© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
Hong Kong Society of Clinical Blood
Management recommendations for
implementation of patient blood management
YF Chow, FHKAM (Anaesthesiology)1; Benny CP Cheng, FHKAM (Anaesthesiology)2; HK Cheng, FHKAM (Anaesthesiology)3; Betty Ho, FHKAM (Anaesthesiology)4; CK Lee, FHKAM (Medicine)5; SK Ng, FHKAM (Anaesthesiology)6; Rita So, FHKAM (Anaesthesiology)7; KC Tse, FHKAM (Anaesthesiology)3; Cindy Tsui, FHKAM (Anaesthesiology)8; Ryan Wan, FHKAM (Anaesthesiology)6; Steven Wong, FHKAM (Anaesthesiology)1, for the Hong Kong Society of Clinical Blood Management Limited
1 Department of Anaesthesiology and OT Services, Queen Elizabeth Hospital, Hong Kong
2 Department of Anaesthesia and Operating Theatre Services, Tuen Mun Hospital, Hong Kong
3 Department of Anaesthesia and Operating Theatre Services, Tseung
Kwan O Hospital, Hong Kong
4 Department of Anaesthesiology, Pamela Youde Nethersole Eastern
Hospital, Hong Kong
5 Hong Kong Red Cross Blood Transfusion Service, Hong Kong
6 Private Practice, Hong Kong
7 Department of Anaesthesia, Princess Margaret Hospital, Hong Kong
8 Department of Anaesthesia and Intensive Care, Prince of Wales
Hospital, Hong Kong
Corresponding author: Dr YF Chow (yfchowhk@yahoo.com.hk)
Abstract
Patient blood management (PBM) is a patient-centred,
multidisciplinary approach to optimise
red cell mass, minimise blood loss, and manage
tolerance to anaemia in an effort to improve patient
outcomes. Well-implemented PBM improves patient
outcomes and reduces demand for blood products.
The multidisciplinary approach of PBM can often
allow patients to avoid blood transfusions, which are
associated with less favourable clinical outcomes.
In Hong Kong, there has been increasing demand
for blood in the ageing population, and there are
simultaneous blood safety and donor issues that are
adversely affecting the blood supply. To address these
challenges, the Hong Kong Society of Clinical Blood
Management recommends implementation of a
PBM programme in Hong Kong, including strategies
such as optimising red blood cell mass, improving
anaemia management, minimising blood loss, and
rationalising the use of blood and blood products.
Introduction
Clinical blood transfusion remains an essential and
irreplaceable part of modern medicine, either as an
independent therapeutic modality or an additional
support to other clinical therapies. Anaemia, a
serious disease with a worldwide burden on both
hospitalised patients and society,1 2 3 is often managed
with blood transfusion as part of the treatment.
Without a reliable substitute, sourcing of the blood
used in transfusion relies solely on donations from
voluntary, non-remunerated blood donors. Because
blood is a biological substance, it is impossible to
completely eliminate adverse outcomes during
and after transfusion. Worldwide, particularly in
developed countries, the ageing of the population
and emerging infectious diseases are the two most
important and ongoing threats to the sustainability
of the safe blood supply. Ageing populations tend
to have increased numbers of complex surgeries
and cancer treatments requiring increased blood
transfusions.4 In 2016, the mean per capital blood use in high-income countries was 32 units of red
cell components per 1000 population. Moreover,
the most frequently transfused patient group is
aged >60 years, accounting for up to 79% among
these transfusions.5 Infectious pathogens continue
to emerge rapidly, which could adversely affect
transfusion safety both directly (if the pathogen
is transmitted through blood transfusion) and
indirectly (if outbreaks reduce the pool of available
donors).6 Recent examples include the Zika virus
outbreak in South America and the dengue,
hepatitis E, and chikungunya virus outbreaks
in Southeast Asia.7 Therefore, maintenance of a
sustainable and safe blood supply continues to be a
challenging task that requires a substantial amount
of effort and resources.
