Hong Kong Med J 2020 Oct;26(5):372–81 | Epub 9 Jul 2020
Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Burden of pneumococcal disease: 8-year
retrospective analysis from a single centre in Hong Kong
MY Man, MB, BS, FHKAM (Medicine)1; HP Shum, MB, BS, MD1; Judianna SY Yu, MB, BS, MRCP (UK)2; Alan Wu, MB, ChB, FRCPath (UK)3; WW Yan, FRCP, FHKAM (Medicine)1
1 Department of Intensive Care, Pamela Youde Nethersole Eastern Hospital, Hong Kong
2 Department of Medicine and Geriatrics, Ruttonjee and Tang Shiu Kin Hospital, Hong Kong
3 Department of Clinical Pathology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
Corresponding author: Dr MY Man (mmy553@ha.org.hk)
Abstract
Purpose: Streptococcus pneumoniae is a common
pathogen involved in community-acquired
pneumonia. Invasive pneumococcal disease is often
associated with higher co-morbidity rates, but
mortality-related findings have been inconclusive.
This study investigated predictors of 30-day mortality
and invasive pneumococcal disease.
Methods: This retrospective analysis included adults
with pneumococcal disease who were admitted to
Pamela Youde Nethersole Eastern Hospital from
1 January 2011 to 31 December 2018. Demographics,
microbiological characteristics, and outcomes
were compared between 30-day survivors and
non-survivors, and between patients with invasive
disease and those with non-invasive disease.
Intensive care unit (ICU) subgroup analysis was
performed. The primary outcome was 30-day
all-cause mortality; secondary outcomes were ICU
and hospital mortalities, and ICU and hospital
lengths of stay.
Results: In total, 792 patients had pneumococcal
disease; 701 survived and 91 (11.5%) died within
30 days. Notably, 106 (13.4%) patients had invasive
pneumococcal disease and 170 (21.5%) patients
received intensive care. Vasopressor use (odds
ratio [OR]=4.96, P<0.001), chronic kidney disease
(OR=3.62, P<0.001), positive urinary antigen test
results (OR=2.57, P=0.001), and advanced age (OR=2.19, P=0.010) were independent predictors
for 30-day mortality by logistic regression analysis.
Among critically ill patients, chronic kidney disease
(OR=4.64, P<0.001), higher APACHE IV score
(OR=3.73, P=0.016), and positive urinary antigen
test results (OR=2.94, P=0.008) were predictors
for 30-day mortality. Logistic regression analysis
revealed that chronic kidney disease (OR=3.10,
P<0.001) was a risk factor for invasive pneumococcal
disease.
Conclusion: Advanced age, vasopressor use,
chronic kidney disease, and positive urinary antigen
test results were independent predictors for 30-day
mortality in patients with pneumococcal disease.
New knowledge added by this study
- This is one of the largest studies thus far regarding pneumococcal infection in Hong Kong; it also includes an analysis of critically ill patients.
- Invasive pneumococcal disease was associated with greater disease severity and higher rates of invasive organ support. Positive urinary pneumococcal antigen test results were associated with increased 30-day mortality rates in all patients, as well as patients in the intensive care unit.
- The 30-day mortality predictors of pneumococcal disease included vasopressor use, chronic kidney disease, positive urinary antigen test results, and advanced age.
- Invasive pneumococcal disease is associated with more severe disease and higher mortality rates. Rapid identification and treatment can improve patient outcomes.
- Increasing use of the urinary antigen test was observed during the study period. A positive urinary antigen test result can serve as an independent predictor for 30-day mortality in all patients, as well as patients in the intensive care unit.
Introduction
Streptococcus pneumoniae causes a wide range
of diseases that include middle ear infection,
sinusitis, pneumonia, and meningitis. As one of the most common pathogens in community-acquired
pneumonia (especially in Western countries),
S pneumoniae infection contributed to 1.6 million
deaths in 2010 and 3.7 million severe pneumococcal infections worldwide in 2015.1 2 3
Streptococcus pneumoniae is a gram-positive
encapsulated bacterium that colonises human
nasopharynx and is mainly transmitted via
respiratory droplets, which cause middle ear and
respiratory tract infection. Thus far, more than
90 serotypes of S pneumoniae have been identified.
Streptococcus pneumoniae infection can be stratified
into invasive and non-invasive disease.4 5 Invasive
pneumococcal disease (IPD) is a notifiable disease
in Hong Kong. In 2019, there were 187 cases;
the incidence has remained similar over the past
few years.6 Worldwide, there is growing concern
regarding drug-resistant S pneumoniae strains (eg,
strains resistant to macrolide, penicillin, and/or
fluoroquinolone). However, drug-resistant strains
have not been associated with higher mortality rates.7
The prevalence of drug-resistant S pneumoniae is
lower in Southeast Asia than in Western countries.1
Despite inconclusive evidence in the literature
regarding its association with mortality, IPD is often
associated with more severe disease and requires
more invasive organ support.8
In this study, we aimed to identify the predictors
for 30-day mortality in patients with S pneumoniae
infection, as well as predictors for IPD. We also
performed subgroup analysis of patients in the intensive care unit (ICU) and identified risk factors
for 30-day mortality and IPD in those patients, as
well as all patients with S pneumoniae infection.
