib S, Kalish-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC
Geriatr 2017;17:230. Crossref
9. Corsonello A, Pranno L, Garasto S, Fabietti P, Bustacchini S, Lattanzio F. Potentially inappropriate medications in elderly
hospitalized patients. Drugs Aging 2009;26 Suppl 1:31-9. Crossref
10. Rochon PA, Gurwitz JH. Optimising drug treatment
for elderly people. The prescribing cascade. BMJ
1997;315:1096-9. Crossref
11. Lam DP, Mak CF, Chan SM, Yao RW, Leung SS, You JH.
Polypharmacy and inappropriate prescribing in
elderly Hong Kong Chinese patients. J Am Geriatr Soc
2010;58:203-5. Crossref
12. Lam MP, Cheung BM, Wong IC. Prevalence of potentially
inappropriate prescribing among Hong Kong older adults:
a comparison of the Beers 2003, Beers 2012, and screening
tool of older person’s prescriptions and screening tool to
alert doctors to right treatment criteria. J Am Geriatric Soc
2015;63:1471-2. Crossref
13. Chiu P, Lee A, See T, Chan F. Outcomes of a pharmacist-led
medication review programme for hospitalised elderly
patients. Hong Kong Med J 2018;24:98-106.
14. Ko CF, Ko PS, Tsang ML. A survey on the polypharmacy
and use of inappropriate medication in a geriatric
outpatient clinic. J HK Geriatr Soc 1996;7:28-31.
15. Maher RL, Hanlon J, Hajjar ER. Clinical consequence of polypharmacy in elderly. Expert Opin Drug Saf 2014;13:57-
65. Crossref
16. Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly
patients. Am J Geriatr Pharmacother 2007;5:345-51. Crossref
17. Onda M, Imai H, Takada Y, Fujii S, Shono T, Nanaumi Y.
Identification and prevalence of adverse drugs events
caused by potentially inappropriate medication in
homebound elderly patients: a retrospective study using
a nationwide survey in Japan. BMJ Open 2015;5:e007581. Crossref
18. Fu Az, Liu GG, Christensen DB. Inappropriate medication
use and health outcomes in the elderly. J Am Geriatr Soc
2002;52:1934-9. Crossref
19. Laroche ML, Charmes JP, Nouaille Y, Picard N, Merle L. Is
inappropriate medication use a major cause of adverse drug
reactions in the elderly? Br J Clin Pharmacol 2007;63:177-86. Crossref
20. American Geriatrics Society 2015 Beers Criteria Update
Expert Panel. American Geriatrics Society 2015 updated
Beers Criteria for potentially inappropriate medication use
in older adults. J Am Geriatr Soc 2015;63:2227-46. Crossref
21. American Geriatrics Society 2019 Beers Criteria Update
Expert Panel. American Geriatrics Society 2019 updated
AGS Beers Criteria for potentially inappropriate medication
use in older adults. J Am Geriatr Soc 2019;67:674-94. Crossref
22. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D.
STOPP (Screening Tool of Older Person’s Prescriptions)
and START (Screening Tool to Alert doctors to Right
Treatment). Consensus validation. Int J Clin Pharmacol
Ther 2008;46:72-83. Crossref
23. O’Mahony D, O’Sullivan D, Byne S, O’Connor MN, Ryan C,
Gallagher P. STOPP/START criteria for potentially
inappropriate prescribing in older people: version 2. Age
Ageing 2015;44:213-8. Crossref
24. Hanlon JT, Schmader KE, Samsa GP, et al. A method for
assessing drug therapy appropriateness. J Clin Epidemiol
1992;45:1045-51. Crossref
25. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and
preventability of adverse drug events among older persons
in the ambulatory setting. JAMA 2003;289:1107-16. Crossref
26. Gurwitz JH, Field TS, Avorn J, et al. Incidence and
preventability of adverse drug events in nursing homes.
Am J Med 2000;109:87-94. Crossref
27. Kalisch LM, Caughey GE, Roughead EE, Gilbert AL. The
prescribing cascade. Aust Prescr 2011;34:162-6. Crossref
28. Bates DW, Leape LL, Cullen DJ, et al. Effect of computerised
physician order entry and a team intervention on prevention of serious medication error. JAMA 1998;280:1311-6. Crossref
29. de Oliverira AM, Radanovic M, de Mello PC, et al.
Nonpharmacological interventions to reduce behavioural
and psychological symptoms of dementia: a systematic
review. Biomed Res Int 2015;2015:218980. Crossref
30. Leape LL. Error in medicine. JAMA 1994;272:1851-7. Crossref
31. Leung DY, Bai X, Leung AY, Lou BC, Chi I. Prevalence of
medication adherence and its associated factors among
community-dwelling Chinese older adults in Hong Kong.
Geriari Gerontol Int 2015;15:789-96. Crossref
32. Lam PW, Lum CM, Leung MF. Drug non-adherence and
associated risk factors among Chinese geriatric patients in
Hong Kong. Hong Kong Med J 2007;13:284-92.
33. Chong CK, Chan JC, Chang S, Yuen YH, Lee SC, Critchley JA.
A patient compliance survey in a general medical clinic. J
Clin Pharm Ther 1997;22:323-6. Crossref
34. Hale LS, Calder DR. Managing medication nonadherence.
In: Muma RD, Lyons BA, editors. Patient Education: a
Practical Approach. 2nd ed. Boston, MA: Jones & Barlett
Learning; 2012: 41-7.