There is evidence associating blood
transfusion with less favourable clinical outcomes.8 9
This evidence includes a higher incidence of
recurrence in cancer surgeries, higher operative
mortality, failure to rescue from sepsis, and other serious complications like renal, neurological,
cardiac, and pulmonary dysfunction.10 Patient
blood management (PBM) is a patient-centred and
multidisciplinary framework that has been rapidly
developing throughout the last decade in Western
countries to improve the treatment outcomes of
patients who may need blood transfusions during
their treatment. It refers to evidence-based medical
and surgical concepts designed to improve patient
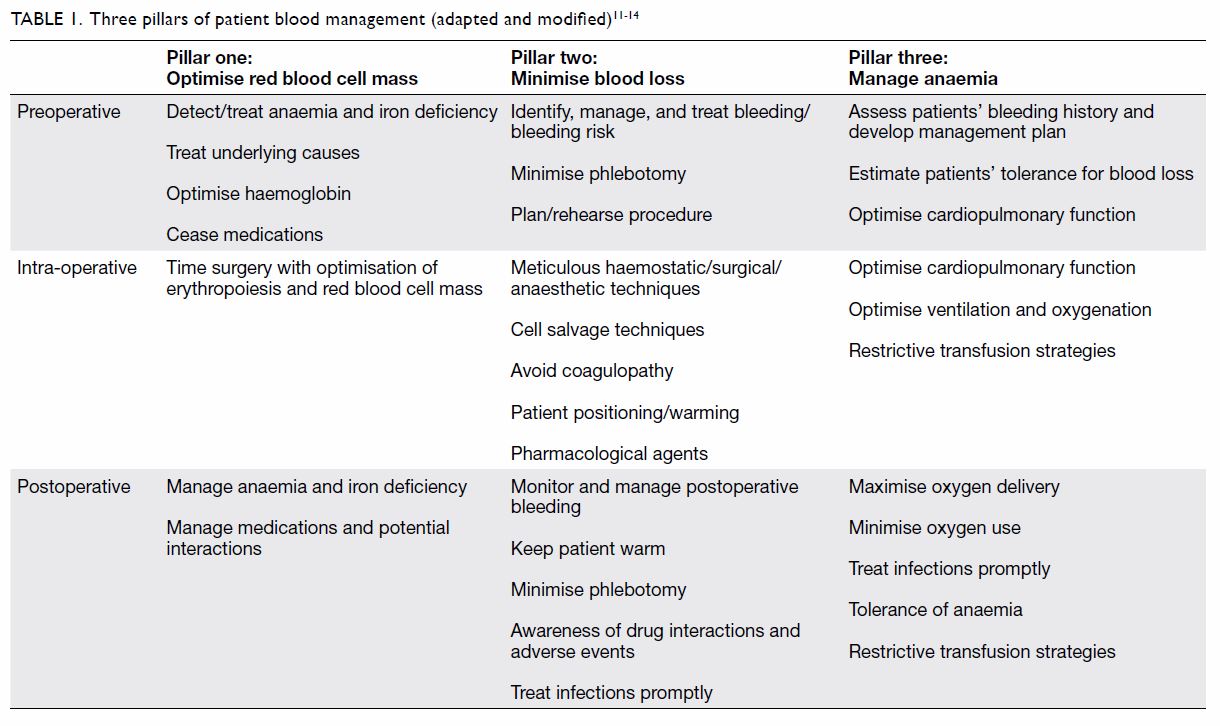
outcomes. Commonly, PBM employs a three-pillar
approach: (1) optimise red blood cell mass;
(2) minimise blood loss; and (3) manage anaemia
(Table 111 12 13 14). Indeed, PBM optimises the patient’s
condition before, during, and after the procedure
and only recommends transfusion when indicated.
It directly addresses the triad of independent
risk factors that can affect patient outcomes:
anaemia, blood loss, and transfusion. Anaemia is
appropriately and timely managed according to its
aetiology instead of being bluntly corrected by blood
transfusion. Thereby, blood loss is minimised, and
the harm associated with inappropriate transfusions
is avoided.15 Therefore, countries that have
implemented PBM have shown improvement of
patients’ outcomes, such as overall survival, disease
recurrence, infection rate, length of stay in intensive
care unit and hospital, cost, and blood utilisation.16 17 18 19 20 21 22
During the Sixty-third World Health Assembly
in 2010, member states were urged to establish or
strengthen systems for the safe and rational use
of blood products and to provide training for all
staff involved in clinical transfusion, to implement
potential solutions to minimise transfusion errors
and promote patient safety, and to promote the
availability of transfusion alternatives including,
where appropriate, autologous transfusion and
PBM.23
Blood supply and transfusion
demand in Hong Kong
Although Hong Kong has a long history of self-sufficiency
in terms of blood supply, population
ageing has brought a significant increase in demand
for blood over the last decade.24 Since 2015, the Hong
Kong Blood Transfusion Service has experienced
excessive difficulties at mobilising citizens to
maintain a stable, safe blood supply. As a result, the
Blood Transfusion Service faces a number of blood
safety and donor issues that affect the blood supply.
Emerging infectious diseases like Zika virus and low
pre-donation haemoglobin due to iron deficiency
are typical examples that may prevent apparently
healthy persons from donating blood.
As blood is irreplaceable and has a limited shelf
life, securing a sustainable and safe blood supply is
of paramount importance in the modern healthcare
system to ensure that patients’ transfusion needs are
met promptly and appropriately. Strategies to enhance
new donor recruitment and existing donor retention
should be undertaken by the Blood Transfusion
Service to increase the blood supply. However,
demand control measures should be simultaneously
implemented to reduce the pressure on the supply
side. In Hong Kong, there has been increasing
awareness of the concept of PBM beginning in the
past 2 years, and small-scale projects have been
initiated. One local project was able to increase
the preoperative haemoglobin concentration and
reduce the transfusion rate after implementation of
PBM.25 In the UK, the National Institute for Health
and Care Excellence recommended consideration
of single-unit transfusions for adults without active
bleeding in November 2015.26 On the basis of this
recommendation, some medical departments in
Hong Kong have implemented single-unit blood
transfusions over the past 2 years, and unpublished
audit results demonstrate an overall reduction of red
blood cell transfusions in general medical in-patients
over that period.