Methods
Study design and data collection
This retrospective cohort study included adults who
were admitted to Pamela Youde Nethersole Eastern
Hospital, Hong Kong, with pneumococcal infection
from 1 January 2011 to 31 December 2018. Patients
who were aged <18 years or had incomplete data
were excluded.
Patient medical records and data were
extracted from clinical management systems and
clinical information systems (IntelliVue Clinical
Information Portfolio; Philips Medical, Amsterdam,
The Netherlands). Baseline demographics, clinical
characteristics, and microbiological data were
identified. For patients in the ICU, disease severity
was quantified using APACHE (Acute Physiology
and Chronic Health Evaluation) IV scores. The use
of invasive organ support was recorded, including
continuous renal replacement therapy, inotropes,
invasive mechanical ventilation, and extracorporeal
membrane oxygenation. The primary outcome was
30-day all-cause mortality; secondary outcomes
were ICU and hospital mortalities, ICU and hospital
length of stay (LOS), and ICU ventilator days.
Definitions
Pneumococcal infection was determined by positive
culture of S pneumoniae. Invasive pneumococcal
disease was defined as the presence of S pneumoniae
in sterile sites (eg, pleural fluid, cerebrospinal fluids,
and blood).4 8 Non-invasive pneumococcal disease
was defined as the presence of S pneumoniae in
non-sterile sites, or a positive urinary antigen test
(UAT) result. Medical co-morbidities (eg, diabetes
mellitus, chronic kidney disease, heart failure,
and haematological malignancies) were coded in
accordance with the International Classification
of Diseases, Ninth Revision, Clinical Modification.
Smokers were defined as those who had ever smoked.
Advanced age was defined as age >65 years.
Microbiology
Antibiotic resistance was determined based on
Clinical and Laboratory Standards Institute testing
criteria for minimal inhibitory concentrations.
Breakpoints adopted for determination of parenteral
penicillin resistance in non-meningitis S pneumoniae
isolates were susceptible, ≤2 μg/mL; intermediate,
4 μg/mL; and resistance, ≥8 μg/mL.9 Breakpoints
adopted for determination of parenteral penicillin
resistance in meningitis S pneumoniae isolates
were susceptible, ≤0.06 μg/mL and resistance, ≥0.12 μg/mL; breakpoints adopted for determination
of levofloxacin resistance in S pneumoniae were
susceptible, ≤2 μg/mL; intermediate, 4 μg/mL; and
resistance, ≥8 μg/mL.9
Urinary antigen test (Alere 710-012
BinaxNOW Streptococcus) results were evaluated in
accordance with the manufacturer’s instructions.
Statistical analysis
Characteristics and clinical parameters were
compared between patients with IPD and those
with non-invasive pneumococcal disease, as well as
between 30-day survivors and non-survivors. Results
were expressed as median (interquartile range) or
as numbers (percentages) of cases, as appropriate.
For univariate analysis, categorical variables were
compared by Pearson Chi squared tests or Fisher’s
exact test, as appropriate; continuous variables
were compared by using the Mann-Whitney U test.
Variables with P<0.2 in univariate analysis or
with known clinical significance from previous
studies were entered into multivariate analysis.
Independent predictors for 30-day mortality and
independent predictors for IPD were assessed by
logistic regression analysis.8 10 11 12 Subgroup analysis
was performed regarding IPD and disease severity
among patients in the ICU. Hosmer-Lemeshow
test was performed for goodness-of-fit for logistic
regression models. Kaplan-Meier survival plots
were used to compare cumulative survival between
patients with IPD and those with non-invasive
pneumococcal disease. SPSS (Mac version 24.0;
IBM Corp, Armonk [NY], United States) was used
for all statistical analyses.
