Hong Kong Med J 2020 Aug;26(4):311–7 | Epub 2 Jul 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Factors associated with depression in people with
epilepsy: a retrospective case-control analysis
PH Ho, MB, BS1; William CY Leung, MRCP (UK)2; Ian YH Leung, MRCP (UK)2; Richard SK Chang, FHKCP2
1 Department of Medicine, Queen Mary Hospital, Hong Kong
2 Division of Neurology, Department of Medicine, Queen Mary Hospital, Hong Kong
Corresponding author: Dr Richard SK Chang (changsk@ha.org.hk)
Abstract
Purpose: This study investigated factors associated
with depression in people with epilepsy.
Methods: All adult patients attending our epilepsy
clinic in 2018 were screened for inclusion in this
study. Eligible patients were divided into case
and control groups, depending on the presence
of co-morbid depression. Depressive disorders
were diagnosed by a psychiatrist. Demographics
and clinical characteristics, including epilepsy
features and antiepileptic drug use, were compared
between groups. The factors contributing to onset of
depression after diagnosis of epilepsy were further
analysed by binomial logistic regression. Statistical
significance was set at P<0.05.
Results: Forty four patients with epilepsy who had
depression and 514 patients with epilepsy who did
not have depression were included in this study
(occurrence rate=7.9%). Female sex (P=0.005), older
age (P<0.001), temporal lobe epilepsy (P=0.01), and
higher number of antiepileptic drugs used (P=0.003)
were associated with depression in patients with
epilepsy. No differences were observed in other
epilepsy-related factors including aetiology, seizure
type, and laterality of epileptic focus. Binomial
logistic regression showed that female sex (P=0.01; odds ratio [OR]=3.56), drug-resistant epilepsy
(P<0.001; OR=4.79), and clonazepam use (P<0.001;
OR=14.41) were significantly positively associated
with risk of depression after epilepsy diagnosis,
whereas valproate use (P=0.03; OR=0.37) was
significantly negatively associated with risk of
depression.
Conclusion: Female sex, refractoriness, and
clonazepam use may be risk factors for depression
after epilepsy diagnosis. Valproate may protect
against depression in people with epilepsy. Better
understanding of clinical features may aid in medical
management or research studies regarding co-morbid
depression in people with epilepsy.
New knowledge added by this study
- This retrospective study investigated factors associated with depression in people with epilepsy, including a subgroup of patients who experienced depression onset after epilepsy diagnosis.
- Female sex, drug-resistant epilepsy, and clonazepam use were significantly positively associated with depression in people with epilepsy.
- Valproate use was significantly negatively associated with depression in people with epilepsy.
- Clinicians who treat patients with epilepsy should be aware of the potential for co-morbid depression, especially in patients with potential risk factors (eg, female sex, drug-resistant epilepsy, and temporal lobe epilepsy).
- Psychotropic properties of antiepileptic drugs should be carefully considered when choosing treatment agents for people with epilepsy; clonazepam may promote depression, whereas valproate may protect against depression.
Introduction
People with epilepsy are susceptible to psychiatric
disorders. Depression is arguably the most
common psychiatric co-morbidity, which affects
approximately 25% to 30% of people with epilepsy.1 2
Depression disorders increase the risk of suicide
among people with epilepsy.3 Notably, co-morbid
depression can greatly impact clinical outcomes and
quality of life for people with epilepsy.4
The relationship between epilepsy and
depression is more complex than simple
psychological stress related to chronic illness.
Structural and functional changes in the brain may
explain the underlying pathogenic mechanism.5 6
Furthermore, people with epilepsy who exhibit
co-morbid depression also demonstrate worse
seizure control, compared with people with epilepsy
who do not have depression.7 Suboptimal drug adherence and higher rates of adverse effects from
antiepileptic drugs (AEDs) have also been reported
among people with epilepsy who exhibit co-morbid
depression.8 9
As discussed in a recent systematic review,
many studies have attempted to evaluate the roles
of various epilepsy-related factors in the onset of
depression; however, most showed no associations
with depression or demonstrated inconsistent
results.10 The present study was performed to
investigate the relationships of clinical factors,
including use of AEDs, with depression in people
with epilepsy.
Methods
Patients and study design
This was a retrospective study. All adult patients,
aged ≥18 years, attending the epilepsy clinic of
Queen Mary Hospital, Hong Kong, from January
to December 2018, were screened for inclusion in
the study. Relevant data were retrieved from the
computerised medical records system. Diagnoses of
epilepsy were made or confirmed by a neurologist.