With the objective of improving patients’
outcomes and better managing transfusion
demand, a group of experienced clinicians from
different specialties and hospitals in Hong Kong
has established the Hong Kong Society of Clinical
Blood Management to continuously promote PBM
in Hong Kong. The Society aims to discuss and make
recommendations regarding the implementation of
PBM in Hong Kong. Below are three areas of focus
that the Society intends to address.
I. Optimising patients’ red blood
cell mass and better managing
anaemia
Anaemia is a serious disease burden in both
hospitalised patients and society.1 2 3 Haematopoiesis and anaemia management are important modifiable
risk factors for adverse outcomes.9 27 28 Beneficial
outcomes in this important pillar of PBM are seen
in not only surgical patients but also other patient
groups, such as those with underlying medical,
obstetric, or gynaecological problems. Therefore,
clinical guidelines have recommended that anaemia
be promptly recognised and the underlying causes
identified and managed appropriately.29 Because
some surgical patients have an increased risk of
bleeding, haemoglobin measurement well before
operation in all patients could provide adequate time
to manage any anaemia before surgery and improve
outcomes.29
Red blood cell transfusion should be restricted
to the minimal amount necessary to achieve clinical
stability and to patients presenting with severe
iron deficiency anaemia and alarming symptoms
(eg, haemodynamic instability) and/or risk criteria
(eg, coronary heart disease).30 31 As iron deficiency
(whether absolute or functional) is commonly
found in anaemic patients, its correction should
be promptly instituted. Oral iron supplements,
provided as ferrous or ferric salts, are usually the
first line of treatment for uncomplicated iron
deficiency anaemia because of their availability,
ease of administration, and relatively low cost.
However, because of these supplements’ notorious
gastrointestinal adverse effects, intravenous iron
should be considered in patients with intolerance
to oral iron and when more rapid restoration of
the iron store is expected. ‘Newer’ intravenous
iron formulations with safer profiles, such as ferric
carboxymaltose or iron isomaltoside, which allow
for a short-time (15-60 min) infusion of high iron
doses (≥1000 mg), are now available for use in both
in-patients and out-patients. Such intravenous iron
formulations can rapidly correct iron deficiency
and anaemia within a few weeks (vs the few months
needed for correction via oral iron). At the University
Hospitals Plymouth, UK, intravenous iron is given
to successfully treat iron deficiency anaemia when
surgery with anticipated blood loss of >500 mL is
anticipated within 6 weeks.32 As a result, intravenous
iron has become an important component of PBM
management strategies.
In Hong Kong, the Blood Transfusion Service
and the Hong Kong Medical Association have
recently issued a simple algorithm to aid general
practitioners with early and prompt recognition
of anaemia and its management.33 Further work
is required to enhance the general population’s
awareness of anaemia and iron deficiency issues,
their diagnosis, and improving their management.
II. Minimising blood loss
Reducing or minimising blood loss in hospitalised
patients is another approach to reduce the need for blood transfusion and improve patients’ outcomes.
Some might consider that this type of planning
should only occur in surgical or operative settings,
but reducing iatrogenic blood loss in non-operative
settings has also been shown to improve patients’
outcomes. Table 234 35 36 37 38 39 40 41 42 43 highlights the measures that
have been shown to be effective at minimising blood
loss. These measures are safe and have not affected
organ function or caused other complications.
Instead, they reduce iatrogenic blood loss and avoid
blood transfusions.
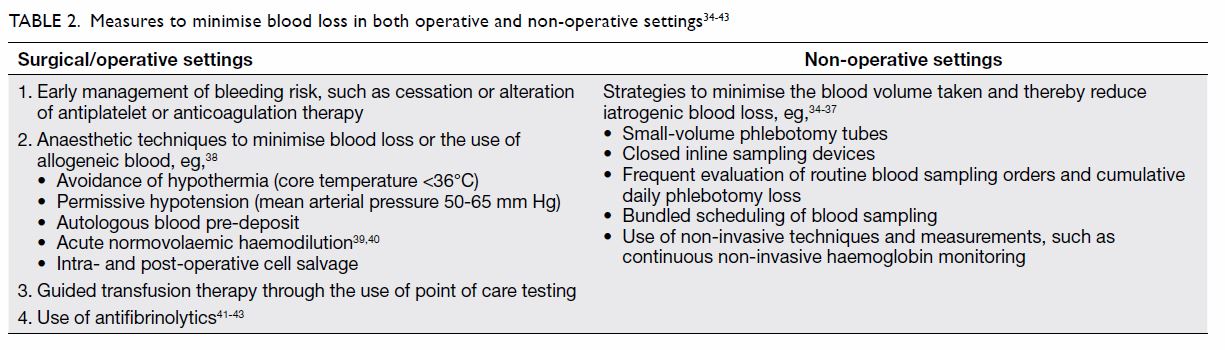
Table 2. Measures to minimise blood loss in both operative and non-operative settings34 35 36 37 38 39 40 41 42 43
Temporary cessation of antiplatelet and
anticoagulant medications in the perioperative
period may lead to reduced blood loss and
transfusion requirements if the risks of perioperative
thromboembolic events and bleeding are balanced.