Results
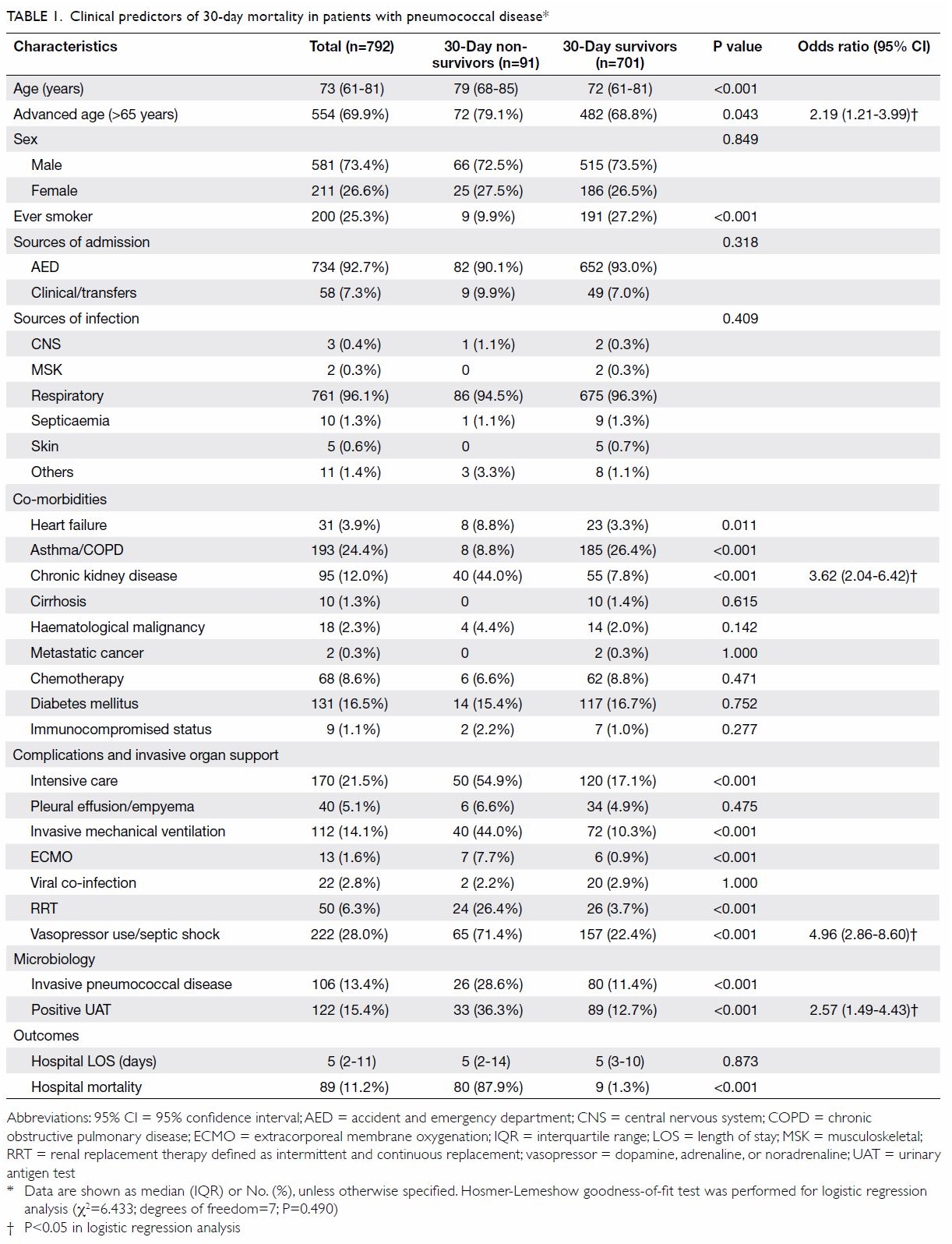
Patient demographic and clinical characteristics,
including co-morbidities and use of invasive organ
support, are shown in Table 1. In total, 792 patients
with pneumococcal disease were identified during
the 8-year study period. The median age was 73 years;
patients were predominantly men. Most patients
exhibited respiratory tract infection (96.1%) and
approximately one quarter of patients had
asthma/chronic obstructive pulmonary disease
(24.4%). In total, 170 patients received intensive
care and 14.1% required invasive mechanical
ventilation; 28% required vasopressor use. Invasive
pneumococcal disease was present in 13.4% of the
patients. The overall hospital mortality rate was
11.2%, while the mortality rate among patients in the
ICU was 22.9%.
Invasive pneumococcal disease was associated
with a higher 30-day mortality rate (28.6% vs 11.4%,
P<0.001); a positive UAT result was also associated
with a higher 30-day mortality rate (36.3% vs 12.7%,
P<0.001). Logistic regression analysis identified statistically significant predictors for 30-day
mortality, which are shown in Table 1. Patients with
vasopressor use (odds ratio [OR]=4.96, P<0.001),
chronic kidney disease (OR=3.62, P<0.001), a positive
UAT result (OR=2.57, P=0.001), and older age
(OR=2.19, P=0.010) exhibited comparatively higher
30-day mortality rates; however, asthma/chronic
obstructive pulmonary disease was not an independent
predictor for mortality in logistic regression analysis.
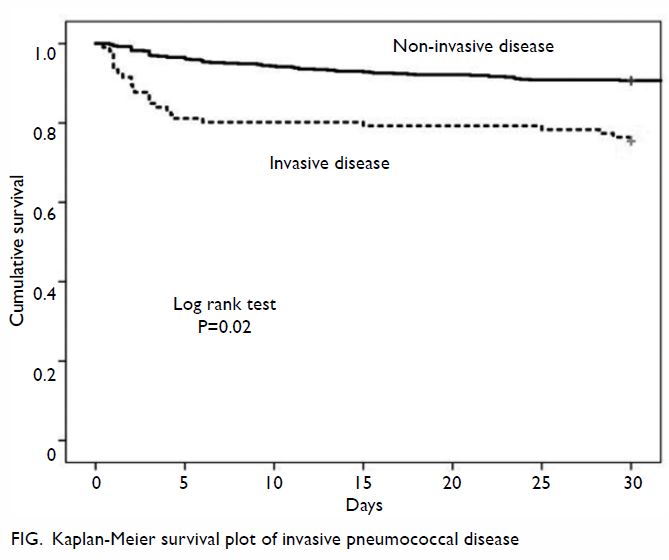
The Figure depicts the results of Kaplan-Meier
survival analysis comparing patients with IPD and
those with non-invasive pneumococcal disease.