Patients with both epilepsy and depression were
included in the case group, while those with epilepsy
alone were included in the control group. Depression
was defined as the presence of depressive disorders
as described in the International Classification of
Diseases 10th Revision, diagnosed by a psychiatrist. Patients with intellectual disability were excluded
due to potential difficulties in determining diagnoses
of mood disorders in this group11; patients with other
psychiatric disorders were also excluded to avoid
confounding effects.
Collection of data
The following data were collected from medical
records: basic demographic characteristics, epilepsy
and depression details, and use of AEDs. Major
neurological and medical conditions that had been
present before the epilepsy and depression diagnoses
were also recorded. The categorisation of epilepsy
was performed in accordance with the International
League Against Epilepsy 2017 classification
scheme.12 Patients were determined to have drug-resistant
epilepsy when adequate trials of two
tolerated, appropriately chosen, and appropriately
used AED schedules (whether as monotherapies or
in combination) failed to achieve sustained seizure
freedom.13 Seizure freedom was defined as the
absence of seizures for 1 year. Seizure type, location,
and laterality of epileptic focus were determined
by seizure semiology, any previous neuroimaging
findings, and electroencephalography results.
Locations of epileptic foci were classified according
to cerebral lobes. Any AED used for >6 months was
recorded in this analysis; the maximum number of
AEDs used was also recorded. This study followed
the STROBE guidelines for study reporting.14
Statistical analysis
Clinical features were compared between the
groups with and without co-morbid depression.
The Chi squared test was used to detect statistically
significant differences in categorical data, and the
t test was used to detect any statistically significant
differences in continuous data. Sample size was
based on the existing patient number during the
study period; thus, no sample size calculations were
performed.
To investigate the effects of AEDs on
development of depression in people with epilepsy,
further analysis was performed regarding the
subgroup of patients in whom depression was
diagnosed after epilepsy onset. In particular, patients
were selected for whom depression diagnosis
occurred in the calendar year (or later) after the year
of epilepsy diagnosis. Relevant factors, particularly
use of AEDs, were analysed for their predictive value
in terms of depression development, using binomial
