Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Autochthonous Emergomyces pasteurianus
pneumonia in an immunocompromised patient
in Hong Kong: a case report
KK Chik, FHKPath, FHKPaed; WK To, FHKPath
Department of Pathology (Clinical Infection and Microbiology), Princess Margaret Hospital, Hong Kong
Corresponding author: Dr KK Chik (chikkk@ha.org.hk)
Case
A 61-year-old man with end-stage renal failure
secondary to immunoglobulin A nephropathy
underwent a cadaveric kidney transplant. His post-transplantation
course was uneventful until he had
very poor compliance to the immunosuppressants
since May 2017 and admitted that he had stopped
all immunosuppressants in February 2018. He
developed acute antibody-mediated graft rejection
in May 2018. His medications were adjusted,
tacrolimus 4 mg/day, mycophenolate 360 mg twice
daily and prednisolone were started. He developed
a chest infection in October 2018. He denied any
travel history or significant contact history. On
physical examination he had cushingoid features
and right-sided crepitation. Investigations revealed
a low white cell count (1.8 × 109/L) and neutrophil
count (0.7 × 109/L). Chest X-ray showed right basal
infiltrates (Fig 1a). Blood and sputum for bacterial
culture was negative, as was sputum for acid-fast
bacilli. The patient’s condition deteriorated despite
use of vancomycin, meropenem, azithromycin, and
fluconazole. Computed tomography of the thorax
revealed extensive collapse and consolidation over
the right lower lobe and bilateral pleural effusions
(Fig 1b). Bronchoscopy and transbronchial lung
biopsy showed granulomatous inflammation,
with granular eosinophilic material and narrow-based
small budding yeasts grouped in clusters
inside macrophages in the alveolar spaces. Both
mucicarmine staining and immunohistochemical
staining for Pneumocystis, Cytomegalovirus, and
herpes simplex virus were negative (Fig 1d-g).
In view of the histopathological findings,
bronchoalveolar lavage was sent for fungal culture.
After 7 days of incubation at 25°C, a tiny colony
of mould was seen. 21 Days later, there was a
white-coloured mould colony with a velvety texture,