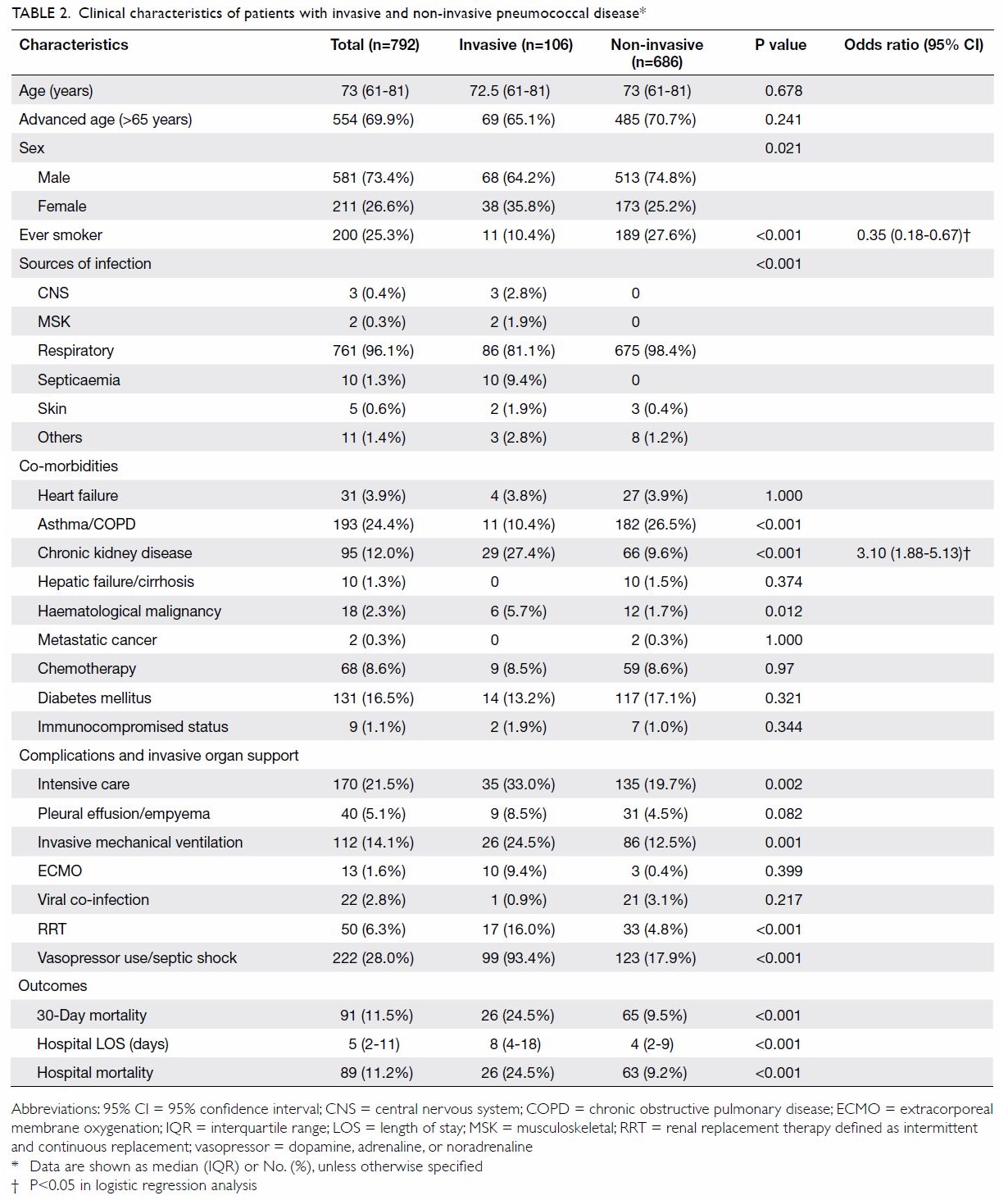
Table 2 shows the characteristics of patients
with IPD and those with non-invasive pneumococcal
disease. More patients with asthma/chronic
obstructive pulmonary disease exhibited non-invasive
pneumococcal disease (26.5% vs 10.4%, P<0.001).
Invasive pneumococcal disease was more likely to be
associated with renal failure (27.4% vs 9.6%, P<0.001)
and haematological malignancy (5.7% vs 1.7%,
P=0.012). Additionally, IPD was associated with
higher rates of ICU admission (33.0% vs 19.7%,
P=0.002), renal replacement therapy (16.0% vs 4.8%,
P<0.001), and vasopressor use (93.4% vs 17.9%,
P<0.001). Patients with IPD had a higher 30-day
mortality rate (24.5% vs 9.5%, P<0.001) and longer
hospital LOS (8 vs 4 days, P<0.001). Independent
risk factors for IPD by logistic regression analysis
are shown in Table 2, along with their ORs. Notably,
chronic kidney disease (OR=3.10, P<0.001) was the
sole independent predictor for IPD.