Meticulous surgical techniques such as performing
minimally invasive surgery, judicious use of
electrocautery, tourniquets, topical haemostatic
agents, and intra-operative blood salvage can
minimise surgery-related blood loss.44 45 46 47 48 49
A number of anaesthetic techniques can also
help to reduce blood loss. Permissive hypotension
refers to the lowering of mean arterial pressure to
values between 50 and 65 mm Hg with the goal of
reducing blood flow to the surgical field, thereby
reducing blood loss and improving visibility in the
surgical field.50 Studies have shown that permissive
hypotension during anaesthesia reduced blood loss
in spinal surgery, radical prostatectomy, functional
endoscopic sinus surgery, and orthopaedic
surgery.51 52 53 It can also reduce blood loss and blood
product utilisation in adult trauma patients with
haemorrhagic shock.54 Organ hypoperfusion is the
major drawback, and therefore, this strategy may not
be suitable for patients with coronary artery disease,
cerebrovascular disease, traumatic brain injury, or
spinal injury.
Prevention of perioperative hypothermia is
another strategy that can help to reduce blood loss.
Hypothermia is defined as a core temperature <36°C
and is a common consequence of anaesthesia.55 Even
mild hypothermia, defined as a core temperature
between 35°C and 36°C, significantly increases
perioperative blood loss and augments the
transfusion requirement.56 Therefore, measures
should be taken to prevent inadvertent hypothermia,
including identification of high-risk patients, pre-warming
before surgery, intra-operative monitoring
of body temperature, using warm intravenous/irrigation fluid and forced-air warming devices, and
avoidance of unnecessary body exposure.57
Another method to minimise blood loss
is acute normovolaemic haemodilution. Acute
normovolaemic haemodilution involves withdrawal
of whole blood with concurrent infusion of fluids to
maintain normovolaemia.58 The autologous blood
is re-infused at the conclusion of the surgery. This
method has been shown to significantly reduce
the incidence and volume of allogeneic blood
transfusion, and its use should be considered in adult
patients who undergo surgery in which substantial
blood loss is anticipated.44 However, relatively
profound anaemia is expected during the surgery,
which may induce tissue ischaemia, particularly
in the myocardium.45 Furthermore, the effects of
normovolaemic haemodilution on morbidity and
mortality are uncertain.
Appropriate patient positioning during the
intra-operative period may also help to reduce
surgery-related blood loss. Elevation of the surgical
site above the right atrium facilitates venous return
and reduces venous engorgement. For example, the
reverse Trendelenburg position has been shown
to reduce intra-operative blood loss in endoscopic
sinus surgery.46
Using the wide pad support widths of the Wilson
frame, when compared with narrow pad support
widths, significantly decreased intra-abdominal
pressure and intra-operative blood loss in patients
undergoing spine surgery in the prone position.47
Pharmacological agents can also be used to facilitate
haemostasis. Tranexamic acid has been studied
extensively in a wide range of surgeries and has
been shown to reduce blood loss effectively without
increasing the risk of thromboembolic events.48 49 In case of significant haemorrhage that is refractory to
standard treatment, the use of recombinant factor
VIIa should also be considered.59
Diagnostic phlebotomy for laboratory testing
can also be a significant source of blood loss,
especially in critically ill patients.60 Such blood
loss has been associated with the development of
anaemia and the need for transfusion.61 Therefore,
blood tests should be ordered only when necessary,
and the volume of blood collected should be the
minimum required. Paediatric bottles can be used
to minimise the blood volume collected for testing,
which in turn reduces iatrogenic blood loss and
transfusion requirements.60 Point-of-care testing
devices require smaller blood volumes for analysis
and serve as an alternative to traditional laboratory
testing. Blood sampling from arterial and central
venous lines traditionally involves discarding the
initial blood sample. The method of returning the
initial blood sample back to the patients has been
used to significantly reduce iatrogenic blood loss,62
and this measure should be considered.