logistic regression. Variables were entered into the
regression model by forward selection, based on
likelihood ratios. Statistical analyses were carried
out using SPSS Statistics for Windows (version 25.0;
IBM Corp, Armonk [NY], United States). Statistical
significance was set at P<0.05.
Results
Patient characteristics
Forty four patients with epilepsy who had co-morbid
depression were selected as the case group, while
558 patients with epilepsy who did not exhibit
depression were selected as the control group; the
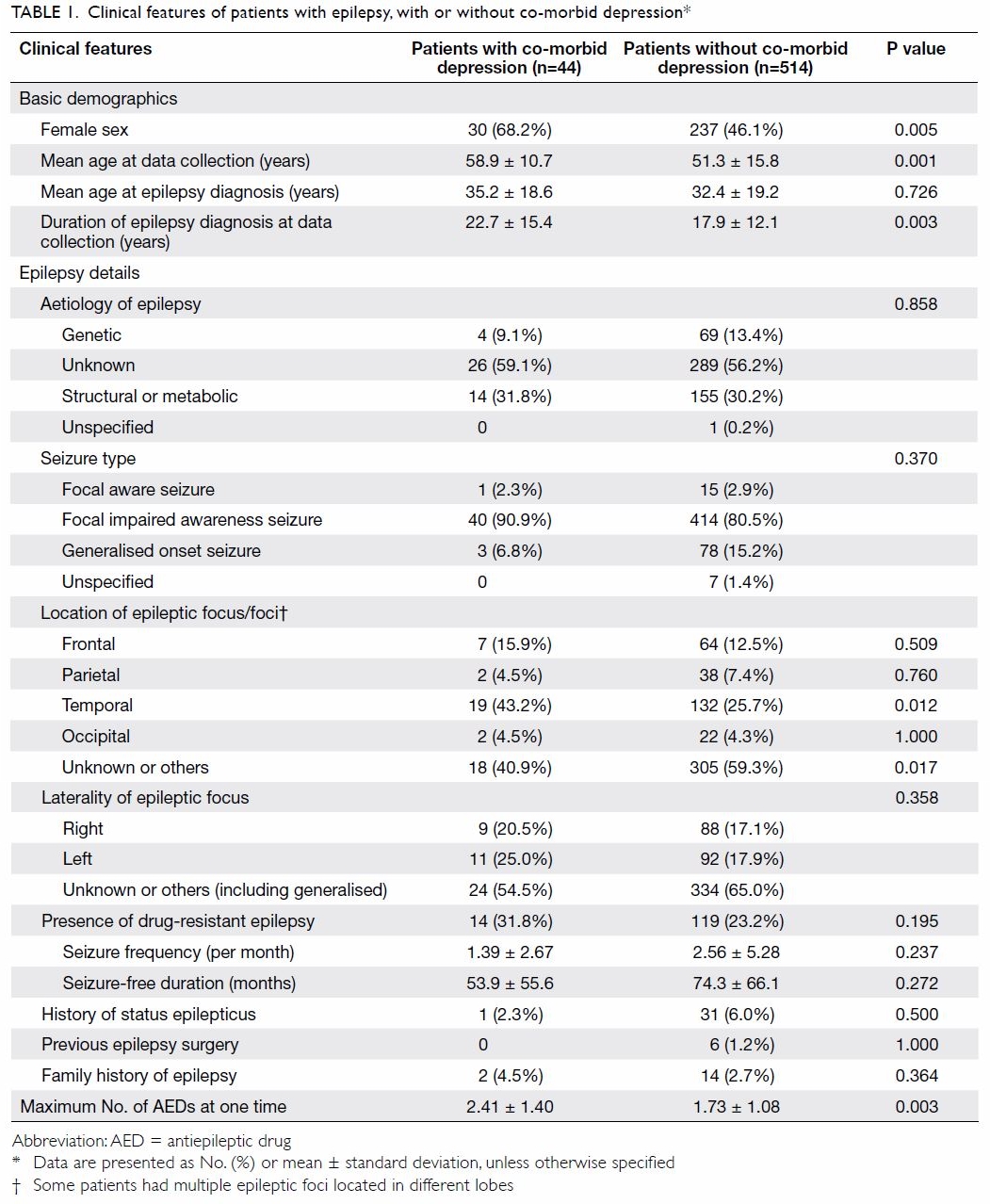
patient characteristics are summarised in Table 1.
Among the patients with co-morbid depression,
32 (73%) experienced onset of epilepsy before the diagnosis of depression, while 12 (27%) had a
diagnosis of depression before the onset of epilepsy;
furthermore, 14 (32%) exhibited drug-resistant
epilepsy. Most patients with co-morbid depression
had a diagnosis of major depression (36/44, 82%); of
the remaining eight patients, one (2%) had dysthymia,
two (5%) had mixed anxiety and depressive disorder,
and five (11%) had a diagnosis of unspecified
depression. The mean age (±standard deviation)
at depression diagnosis was 46±13 years, while the mean age at epilepsy diagnosis was 35±19 years.
The mean duration between onset of epilepsy and
diagnosis of co-morbid depression was 13±13 years.
Most patients with epilepsy who had co-morbid
depression did not exhibit other major neurological
(36/44, 82%) or medical conditions (41/44, 93%). Of
the remaining eight patients with major neurological
conditions, six (14%) had stroke, one (2%) had
traumatic brain injury, and one (2%) had migraine.
Of the remaining three patients with major medical
conditions, two (5%) had malignancy and one (2%)
had rheumatoid arthritis. Notably, one patient had
both stroke and malignancy, while another patient
had both stroke and rheumatoid arthritis.
Comparison between case and control groups
Clinical features were compared between the case
and control groups (Table 1). Patients with depression
were older and included more women. A significantly
longer duration of epilepsy was observed in patients
with depression; however, the mean age at epilepsy
onset did not significantly differ between the groups.
Temporal lobe epilepsy was more common in
patients with depression; moreover, patients with
depression used a greater mean number of AEDs.
No differences were noted in other epilepsy-related
factors, including family history, drug-resistant
disease, seizure frequency, aetiology, seizure type,
or history of status epilepticus. Laterality in focal
epilepsy also showed no association with co-morbid
depression.