wrinkled surface, acquired splits on the surface with
no diffusible pigment (Fig 2a and b). Lactophenol
cotton blue stain of the wet mount revealed a
classic floret pattern (Fig 2c and d). Subculture was
performed at 35°C. After 10 days of incubation at
35°C, a 25-mm-diameter yeast colony was evident.
Based on the characteristic growth morphology, infection with the thermally dimorphic fungus was
established. Subsequent molecular genetic analysis
by sequencing of the internal transcribed spacer
and D1/D2 regions was performed and identified
Emergomyces pasteurianus, a rare thermally
dimorphic fungus not previously reported in our
locality. Liposomal amphotericin B was commenced
and continued for 8 weeks. The patient responded
well both clinically and radiologically (Fig 1c) and
treatment was switched to oral voriconazole 200 mg
twice daily. The patient succumbed to his medical
illness 10 weeks after being discharged home.
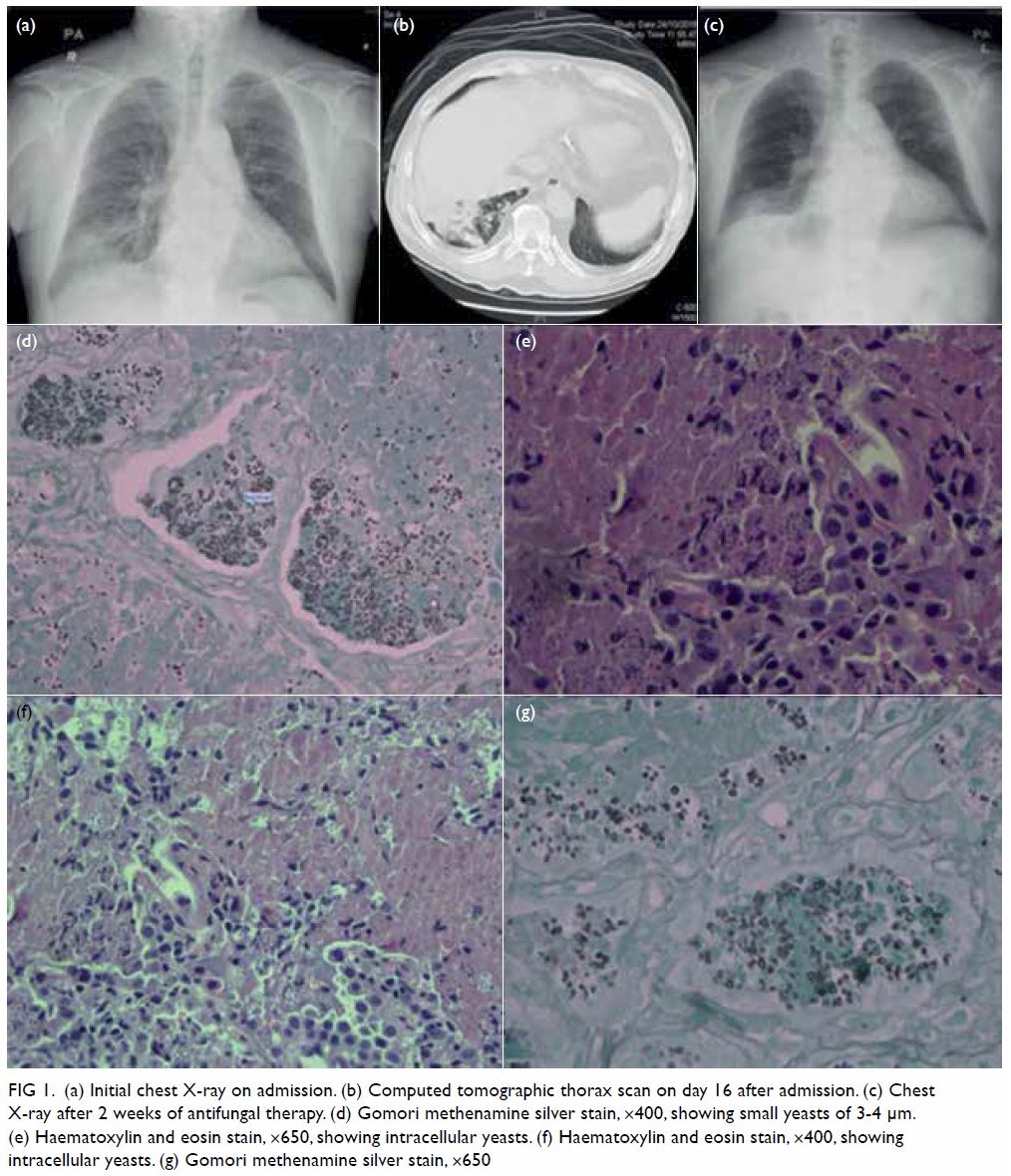
Figure 1. (a) Initial chest X-ray on admission. (b) Computed tomographic thorax scan on day 16 after admission. (c) Chest X-ray after 2 weeks of antifungal therapy. (d) Gomori methenamine silver stain, ×400, showing small yeasts of 3-4 μm. (e) Haematoxylin and eosin stain, ×650, showing intracellular yeasts. (f) Haematoxylin and eosin stain, ×400, showing intracellular yeasts. (g) Gomori methenamine silver stain, ×650
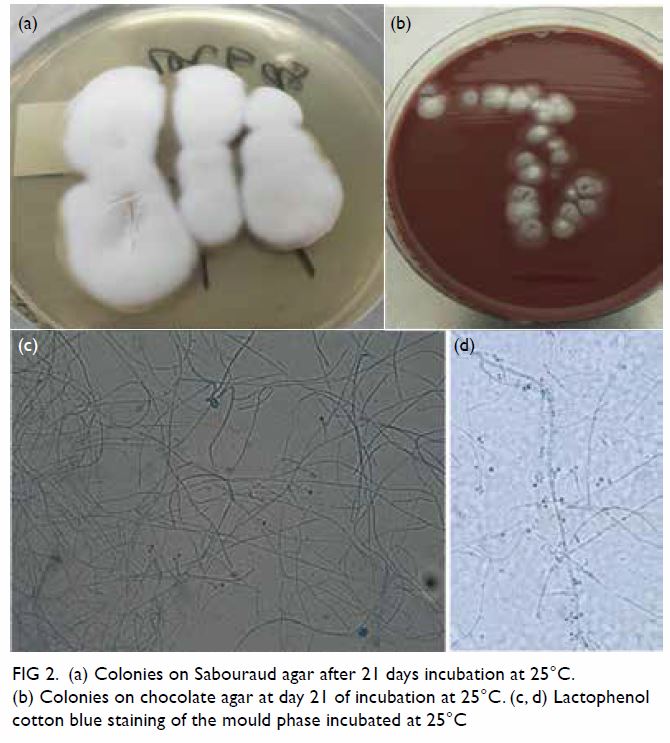
Figure 2. (a) Colonies on Sabouraud agar after 21 days incubation at 25°C. (b) Colonies on chocolate agar at day 21 of incubation at 25°C. (c, d) Lactophenol cotton blue staining of the mould phase incubated at 25°C
Discussion
Thermally dimorphic fungal pathogens cause a
significant human disease but are rarely reported
in our locality with the exception of Talaromyces
(Penicillium) marneffei. To the best of our
knowledge, this is the first report of Emergomyces
infection in Hong Kong. Emergomyces shares the
characteristics of other thermally dimorphic fungi:
filamentous forms at 25°C that becomes an invasive
yeast-like form at 35°C.1 Emergomyces pasteurianus
was previously known as Emmonsia pasteuriana.