III. Rationalising use of blood and
blood components
As blood transfusion is not without risks, consideration
should be given to the balance of benefits against
risks. Most would advocate the adoption of a quality
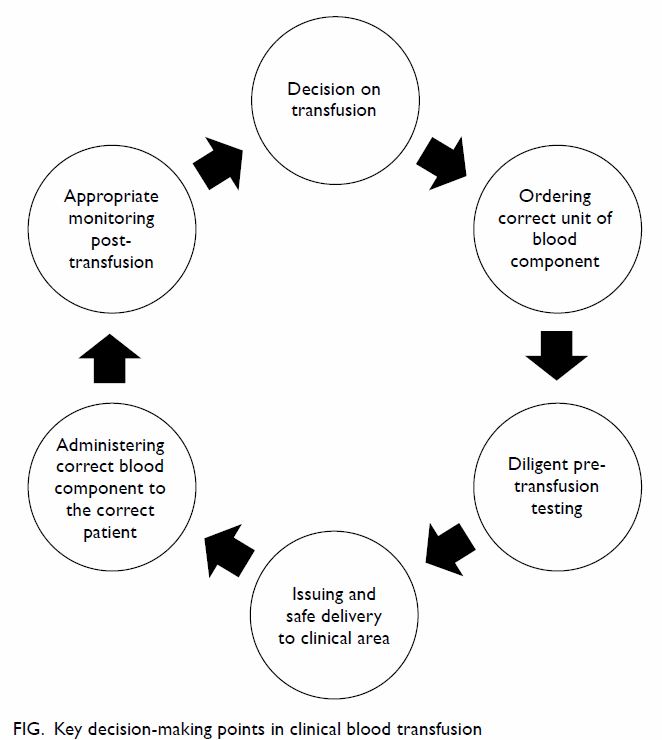
clinical transfusion process, ie, “transfusion of the
right number of units of blood to the right patient at
the right time, in the right conditions, and according
to appropriate guidelines”.63 Thus, clinicians should
proceed through a chain of related events by making
appropriate decisions (Fig).
Recommendations for
implementation of patient blood
management in Hong Kong
On the basis of the above three areas for consideration,
as well as advice from the Joint United Kingdom
(UK) Blood Transfusion and Tissue Transplantation
Services Professional Advisory Committee64 and
the World Health Organization,65 the Hong Kong
Society of Clinical Blood Management makes the
following recommendations:
1. A PBM framework, covering primary, hospital, research, audit, and public health measures, should be developed for use in Hong Kong after engagement of different stakeholders;
2. Healthcare professionals, patients, and the public should be educated on the appropriateness of blood transfusion, and PBM programmes; and
3. A PBM framework should be developed for application in Hong Kong, including early recognition and better management of anaemia and iron deficiency in patients and the general population; optimisation of patients’ haematopoiesis and correction of coagulation before surgical procedures; and application of various blood-saving technologies/techniques and point-of-care testing to optimise patients’ outcomes with less transfusion.
1. A PBM framework, covering primary, hospital, research, audit, and public health measures, should be developed for use in Hong Kong after engagement of different stakeholders;
2. Healthcare professionals, patients, and the public should be educated on the appropriateness of blood transfusion, and PBM programmes; and
3. A PBM framework should be developed for application in Hong Kong, including early recognition and better management of anaemia and iron deficiency in patients and the general population; optimisation of patients’ haematopoiesis and correction of coagulation before surgical procedures; and application of various blood-saving technologies/techniques and point-of-care testing to optimise patients’ outcomes with less transfusion.
The proposed PBM framework is a multi-pronged
approach that encompasses a wide range
of sectors, disciplines, specialties, and departments.
Its implementation will include hospitals, clinics,
healthcare facilities, and public health measures to
provide care to in-patients, out-patients, and the
public of Hong Kong. However, a number of barriers
exist that may hamper PBM implementation in
Hong Kong.66 67 These include misconceptions
related to blood transfusion and difficulties accessing
contemporary evidence and data about PBM. There
are also existing cultural pressures to retain the
status quo, with inadequate incentive for change, as
blood is currently delivered freely and efficiently to
receivers in Hong Kong. Resources may be inadequate
or unequally allocated, such as ferritin assays and
intravenous iron preparations for early diagnosis
and effective treatment of anaemia, or cell savers and
active patient warming equipment for minimising
blood loss and conserving blood during surgery.
Logistical complexities such as timely investigation
and treatment of preoperative anaemia before elective surgery and establishment of point-of-care
testing coagulation management programmes may
also present obstacles. Finally, PBM lacks specific
established quality mechanisms, such as associated
policies, standards, guidelines, documentation,
performance indicators, coordination, monitoring,
evaluation, and feedback.
To overcome these barriers, strong leadership
with central steering and empowerment of PBM
advocates is required to reinforce and coordinate the
current piecemeal and uncoordinated efforts of PBM
promotion. The goal of PBM is not simply to reduce
the amount of blood transfusion. It is a continuing
programme of quality improvement that has the
goal of improving patient outcomes via its different
measures. With reference to other countries’
experiences and the barriers and challenges that
could limit the implementation of PBM in clinical
practice, an appropriate framework with local
interest should be developed to implement PBM
practices at the hospital and territory level.11 68 69
Conclusion
On the basis of the scientific evidence on the
successful implementation of PBM and its
improvement of patient outcomes, the Hong Kong
Society of Clinical Blood Management strongly
recommends that Hong Kong implement PBM as
soon as possible. The Society will continue to work
with relevant professional bodies, patients, and
stakeholders to facilitate the local implementation
of PBM.