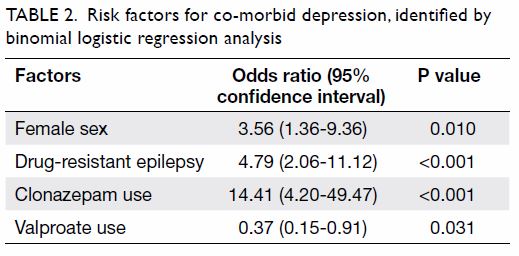
Subgroup analysis of patients in whom
depression was diagnosed after epilepsy onset
Relevant parameters were selectively included in
binomial logistic regression analysis to determine
risk factors for co-morbid depression (Table 2). The
relevant parameters depended on statistical results
in the whole group analysis and clinical judgement.
Parameters that reached statistically significance
in the whole group analysis were selected. Other
parameters considered as clinically important were
included as well. Only patients in whom depression
was diagnosed after epilepsy onset were included
in this analysis. Female sex, drug-resistant epilepsy, and clonazepam use were significantly positively
associated with a risk of co-morbid depression
among patients with epilepsy. In contrast, valproate
use was significantly negatively associated with a risk
of co-morbid depression.
Discussion
Depression is one of the most common psychiatric
co-morbidities among people with epilepsy. Our
study involving a cohort of people with epilepsy
revealed that 7.9% had depression; of these, 73%
had epilepsy onset before depression diagnosis.
This prevalence is much lower than the rate of
approximately 30% previously described in previous
studies of Western and Chinese populations17 16 17;
differences in study design may explain this
discrepancy. Previous studies were commonly
questionnaire or scale-based; generally, they used the
Hospital Anxiety and Depression Scale, Neurological
Disorders Depression Inventory for Epilepsy, or
Patient Health Questionnaire nine-item depression
scale.18 19 20 Importantly, these different scales have
respective strengths and limitations.21 22 23 In our study,
the definition of depression was relatively stringent,
because it was confirmed by a psychiatrist. Patients
with co-existing psychiatric disorders (eg, psychotic
disorders, substance abuse, and personality
disorders) were deliberately excluded. Although
the psychiatric profile in our cohort was relatively
homogeneous because of the stringent criteria,
patients with relatively minor or occult depressive
symptoms might have been excluded. Importantly,
the low prevalence of depression may indicate that
this common affective disorder is often overlooked
by clinicians in our locality. Underdiagnosis of
psychiatric co-morbidities is a major problem
encountered by people with epilepsy.24 25 Awareness
of psychiatric co-morbidities, identified with the aid
of assessment scales, may improve the sensitivity of
diagnosis.
This study placed considerable emphasis
on the temporal relationship between epilepsy
and depression. A number of similar studies
used a cross-sectional design, which present a
methodological problem regarding the unclear
temporal relationship between epilepsy and
co-morbid depression.6 Extraction of data from
clinical records allows assessment of an individual
patient’s clinical features at different timepoints.
In the present study, only patients with depression
onset after epilepsy diagnosis were included in
logistic regression analysis, which enables clearer
assessment of the relationship between epilepsy and
co-morbid depression.
In our study, patients with epilepsy who
exhibited depression were predominantly women.
This finding is consistent with the results of a previous
systemic review.10 Female sex predominance has also been observed in studies of depression alone.26 27
In the present study, patients with depression were
older and had a longer duration of epilepsy diagnosis,
presumably because of accumulation bias. The mean
interval for development of depression was 13 years
after epilepsy onset; however, age at epilepsy onset
was not associated with development of depression.
It remains controversial whether younger epilepsy
onset age is related to a higher risk of subsequent
depression. Some studies have shown positive
associations, whereas others have not.28 29
Previous evidence suggested that focal
epilepsy, rather than generalised epilepsy, was
associated with co-morbid depression in people
with epilepsy.15 30 31 This finding was not observed
in our study; however, temporal lobe epilepsy
was significantly more prevalent in patients with
depression. The underlying pathogenic mechanism
may involve the close relationship of the temporal
lobe with the limbic system, which plays a key
role in emotional control. Frequent epileptic focus
discharge can lead to reduced blood flow and
metabolism in corresponding cerebral regions.5 32
Furthermore, temporal lobe epilepsy is associated
with hippocampal damage and atrophy, typically
comprising hippocampal sclerosis.33 Temporal lobe
hypometabolism and hippocampal volume loss have
also been implicated in depression.34 35 Finally, an
association of depression with epileptic foci in frontal
and left temporal lobes has also been reported,31 but
this phenomenon was not observed in our cohort.