Emmonsia species are ubiquitous, soil-dwelling
saprophytic fungi. Species such as Emmonsia
crescens and Emmonsia parva may rarely cause
adiaspiromycosis in rodents and humans.2 Recently,
Emergomyces has been reclassified as a new genus
within the family Ajellomycetaceae. Emergomyces
and Emmonsia have significant differences in
microbiology, epidemiology, clinical manifestations,
and treatment outcomes. Microbiologically,
Emergomyces is a thermally dimorphic fungus
while Emmonsia does not undergo mould-to-yeast
conversion at 37°C. Emmonsia is rarely cultivated
from clinical specimens. Human infection with
Emmonsia is relatively rare in clinical practice2
but Emergomyces can cause fatal and disseminated
human infection and appropriate antifungal therapy
is essential.
At the time of this report, five Emergomyces
species are described. These species differ in
geographic distribution. Es pasteurianus has been
reported in Europe, Asia, and Africa. Emergomyces canadensis has been reported in Canada and the
United States. Emergomyces africanus has been
reported in South Africa, Emergomyces orientalis in
China, and Emergomyces europaeus in Germany. The
natural reservoir of Emergomyces is soil. Our patient
presumably acquired the infection via inhalation of
conidia in Hong Kong since he had no travel history
outside of the region.
Emergomycosis is a multi-system disease.
According to case reports, patients most often
present with fever, widespread skin lesions, weight loss, and pulmonary disease.3 4 The diagnosis can
be missed due to the slow-growing nature of the
fungus. Histological examination of tissues can
help diagnose the disease but on its own does not
distinguish infection from other dimorphic fungi.
Molecular diagnosis plays an important role in
microbiological investigation. Using broad-range
fungal polymerase chain reaction to sequence D2
large-subunit rDNA gene can help to confirm the
diagnosis in a rapid, sensitive, and specific way.
There are no treatment guidelines for patients with emergomycosis. Guidelines for blastomycosis
and histoplasmosis recommend liposomal
amphotericin B as initial therapy followed by
itraconazole (or other newer azole). Our patient
responded to liposomal amphotericin B and oral
voriconazole but passed away due to his medical
disease.
More patients are now rendered
immunosuppressed by advances in treatment for a
variety of diseases. Clinicians and microbiologists
should be aware of the presence of rare invasive
fungal infections among these susceptible patients. Molecular techniques such as internal transcribed
spacer polymerase chain reaction and sequencing
can aid early and accurate identification of these rare
fungal pathogens.
Author contributions
All authors contributed to the concept or design of the study,
acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgement
The authors would like to thank pathologist Dr WL Lam for providing the histopathology clinical photos and nephrologist
Dr SK Fung and Dr HL Tang for providing clinical information.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This case report was approved by the Hospital Authority Kowloon West Cluster Research Ethics Committee (Ref KW/
EX-19-063(137-04)).
References
1. Gast KB, van der Hoeven A, de Boer MG, et al. Two
cases of Emergomyces pasteurianus infection in
immunocompromised patients in the Netherlands. Med
Mycol Case Rep 2019;24:5-8. Crossref
2. Koneru H, Penupolu S. Pulmonary adiaspiromycosis: an
emerging fungal infection. Chest 2017;152 Suppl:A162. Crossref
3. Schwartz IS, Sanche S, Wiederhold NP, Patterson TF,
Sigler L. Emergomyces canadensis, a dimorphic fungus
causing fatal systemic human disease in North America.
Emerg Infect Dis 2018;24:758-61. Crossref
4. Schwartz IS, Maphanga TG, Govender NP. Emergomyces:
a new genus of dimorphic fungal pathogens causing
disseminated disease among immunocompromised
persons globally. Curr Fungal Infect Rep 2018;12:44-50. Crossref