Author contributions
All authors contributed to the concept or design of the study,
acquisition and analysis or interpretation of data, drafting of
the manuscript, and critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency
in the public, commercial, or not-for-profit sectors.
References
1. GBD 2016 Disease and Injury Incidence and Prevalence
Collaborators. Global, regional, and national incidence,
prevalence, and years lived with disability for 328 diseases
and injuries for 195 countries, 1990-2016: a systematic
analysis for the Global Burden of Disease Study 2016.
Lancet 2017;390:1211-59. Crossref
2. Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic
analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615-24. Crossref
3. Kulier A, Gombotz H. Perioperative Anemia [in German].
Anaesthesist 2001;50:73-86. Crossref
4. Williamson LM, Devine DV. Challenges in the management
of the blood supply. Lancet 2013;381:1866-75. Crossref
5. Global Status Report on Blood Safety and Availability.
World Health Organization; 2017.
6. Marks PW, Epstein JS, Borio LL. Maintaining a safe blood
supply in an era of emerging pathogens. J Infect Dis
2016;213:1676-7. Crossref
7. Faddy HM, Viennet E, Flower RL. Transfusion risk from
emerging pathogens in the Asia-Pacific region. ISBT
Science Series 2016;11:143-8. Crossref
8. Hans G, Zacharowski K, Spahn DR. Patient blood management. 2016. Available from: http://www.thieme-connect.de/products/ebooks/lookinside/10.1055/b-0036-129702. Accessed 15 Nov 2019.
9. Musallam KM, Tamim HM, Richards T, et al.
Preoperative anaemia and postoperative outcomes in
non-cardiac surgery: a retrospective cohort study. Lancet
2011;378:1396-407. Crossref
10. Ferraris VA, Hochstetler M, Martin JT, Mahan A, Saha SP.
Blood transfusion and adverse surgical outcomes: the good
and the bad. Surgery 2015;158:608-17. Crossref
11. Australia National Blood Authority. Patient blood
management. Available from: https://www.blood.gov.au/patient-blood-management-pbm. Accessed 8 Mar 2019.
12. Isbister JP. The three-pillar matrix of patient blood
management—an overview. Best Pract Res Clin
Anaesthesiol 2013;27:69-84. Crossref
13. Spahn DR, Goodnough LT. Alternatives to blood
transfusion. Lancet 2013;381:1855-65. Crossref
14. Hofmann A, Farmer S, Towler SC. Strategies to preempt
and reduce the use of blood products: an Australian
perspective. Curr Opin Anaesthesiol 2012;25:66-73. Crossref
15. Frietsch T, Shander A, Faraoni D, Hardy JF. Patient blood
management is not about blood transfusion: it is about
patients’ outcomes. Blood Transfus 2019;17:331-3.
16. Morden Healthcare. Hospitals see improved outcomes,
lower costs as blood transfusions drop. 2017. Available from:
http://www.modernhealthcare.com/article/20170721/NEWS/170729977. Accessed 15 Nov 2019.
17. Althoff FC, Neb H, Herrmann E, et al. Multimodal patient
blood management program based on a three-pillar
strategy: a systematic review and meta-analysis. Ann Surg
2019;269:794-804. Crossref
18. Leahy MF, Hofmann A, Towler S, et al. Improved outcomes
and reduced costs associated with a health-system-wide
patient blood management program: a retrospective
observational study in four major adult tertiary-care
hospitals. Transfusion 2017;57:1347-58. Crossref
19. Muñoz M, Acheson AG, Auerbach M, et al. International
consensus statement on the peri-operative management of
anaemia and iron deficiency. Anaesthesia 2017;72:233-47. Crossref
20. Shander A, Van Aken H, Colomina MJ, et al. Patient blood
management in Europe. Br J Anaesth 2012;109:55-68. Crossref
21. Vaglio S, Gentili S, Marano G, et al. The Italian regulatory
guidelines for the implementation of patient blood
management. Blood Transfus 2017;15:325-8.
22. Vaglio S, Prisco D, Biancofiore G, et al. Recommendations
for the implementation of a patient blood management
programme. Application to elective major orthopaedic
surgery in adults. Blood Transfus 2016;14:23-65.
23. World Health Organization. Sixty-third World Health
Assembly: availability, safety and quality of blood products.
21 May 2010. Available from: http://apps.who.int/gb/ebwha/pdf_files/wha63/a63_r12-en.pdf. Accessed 8 Mar
2019.
24. Hong Kong Red Cross Blood Transfusion Service. Fact
sheet of blood collection and use. Available from: https://www5.ha.org.hk/rcbts/fs2016?lang=en. Accessed 29 Feb
2020.