In our study, drug-resistant epilepsy was
associated with depression in binomial logistic
regression analysis, but not when analysis was
performed using the Chi squared test. In this study,
patients included in analysis by the Chi squared test
had depression either before or after the diagnosis of
epilepsy. In contrast, the binomial logistic regression
model only included patients in whom depression
was diagnosed after epilepsy onset. Additionally,
the exact seizure frequency did not affect the risk
of depression in this study; conversely, seizure
frequency was associated with co-morbid depression
in a previous systemic review.10 This discrepancy may
be explained by differences in sample composition.
However, it remains unclear whether better epilepsy
control (ie, seizure frequency reduction) will alleviate
the risk of depression.
Use of AEDs also contributes to the onset
of mood disorder in people with epilepsy. This
study showed that clonazepam use was positively
associated with risk of depression in people with
epilepsy; conversely, valproate use was negatively
associated with risk of depression. The impact of
AEDs on psychiatric illness has been extensively
studied. Benzodiazepines, including clonazepam,
have been proposed to induce depression by
overinhibition of the GABAergic pathway.31 36 Valproate, carbamazepine, and lamotrigine are
examples of AEDs with positive psychotropic
effects, whereas benzodiazepine, levetiracetam,
phenobarbital, and topiramate exhibit negative
psychotropic effects.37 38 39 40
The findings of this study have a few important
implications for clinical practice. First, the
recognition of depression in people with epilepsy
can be challenging for clinicians, especially in a
busy out-patient clinic setting.24 The identification
of at-risk patients is essential for improving the
diagnostic yield of affective disorders among people
with epilepsy. Female sex, drug-resistant epilepsy,
and temporal lobe epilepsy may be associated with
co-morbid depression. Clinicians should be vigilant
in searching for depressive features in patients with
these characteristics during clinical consultation.
Second, the use of AEDs plays a role in
co-morbid depression in people with epilepsy.
Psychotropic properties of AEDs should be carefully
considered when choosing treatment agents for
people with epilepsy, especially for patients who
are at risk of depression (eg, women, patients with
drug-resistant epilepsy, and patients with temporal
lobe epilepsy). Clonazepam (ie, a benzodiazepine)
has been associated with depression onset in people
with epilepsy; in contrast, valproate may have a
protective effect against depression onset in people
with epilepsy. This could be due to the mood-stabilising
effect of valproate, which has led to its
use for treatment of patients with manic disorder.
Antiepileptic drugs can have a considerable impact
on quality of life in people with epilepsy, in addition
to their seizure control effects. The impact of newer-generation
AEDs requires further analysis, because
these drugs were inadequately represented among
the limited number of patients in the current study.
There were some limitations in this study.
First, there were inherent limitations due to the
retrospective nature of the analysis. In particular, the
information documented in medical records may not
be uniform and may be subject to recall bias. Second,
the diagnosis of depression in our study tended to
be stringent, because it relied on a psychiatrist’s
diagnosis, instead of more widely used assessment
tools (eg, depressive scales). However, this approach
may have led to underestimation regarding the
extent of depressive disorders among patients in this
cohort. Third, the AEDs were required to be used for
>6 months to be included in the analysis. However,
the durations, dosages, or serum drug levels of AEDs
were not considered, because they may have varied
during the course of epilepsy. Fourth, relatively few
socio-economic and psychological factors were
included in this analysis. Some previous studies
showed that these factors were associated with
co-morbid depression; however, they have been less
frequently investigated than other epilepsy-related factors (eg, employment, marital status, and stressful
life events).1 Further prospective studies that include
examinations of these psychosocial factors may
provide more complete information regarding
depression in people with epilepsy.
Conclusions
Depression is a common mood disorder in people
with epilepsy. This study showed that depression
tends to affect a subgroup of people with epilepsy who
exhibit specific demographic and epilepsy-related
factors. Notably, the use of AEDs may also influence
the risk of depression in people with epilepsy. This
study may contribute to better understanding of
clinical features, thereby aiding in future clinical
management or basic science studies regarding
co-morbid depression in people with epilepsy.
Author contributions
Concept or design: PH Ho.
Acquisition of data: PH Ho, RSK Chang.
Analysis or interpretation of data: PH Ho, RSK Chang.
Drafting of the manuscript: PH Ho, RSK Chang.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: PH Ho, RSK Chang.