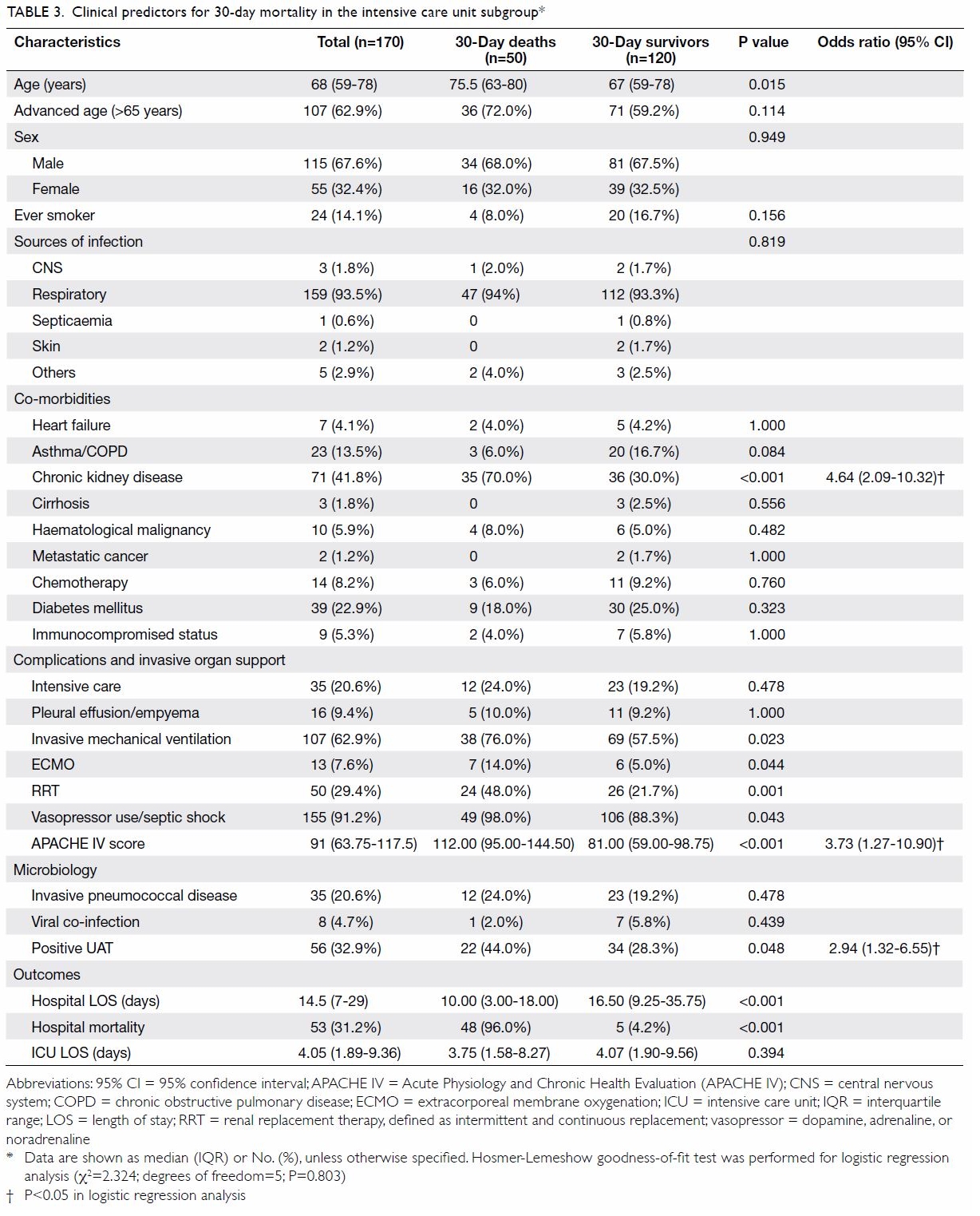
The results of ICU subgroup analysis are
shown in Table 3. Respiratory tract infection
constituted 93.5% of all S pneumoniae infections.
The rate of IPD was 20.6% among patients in the
ICU with S pneumoniae infection, which was higher
than the rate among all patients with S pneumoniae
infection. Further analysis revealed that IPD was
associated with higher rates of complications and
invasive organ support; in particular, more patients
with IPD required renal replacement therapy
(48.6% vs 24.4%, P=0.005) and vasopressor use
(100% vs 88.9%, P=0.039). Additionally, more
patients with IPD tended to exhibit pleural
effusion/empyema, although this difference was
not statistically significant. Patients who required
invasive mechanical ventilation (76.0% vs 57.5%,
P=0.023), extracorporeal membrane oxygenation
(14.0% vs 5.0%, P=0.044), renal replacement therapy
(48.0% vs 21.7%, P=0.001), and vasopressor use
(98.0% vs 88.3%, P=0.043) exhibited significantly
higher 30-day mortality rates. Logistic regression
analysis showed that chronic kidney disease
(OR=4.64, P<0.001), higher APACHE IV score
(OR=3.73, P=0.016), and a positive UAT result
(OR=2.94, P=0.008) were independent predictors for
30-day mortality among patients in the ICU who had
IPD (Table 3).