25. Fong CM. Hospital Authority Convention 2019
masterclass 1.4 patient blood management: experience
from Tseung Kwan O Hospital. 2019. Available from:
https://dryfta-assets.s3.eu-central1.amazonaws.com/assets/haconvention2019/editorimages/1562116855M1.4PatientBloodManagementv.2.pdf. Accessed 29 Feb 2020.
26. National Institute for Health and Care Excellence. Blood
transfusion. NICE guideline 24. 2015. Available from:
https://www.nice.org.uk/guidance/ng24. Accessed 20 Apr
2020.
27. Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA,
Pearse RM. Meta- analysis of the association between
preoperative anaemia and mortality after surgery. Br J Surg
2015;102:1314-24. Crossref
28. Mueller MM, Van Remoortel H, Meybohm P, et al. Patient
blood management: recommendations from the 2018
Frankfurt Consensus Conference. JAMA 2019;321:983-97. Crossref
29. De Hert S, Staender S, Fritsch G, et al. Pre-operative
evaluation of adults undergoing elective noncardiac
surgery: updated guideline from the European Society of
Anaesthesiology. Eur J Anaesthesiol 2018;35:407-65. Crossref
30. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice
guidelines from the AABB: red blood cell transfusion
thresholds and storage. JAMA 2016;316:2025-35. Crossref
31. Franchini M, Marano G, Mengoli C, et al. Red blood
cell transfusion policy: a critical literature review. Blood
Transfus 2017;15:307-17.
32. Cleland SR, Thomas W. Iron homeostasis and perioperative
management of iron deficiency. BJA Education
2019;19:390-7. Crossref
33. Lau CW, Lee CK. A practical guide of anaemia management
for general practitioners. HKMA CME Bulletin July 2018:
2-9.
34. Australia Victoria State Government. Reducing iatrogenic
blood loss—clinical practice guideline template.
Available from: https://www2.health.vic.gov.au/Api/downloadmedia/%7BF18A2239-E96F-4A49-98F1-C9124E81163F%7D. Accessed 8 Mar 2019.
35. Fischer DP, Zacharowski KD, Meybohm P. Savoring every
drop—vampire or mosquito? Crit Care 2014;18:306. Crossref
36. Stefanini M. Iatrogenic anemia (can it be prevented?). J
Thromb Haemost 2014;12:1591. Crossref
37. Ullman AJ, Keogh S, Coyer F, Long DA, New K, Rickard CM.
‘True blood’ the critical care story: an audit of blood
sampling practice across three adult, paediatric and
neonatal intensive care settings. Aust Crit Care 2016;29:90-5. Crossref
38. Squire Y, Laxton C. Blood conservation techniques.
Tutorial 390. Available from: https://www.wfsahq.org/components/com_virtual_library/media/8d6712e97e44cd3c3c2af5cc1cbffc00-atow-390-00-01.pdf. Accessed 15 Nov
2019.
39. Jamnicki M, Kocian R, van der Linden P, Zaugg M,
Spahn DR. Acute normovolemic hemodilution: physiology,limitations, and clinical use. J Cardiothoracic Vasc Anesth
2003;17:747-54. Crossref
40. Pasternak J, Nikolic D, Milosevic D, Popovic V, Markovic V.
An analysis of the influence of intra-operative blood
salvage and autologous transfusion on reducing the need
for allogeneic transfusion in elective infrarenal abdominal
aortic aneurysm repair. Blood Transfus 2014;12 Suppl
1:s182-6.
41. Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis
and meta-regression of the effect of tranexamic
acid on surgical blood loss. Br J Surg 2013;100:1271-9. Crossref
42. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS,
Mason JM. A systematic review and meta-analysis of the
topical administration of tranexamic acid in total hip and
knee replacement. Bone Joint J 2014;96-B:1005-15. Crossref
43. Cheriyan T, Maier SP 2nd, Bianco K, et al. Efficacy of
tranexamic acid on surgical bleeding in spine surgery: a
meta-analysis. Spine J 2015;15:752-61. Crossref
44. National Blood Authority Australia. Patient Blood
Management Guidelines: Module 2: Perioperative.
Available from: https://www.blood.gov.au/pubs/pbm/module2/3-clinical-guidance/3.6.5-acute-normovolemic-haemodilution.
html. Accessed 27 Apr 2020.
45. Murray D. Acute normovolemic hemodilution. Eur Spine J
2004;13 Suppl 1:S72-5. Crossref
46. Ko MT, Chuang KC, Su CY. Multiple analyses of factors
related to intraoperative blood loss and the role of reverse
Trendelenburg position in endoscopic sinus surgery.
Laryngoscope 2008;118:1687-91. Crossref
47. Park CK. The effect of patient positioning on intraabdominal
pressure and blood loss in spinal surgery. Anesth Analg
2000;91:552-7. Crossref
48. Franchini M, Mengoli C, Marietta M, et al. Safety of
intravenous tranexamic acid in patients undergoing major
orthopaedic surgery: a meta-analysis of randomised
controlled trials. Blood Transfus 2018;16:36-43.