35. Yap AF, Thirumoorthy T, Kwan YH. Systematic review of
the barriers affecting medication adherence in older adults.
Geriatric Gerontol Int 2016;16:1093-101. Crossref
36. Smaje A, Weston-Clark M, Raj R, Orlu M, Davis D, Rawle M.
Factors associated with medication adherence in older
patients: a systematic review. Aging Med (Milton)
2018;1:245-66. Crossref
37. Barber N, Parsons J, Clifford S, Darracott R, Horne R.
Patients’ problems with new medication for chronic
conditions. Qual Saf Health Care 2004;13:172-5. Crossref
38. Wilhelmsen NC, Eriksson T. Medication adherence
intervention and outcomes: an overview of systematic
reviews. Eur J Hosp Pharm 2019;26:187-92. Crossref
39. Mixon AS, Myers AP, Leak CL, et al. Characteristics
associated with post-discharge medications errors. Mayo
Clin Proc 2014;89:1041-51. Crossref
40. Lindquist LA, Go L, Fleisher J, Jain N, Friesema E,
Baker DW. Relationship of health literacy to intentional
and unintentional non-adherence of hospital discharge
medications. J Gen Intern Med 2012;27:173-8. Crossref
41. Mulhem E, Lick D, Varugese J, Barton E, Ripley T, Haveman J.
Adherence to medications after hospital discharge in the
elderly. Int J Family Med 2013;2013:901845. Crossref
42. Coleman EA, Smith JD, Raha D, Min SJ. Posthospital
medication discrepancies: prevalence and contributing
factors. Arch Intern Med 2005;165:1842-7. Crossref
43. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW.
The incidence and severity of adverse events affecting
patient after discharge from the hospital. Ann Intern Med
2003;138:161-7. Crossref
44. Caleres G, Modig S, Midlöv P, Chalmers J, Bondesson A. Medication discrepancies in discharge summaries and
associated risk factors for elderly patients with many drugs.
Drugs Real World Outcomes 2020;7:53-62. Crossref
45. National Institute for Health and Care Excellence, UK
Government. Medicines optimisation: the safe and
effective use of medicine to enable the best possible outcomes. NICE guideline [NG5]. 2015. Available from:
https://www.nice.org.uk/guidance/ng5/chapter/1-Recommendations#medicines-related-models-of-organisational-and-cross-sector-working. Accessed 23
Nov 2020.
46. Kripalani S, Roumie CL, Dala AK, et al. Effect of
a pharmacist intervention on clinically important
medication errors after hospital discharge: a randomized
controlled trial. Ann Intern Med 2012;157:1-10. Crossref
47. Iyer S, Naganathan V, McLachlan AJ, Le Couteur DG.
Medication withdrawal trials in people aged 65 years and
older: a systematic review. Drugs Aging 2008;25:1021-31. Crossref
48. van der Cammen TJ, Rajkumar C, Onder G, Sterke CS,
Petrovic M. Drug cessation in complex older adults: time
for action. Age Ageing 2014;43:20-5. Crossref
49. Declercq T, Petrovic M, Azermai M, et al. Withdrawal
versus continuation of chronic antipsychotic drugs
for behavioural and psychological symptoms in older
people with dementia. Cochrane Database Syst Rev
2013;(3):CD007726. Crossref
50. Page AT, Potter K, Clifford R, Etherton-Beer C.
Deprescribing in older people. Maturitas 2016;91:115-34. Crossref
51. NHS Derby and Derbyshire. Clinical Commissioning Group.
Deprescribing: a practical guide. NHS 2019. Available
from: http://www.derbyshiremedicinesmanagement.nhs.uk/assets/Clinical_Guidelines/clinical_guidelines_front_page/Deprescribing.pdf. Accessed 23 Nov 2020.
52. McGrath K, Hajjar ER, Kumar C, Hwang C, Salzman B.
Deprescribing: a simple method for reducing
polypharmacy. J Fam Pract 2017;66:436-45.
53. Deprescribing.org. Deprescribing guidelines and
algorithms. Available from: https://deprescribing.org/resources/deprescribing-guidelines-algorithms/. Accessed
23 Nov 2020.
54. Farrell B, Tsang C, Raman-Wilms L, Irving H, Conklin J,
Pottie K. What are priorities for deprescribing for elderly
patients? Capturing the voice of practitioners: a modified
delphi process. PLoS One 2015;10:e0122246. Crossref
55. Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD.
Patient barriers to and enablers of deprescribing: a
systematic review. Drug Aging 2013;30:793-807. Crossref
56. Topinková E, Baeyens JP, Michel JP, Lang PO. Evidence-based
strategies for the optimization of pharmacotherapy
in older people. Drugs Aging 2012;29:477-94. Crossref
57. Bonetti AF, Reis WC, Mendes AM, et al. Impact of
pharmacist-led discharge counselling on hospital
readmission and emergency department visits: a systematic
review and meta-analysis. J Hosp Med 2020;15:52-9. Crossref
58. Renaudin P, Boyer L, Esteve MA, Bertault-Peres P,
Auquier P, Honore S. Do pharmacist-led medication
reviews in hospitals help reduce hospital readmission? A
systematic review and meta-analysis. Br J Clin Pharmacol
2016;82:1660-73. Crossref
59. Chung AY, Anand S, Wong I, et al. Improving medication
safety and diabetes management in Hog Kong: a
multidisciplinary approach. Hong Kong Med J 2017;23:158-67. Crossref