Discussion
Medical co-morbidity and mortality
The overall mortality rate was 28.6% for patients with
IPD and 11.4% for patients without IPD. The case
fatality rate in our cohort was higher than that in a
previous cohort from the Netherlands, but similar to
the rate in a previous study from Korea.5 13 A higher
number of co-morbid diseases, worse immune
function, impaired mucociliary clearance, and older
age are associated with a higher risk of mortality in
patients with pneumococcal infection.4
Chronic conditions such as chronic lung
disease, heart failure, and diabetes, as well as
smoking status, were previously shown to be
associated with pneumococcal disease and IPD.10 11
Consistent with the results of prior studies, we
found that patients with heart failure (8.8% vs 3.3%,
P=0.011) and haematological malignancies
(5.7% vs 1.7%, P=0.012) exhibited significantly
higher 30-day mortality rates in univariate analysis.
Surprisingly, we found a negative association
between chronic lung disease and mortality. In
post-hoc analysis, we found that patients with
chronic lung disease (ie, asthma/chronic obstructive
pulmonary disease) also had a lower rate of invasive
organ support (15.0% vs 32.7%, P<0.001). This
group of patients may be under constant medical
surveillance; thus, they may seek medical attention
and receive antibiotics earlier than patients without
chronic lung disease. Importantly, we did not
examine the management and status of underlying
lung conditions, which may have affected mortality
in these patients.
Pneumococcal urinary antigen test
In our cohort, 122 patients were diagnosed with
pneumococcal infection by using the UAT. In our
hospital, the first patient was diagnosed by using
the UAT in 2015. Use of the UAT in diagnosing
community-acquired pneumonia has since
increased; thus, in 2018, 71 of 146 patients (48.6%)
were diagnosed by using the UAT. A positive UAT
result was a consistent independent predictor for
30-day mortality among patients in the ICU, as well
as among all patients. Post-hoc analysis showed that
a positive UAT result was significantly associated
with ICU admission (34.7% vs 10.1%, P<0.001).
However, it was not significantly associated with
ICU LOS (6.16 vs 8.43 days, P=0.515) or hospital
LOS (21.46 vs 29.78 days, P=0.415).
The pneumococcal UAT assay detects
the C-polysaccharide antigen of S pneumoniae,
which is present in all serotypes, from urine
samples.14 Fluorescence immunoassay and
immunochromatographic test methods provide
similar results in terms of diagnosing pneumococcal
disease.15 While the UAT result remains positive for up to 3 days after initiation of antibiotic
treatment, the UAT increases the diagnostic yield of
pneumococcal disease relative to the yield of sputum
culture of S pneumoniae; notably, the yield of such
sputum cultures markedly decreases after initiation
of antibiotic treatment.16 This test provides a rapid
and simple method for diagnosis of patients with
suspected S pneumoniae infection; it is particularly
helpful in the diagnosis of patients who cannot
produce sputum for cultures. The test sensitivity
and specificity were approximately 60% and 99%,
respectively.16 Because of the high test specificity, the
UAT helps to reduce the costs of further diagnostic
tests and aids in selection of empirical antibiotic
treatment. It is recommended in the Infectious
Diseases Society of America/American Thoracic
Society guidelines for aiding the rapid identification
of pneumococcal disease in adults.17 Urinary antigen
tests were also found to predict the severity and
outcomes of pneumonia. A Korean group found
that patients with positive UAT results exhibited
greater severity of disease; however, the test results
were associated with rates of ICU admission and
mortality.14
Counterindications for the UAT include recent
pneumococcal disease within 3 months; moreover,
it may cross-react with antigens from other
streptococcal bacteria.16 18 Patients with acute kidney
injury due to sepsis, as well as those with oliguria
or anuria of various aetiologies may not be able to
provide urine samples for use in the UAT.
Invasive pneumococcal disease
In our cohort, IPD was associated with a higher
30-day mortality rate; however, this association
did not remain statistically significant in logistic
regression analysis. Consistent with the results of
previous studies,8 12 we found that patients with IPD
exhibited more severe disease and worse outcomes.
Moreover, IPD was associated with higher rates
of ICU admission, invasive organ support (ie,
vasopressor use), and renal replacement therapy,
as well as longer hospital LOS. The findings might
be explained by the higher bacterial load in patients
with IPD, which may lead to worse outcomes.
Similar to the study by Ceccato et al,8 we did
not identify a positive relationship between smoking
and IPD. Thus far, results regarding the relationship
of smoking with IPD have been inconsistent; the
association varies according to local smoking
prevalence.11 With the implementation of effective
smoking cessation programmes and corresponding
legislation in Hong Kong, approximately 10% of
individuals >15 years of age report daily cigarette
consumption; this is markedly lower than the
rates in other countries.19 20 In our study, smoking
status information was extracted from patient
records stored in the Hospital Authority Clinical
Management System and nursing notes; thus, we
may have underestimated the number of smokers
in this cohort. Other important aspects of smoking
(eg, number of pack-years and passive smoking)
were not available for inclusion in this analysis.