Analysis or interpretation of data: PH Ho, RSK Chang.
Drafting of the manuscript: PH Ho, RSK Chang.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Declaration
This research was presented as a poster presentation titled
“Clinical features and predictors of depression in people with
epilepsy (PWE)” at the 33rd International Epilepsy Congress,
Bangkok, 22-26 June 2019.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Hospital Authority Hong Kong
West Cluster Institutional Review Board (Ref UW 19-742).
The need for informed consent was waived.
References
1. Hermann BP, Seidenberg M, Bell B. Psychiatric comorbidity
in chronic epilepsy: identification, consequences, and
treatment of major depression. Epilepsia 2000;41 Suppl
2:S31-41. Crossref
2. Asadi-Pooya AA, Kanemoto K, Kwon OY, et al. Depression
in people with epilepsy: how much do Asian colleagues
acknowledge it? Seizure 2018;57:45-9. Crossref
3. Kanner AM. Depression and epilepsy: a new perspective
on two closely related disorders. Epilepsy Curr 2006;6:141-
6. Crossref
4. Lehrner J, Kalchmayr R, Serles W, et al. Health-related
quality of life (HRQOL), activity of daily living (ADL)
and depressive mood disorder in temporal lobe epilepsy
patients. Seizure 1999;8:88-92. Crossref
5. Bromfield EB, Altshuler L, Leiderman DB, et al. Cerebral
metabolism and depression in patients with complex
partial seizures. Arch Neurol 1992;49:617-23. Crossref
6. Kanner AM, Schachter SC, Barry JJ, et al. Depression and
epilepsy: epidemiologic and neurobiologic perspectives
that may explain their high comorbid occurrence. Epilepsy
Behav 2012;24:156-68. Crossref
7. Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ.
Predictors of pharmacoresistant epilepsy. Epilepsy Res
2007;75:192-6. Crossref
8. Ettinger AB, Good MB, Manjunath R, Edward Faught R,
Bancroft T. The relationship of depression to antiepileptic
drug adherence and quality of life in epilepsy. Epilepsy
Behav 2014;36:138-43. Crossref
9. Cramer JA, Blum D, Reed M, Fanning K, Epilepsy Impact
Project Group. The influence of comorbid depression on
quality of life for people with epilepsy. Epilepsy Behav
2003;4:515-21. Crossref
10. Lacey CJ, Salzberg MR, D'Souza WJ. Risk factors for
depression in community-treated epilepsy: systematic
review. Epilepsy Behav 2015;43:1-7. Crossref
11. Walton C, Kerr M. Severe intellectual disability: systematic
review of the prevalence and nature of presentation
of unipolar depression. J Appl Res Intellect Disabil
2016;29:395-408. Crossref
12. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE
classification of the epilepsies: position paper of the ILAE
Commission for Classification and Terminology. Epilepsia
2017;58:512-21. Crossref
13. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug
resistant epilepsy: consensus proposal by the ad hoc Task
Force of the ILAE Commission on Therapeutic Strategies.
Epilepsia 2010;51:1069-77. Crossref
14. Equator Network. The Strengthening the Reporting
of Observational Studies in Epidemiology (STROBE)
Statement: guidelines for reporting observational studies.
Available from: http://www.equator-network.org.
Accessed 15 Nov 2019.
15. Chen K, Pan Y, Xu C, Wu W, Li X, Sun D. What are the
predictors of major depression in adult patients with
epilepsy? Epileptic Disord 2014;16:74-9. Crossref
16. Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S.