49. Houston BL, Uminski K, Mutter T, et al. Efficacy and safety
of tranexamic acid in major non-cardiac surgeries at high
risk for transfusion: a systematic review and meta-analysis.
Transfus Med Rev 2020;34:51-62. Crossref
50. Shah A, Palmer AJ, Klein AA. Strategies to minimize
intraoperative blood loss during major surgery. Br J Surg
2020;107:e26-38. Crossref
51. Albertin A, La Colla L, Gandolfi A, et al. Greater peripheral
blood flow but less bleeding with propofol versus
sevoflurane during spine surgery: a possible physiologic
model? Spine (Phila Pa 1976) 2008;33:2017-22. Crossref
52. Boonmak P, Boonmak S, Laopaiboon M. Deliberate
hypotension with propofol under anaesthesia for functional
endoscopic sinus surgery (FESS). Cochrane Database Syst
Rev 2016;10(10):CD006623. Crossref
53. Paul JE, Ling E, Lalonde C, Thabane L. Deliberate
hypotension in orthopedic surgery reduces blood loss and
transfusion requirements: a meta-analysis of randomized
controlled trials. Can J Anaesth 2007;54:799-810. Crossref
54. Tran A, Yates J, Lau A, Lampron J, Matar M. Permissive
hypotension versus conventional resuscitation strategies
in adult trauma patients with hemorrhagic shock: A
systematic review and meta-analysis of randomized
controlled trials. J Trauma Acute Care Surg 2018;84:802-8. Crossref
55. Harper CM, Andrzejowski JC, Alexander R. NICE and
warm. Br J Anaesth 2008;101:293-5. Crossref
56. Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin
Anaesthesiol 2008;22:645-57. Crossref
57. National Institute for Health and Care Excellence.
Clinical guideline CG65. Hypothermia: prevention and
management in adults having surgery. Available from:
https://www.nice.org.uk/guidance/cg65. Accessed 20 Apr
2020.
58. Segal JB, Blasco-Colmenares E, Norris EJ, Guallar E.
Preoperative acute normovolemic hemodilution: a metaanalysis.
Transfusion 2004;44:632-44. Crossref
59. Hedner U. Recombinant activated factor VII: 30 years of
research and innovation. Blood Rev 2015;29 Suppl 1:S4-8. Crossref
60. Tinmouth AT, McIntyre LA, Fowler RA. Blood conservation
strategies to reduce the need for red blood cell transfusion
in critically ill patients. CMAJ 2008;178(1):49-57. Crossref
61. AuBuchon JP, Puca K, Saxena S, Shulman IA, Waters JH.
Getting started in patient blood management.
2011. Available from: https://www.aabb.org/pbm/Documents/112024DB.pdf. Accessed 8 Mar 2019.
62. Gleason E, Grossman S, Campbell C. Minimizing
diagnostic blood loss in critically ill patients. Am J Crit
Care 1992;1:85-90. Crossref
63. European Directorate for the Quality of Medicines &
HealthCare. Guide to the preparation, use and quality
assurance of blood components. Recommendation No.
R (95) 15. Available from: http://www.ipst.pt/files/IPST/INFORMACAO_DOCUMENTACAO/EDQM_Blood_transfusion_guide_19ed_2017_pub_PUBSD-89.pdf. Accessed 8 Mar 2019.
64. Joint United Kingdom (UK) Blood Transfusion and Tissue
Transplantation Services Professional Advisory Committee.
Transfusion Handbook. 4: Safe transfusion—right blood,
right patient, right time and right place. Available from:
https://www.transfusionguidelines.org/transfusionhandbook/4-safe-transfusion-right-blood-right-patient-right-time-and-right-place. Accessed 8 Mar 2019.
65. World Health Organization. Developing a national
policy and guidelines on the clinical use of blood:
recommendations. Available from: https://www.who.int/bloodsafety/clinical_use/en/who_bct_bts_01_3.pdf?ua=1.
Accessed 8 Mar 2019.
66. Mbanya D. Barriers and enablers to introducing
comprehensive patient blood management in the hospital.
Biologicals 2012;40:205-8. Crossref
67. Delaforce A, Duff J, Munday J, Hardy J. Overcoming
barriers to evidence-based patient blood management: a
restricted review. Implement Sci 2020;15:6. Crossref
68. European Commission. Building national programmes of
patient blood management (PBM) in the EU—a guide for
health authorities. Available from: https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/2017_eupbm_authorities_en.pdf. Accessed 8 Mar 2019.
69. European Commission. Supporting Patient Blood
Management (PBM) in the EU—A practical implementation
guide for hospitals. Mar 2017. Available from: https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/2017_eupbm_hospitals_en.pdf. Accessed 8
Mar 2019.