Chronic kidney disease has been consistently
associated with IPD. A large retrospective
observational cohort of 36 million adults revealed
a risk ratio of 21.67 for development of IPD among
patients with chronic kidney disease.21 A Japanese
registry showed that the relative risk for IPD among
patients with chronic kidney disease ranged from
12.4 to 51.3.10 Notably, chronic kidney disease was
consistently one of the most important predictors
for 30-day mortality among all patients (OR=3.62,
P<0.001) and among patients in the ICU (OR=4.64,
P<0.001).
Intensive care subgroup
Patients with IPD tended to experience a higher rate
of complications and require higher rates of invasive
organ support. In particular, patients with IPD more
frequently exhibited pleural effusion/empyema; they
also more frequently required invasive mechanical
ventilation, extracorporeal membrane oxygenation,
renal replacement therapy, and vasopressor use. Our
sample size may not have been sufficiently powered
to demonstrate statistically significant results
regarding the ICU subgroup; thus, future studies
focused specifically on patients in the ICU may be
needed. Other aspects of IPD and use of rescue therapies for acute respiratory distress syndrome
(eg, prone ventilation, muscle paralytic agents, and
inhaled nitrogen oxide) should be investigated in the
future.
Drug non-susceptible Streptococcus
pneumoniae and viral co-infection
Penicillin non-susceptible S pneumoniae was not
common in the present study; it was only observed in
2.4% of patients. Non-susceptibility to levofloxacin
was observed in 0.9% of patients. Drug non-susceptible
S pneumoniae were not significantly associated
with 30-day mortality (penicillin non-susceptible
S pneumoniae was present in two non-survivors and
14 survivors, P=0.641; levofloxacin non-susceptible
S pneumoniae was present in zero non-survivors
and six survivors, P=1.000). However, these results
should be carefully interpreted, because of the small
number of drug non-susceptible S pneumoniae in our
cohort. According to a recent study in Hong Kong,
the penicillin resistance rate was approximately 7%
and the levofloxacin resistance rate was 0%.22
Viral-bacterial interactions have been
described with respect to pneumococcal disease.23
An epidemiological study regarding the 2009 H1N1
influenza pandemic period showed a significant
increase in the number of pneumococcal pneumonia
hospitalisations.24 However, viral co-infection was
not associated with IPD or mortality in our findings.
Notably, an age-specific interaction was described
between influenza and IPD; specifically, patients
aged 5 to 19 years were significantly more frequently
affected, compared with other age-groups.24 25
Strengths and limitations
Thus far, this is the first and largest study regarding
pneumococcal disease in adults in Hong Kong; it
provides clinical and outcome data in both general
ward and intensive care subgroups to allow a
comprehensive overview of pneumococcal disease in
the locality. It is a standard practice in our centre to
check urinary antigens and perform blood cultures
for nearly all patients with suspected pneumonia
to facilitate accurate diagnosis and avoid missed
diagnoses. By including data regarding invasive organ
support and ICU admission, we were able to identify
and describe complications of pneumococcal disease
and determine the broader clinical characteristics of
affected patients.
However, because of changes in vaccination
programmes, the influenza and pneumococcal
vaccination statuses were not available for analysis
in the current study. Because of the limited
number of patients with drug non-susceptible
S pneumoniae in the present cohort, further robust
analyses regarding antibiotic sensitivity patterns and
appropriateness of antimicrobial treatment could not be performed. Furthermore, capsular serotypes
of S pneumoniae among patients in our cohort were
not available for analysis. Future studies focused on
capsular subtypes of S pneumoniae will facilitate
understanding of pneumococcal disease. Because
this was a retrospective study, it was subject to
potential confounding factors. Finally, the results
of this single-centre study may not be generalisable
to other countries with higher prevalences of drug
non-susceptible S pneumoniae infection.
Conclusion
Pneumococcal disease is associated with high
rates of morbidity and mortality. In this cohort,
vasopressor use, chronic kidney disease, advanced
age, and positive UAT results were predictors for
30-day mortality.
Author contributions
Concept or design: MY Man, HP Shum.
Acquisition of data: MY Man, HP Shum.
Analysis or interpretation of data: MY Man, HP Shum.
Drafting of the manuscript: MY Man, A Wu.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: MY Man, HP Shum.
Analysis or interpretation of data: MY Man, HP Shum.
Drafting of the manuscript: MY Man, A Wu.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Declaration
The abstract of this study was accepted as an oral presentation
at the Annual Scientific Meeting of the Hong Kong Society of
Critical Care Medicine on 8 December 2019.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approval by the Hospital Authority Hong Kong
East Cluster Research Ethics Committee (Ref HKECREC-
2019-065). The requirement for written informed consent
was waived.