Psychiatric comorbidity in epilepsy: a population-based
analysis. Epilepsia 2007;48:2336-44. Crossref
17. Kwong KL, Lam D, Tsui S, et al. Anxiety and depression in
adolescents with epilepsy. J Child Neurol 2016;31:203-10. Crossref
18. Alsaadi T, El Hammasi K, Shahrour TM, et al. Depression
and anxiety among patients with epilepsy and multiple
sclerosis: UAE comparative study. Behav Neurol
2015;2015:196373. Crossref
19. Azuma H, Akechi T. Effects of psychosocial functioning,
depression, seizure frequency, and employment on quality
of life in patients with epilepsy. Epilepsy Behav 2014;41:18-20. Crossref
20. Mohamed S, Gill JS, Tan CT. Quality of life of patients with
epilepsy in Malaysia. Asia Pac Psychiatry 2014;6:105-9. Crossref
21. Mitchell AJ, Meader N, Symonds P. Diagnostic validity
of the Hospital Anxiety and Depression Scale (HADS)
in cancer and palliative settings: a meta-analysis. J Affect Disord 2010;126:335-48. Crossref
22. Kim DH, Kim YS, Yang TW, Kwon OY. Optimal cutoff
score of the Neurological Disorders Depression Inventory
for Epilepsy (NDDI-E) for detecting major depressive
disorder: a meta-analysis. Epilepsy Behav 2019;92:61-70. Crossref
23. Hartung TJ, Friedrich M, Johansen C, et al. The Hospital
Anxiety and Depression Scale (HADS) and the 9-item
Patient Health Questionnaire (PHQ-9) as screening
instruments for depression in patients with cancer. Cancer
2017;123:4236-43. Crossref
24. O’Donoghue MF, Goodridge DM, Redhead K, Sander JW,
Duncan JS. Assessing the psychosocial consequences
of epilepsy: a community-based study. Br J Gen Pract
1999;49:211-4.
25. Boylan LS, Flint LA, Labovitz DL, Jackson SC, Starner K,
Devinsky O. Depression but not seizure frequency predicts
quality of life in treatment-resistant epilepsy. Neurology
2004;62:258-61. Crossref
26. Çakıcı M, Gökçe Ö, Babayiğit A, Çakıcı E, Eş A. Depression:
point-prevalence and risk factors in a North Cyprus
household adult cross-sectional study. BMC Psychiatry
2017;17:387. Crossref
27. Lam LC, Wong CS, Wang MJ, et al. Prevalence, psychosocial
correlates and service utilization of depressive and anxiety
disorders in Hong Kong: the Hong Kong Mental Morbidity
Survey (HKMMS). Soc Psychiatry Psychiatr Epidemiol
2015;50:1379-88. Crossref
28. Kanner AM, Barry JJ. The impact of mood disorders in
neurological diseases: should neurologists be concerned?
Epilepsy Behav 2003;4 Suppl 3:S3-13. Crossref
29. Lacey CJ, Salzberg MR, D'Souza WJ. What factors
contribute to the risk of depression in epilepsy? Tasmanian
Epilepsy Register Mood Study (TERMS). Epilepsia
2016;57:516-22. Crossref
30. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with
demographic and seizure-related variables. Ann Gen
Psychiatry 2007;6:28. Crossref
31. Grabowska-Grzyb A, Jedrzejczak J, Nagańska E, Fiszer U.
Risk factors for depression in patients with epilepsy.
Epilepsy Behav 2006;8:411-7.Crossref
32. Victoroff JI, Benson F, Grafton ST, Engel J Jr,
Mazziotta JC. Depression in complex partial seizures.
Electroencephalography and cerebral metabolic correlates.
Arch Neurol 1994;51:155-63. Crossref
33. Kälviäinen R, Salmenperä T, Partanen K, Vainio P,
Riekkinen P, Pitkänen A. Recurrent seizures may cause
hippocampal damage in temporal lobe epilepsy. Neurology
1998;50:1377-82. Crossref
34. Hosokawa T, Momose T, Kasai K. Brain glucose
metabolism difference between bipolar and unipolar
mood disorders in depressed and euthymic states. Prog
Neuropsychopharmacol Biol Psychiatry 2009;33:243-50. Crossref
35. Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL,
Charney DS. Hippocampal volume reduction in major
depression. Am J Psychiatry 2000;157:115-8. Crossref
36. Luscher B, Shen Q, Sahir N. The GABAergic deficit
hypothesis of major depressive disorder. Mol Psychiatry
2011;16:383-406. Crossref
37. Tao K, Wang X. The comorbidity of epilepsy and
depression: diagnosis and treatment. Expert Rev Neurother
2016;16:1321-33. Crossref
38. Mula M, Agrawal N, Mustafa Z, et al. Self-reported
aggressiveness during treatment with levetiracetam
correlates with depression. Epilepsy Behav 2015;45:64-7. Crossref
39. Klufas A, Thompson D. Topiramate-induced depression.
Am J Psychiatry 2001;158:1736.Crossref
40. Brent DA, Crumrine PK, Varma RR, Allan M, Allman C.
Phenobarbital treatment and major depressive disorder in
children with epilepsy. Pediatrics 1987;80:909-17.