References
1. Aliberti S, Cook GS, Babu BL, et al. International prevalence
and risk factors evaluation for drug-resistant Streptococcus
pneumoniae pneumonia. J Infect 2019;79:300-11. Crossref
2. Black RE, Cousens S, Johnson HL, et al. Global, regional,
and national causes of child mortality in 2008: a systematic
analysis. Lancet 2010;375:1969-87. Crossref
3. Wahl B, O’Brien KL, Greenbaum A, et al. Burden of
Streptococcus pneumoniae and Haemophilus influenzae
type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet
Glob Health 2018;6:e744-57. Crossref
4. Drijkoningen JJ, Rohde GG. Pneumococcal infection in
adults: burden of disease. Clin Microbiol Infect 2014;20
Suppl 5:45-51. Crossref
5. Song JY, Choi JY, Lee JS, et al. Clinical and economic burden
of invasive pneumococcal disease in adults: a multicenter
hospital-based study. BMC Infect Dis 2013;13:202. Crossref
6. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. Number of notifiable infectious
diseases by month. 2019. Available from: https://www.chp.
gov.hk/en/statistics/data/10/26/43/6830.html. Accessed 5
May 2020.
7. Cillóniz C, de la Calle C, Dominedò C, et al. Impact of
cefotaxime non-susceptibility on the clinical outcomes
of bacteremic pneumococcal pneumonia. J Clin Med
2019;8:1150. Crossref
8. Ceccato A, Torres A, Cilloniz C, et al. Invasive disease vs
urinary antigen-confirmed pneumococcal community-acquired
pneumonia. Chest 2017;151:1311-9. Crossref
9. Clinical and Laboratory Standards Institute. M100
Performance standards for antimicrobial susceptibility
testing. 29th ed. CLSI supplement M100. Available from:
https://clsi.org/media/2663/m100ed29_sample.pdf.
Accessed 22 Feb 2020.
10. Imai K, Petigara T, Kohn MA, et al. Risk of pneumococcal
diseases in adults with underlying medical conditions: a
retrospective, cohort study using two Japanese healthcare
databases. BMJ Open 2018;8:e018553. Crossref
11. Torres A, Blasi F, Dartois N, Akova M. Which individuals
are at increased risk of pneumococcal disease and why?
Impact of COPD, asthma, smoking, diabetes, and/or
chronic heart disease on community-acquired pneumonia
and invasive pneumococcal disease. Thorax 2015;70:984-9. Crossref
12. Heo JY, Seo YB, Choi WS, et al. Incidence and case
fatality rates of community-acquired pneumonia and
pneumococcal diseases among Korean adults: catchment
population-based analysis. PLoS One 2018;13:e0194598. Crossref
13. van Mens SP, van Deursen AM, de Greeff SC, et al.
Bacteraemic and non-bacteraemic/urinary antigen-positive
pneumococcal community-acquired pneumonia
compared. Eur J Clin Microbiol Infect Dis 2015;34:115-22. Crossref
14. Kim B, Kim J, Jo YH, et al. Prognostic value of pneumococcal
urinary antigen test in community-acquired pneumonia.
PLoS One 2018;13:e0200620. Crossref
15. Olofsson E, Özenci V, Athlin S. Evaluation of the sofia
S. pneumoniae FIA for detection of pneumococcal antigen
in patients with bloodstream infection. J Clin Microbiol
2019;57:e01535-18. Crossref
16. Molinos L, Zalacain R, Menéndez R, et al. Sensitivity,
specificity, and positivity predictors of the pneumococcal
urinary antigen test in community-acquired pneumonia.
Ann Am Thorac Soc 2015;12:1482-9. Crossref
17. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious
Diseases Society of America/American Thoracic Society
consensus guidelines on the management of community-acquired
pneumonia in adults. Clin Infect Dis 2007;44
Suppl 2:S27-72.
18. Blaschke AJ. Interpreting assays for the detection of
Streptococcus pneumoniae. Clin Infect Dis 2011;52 Suppl 4:
S331-7.Crossref
19. Census and Statistics Department, Hong Kong SAR
Government. Thematic Household Survey Report—Report No. 70—Pattern of smoking. Available from:
https://www.censtatd.gov.hk/hkstat/sub/sp453.
jsp?productCode=C0000047. Accessed 5 May 2020.
20. Wang TW, Asman K, Gentzke AS, et al. Tobacco product
use among adults—United States, 2017. MMWR Morb
Mortal Wkly Rep 2018;67:1225-32. Crossref
21. Zhang D, Petigara T, Yang X. Clinical and economic burden
of pneumococcal disease in US adults aged 19-64 years
with chronic or immunocompromising diseases: an
observational database study. BMC Infect Dis 2018;18:436. Crossref
22. Chan KC, Ip M, Chong PS, Li AM, Lam HS, Nelson EA.
Nasopharyngeal colonisation and antimicrobial resistance
of Streptococcus pneumoniae in Hong Kong children younger than 2 years. Hong Kong Med J 2018;24 Suppl
6:4-7.
23. Blasi F, Mantero M, Santus P, Tarsia P. Understanding the
burden of pneumococcal disease in adults. Clin Microbiol
Infect 2012;18 Suppl 5:7-14. Crossref
24. Weinberger DM, Simonsen L, Jordan R, Steiner C, Miller M,
Viboud C. Impact of the 2009 influenza pandemic on
pneumococcal pneumonia hospitalizations in the United
States. J Infect Dis 2012;205:458-65. Crossref
25. Chiavenna C, Presanis AM, Charlett A, et al. Estimating
age-stratified influenza-associated invasive pneumococcal
disease in England: a time-series model based on
population surveillance data. PLoS Med 2019;16:e1002829. Crossref