Hong Kong Med J 2020 Aug;26(4):294–303 | Epub 30 Jul 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Warfarin control in Hong Kong clinical practice:
a single-centre observational study
Amy SM Lam, BPharm, MSc1; Isis MH Lee, BPharm1; Simon KS Mak, BPharm1; Bryan PY Yan, MB, BS, FRACP2; Vivian WY Lee, PharmD, BCPS (AQ Cardiology)3
1 School of Pharmacy, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong
2 Department of Medicine & Therapeutics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong
3 Centre for Learning Enhancement and Research, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Prof Vivian WY Lee (vivianlee@cuhk.edu.hk)
Abstract
Introduction: Time in therapeutic range (TTR)
assesses the safety and effectiveness of warfarin
therapy using the international normalised ratio.
This study investigated the TTR in Hong Kong
patients using both European and Japanese
therapeutic ranges and patients’ economic and
clinical outcomes. Predictors of poor warfarin
control and patient knowledge concerning warfarin
therapy were assessed.
Methods: A 5-month observational study with
retrospective and prospective components was
conducted in the Prince of Wales Hospital. The study
examined electronic patient records of patients
who received warfarin for at least 1 year during the
period from January 2010 to August 2015. Patient
knowledge was assessed via phone interview using
the Oral Anticoagulation Knowledge (OAK) test.
Results: In total, 259 patients were included; 174
completed the OAK test. The calculated mean TTR
was 40.2±17.1% (European therapeutic range),
compared with 49.1±16.1% (Japanese therapeutic
range) [P<0.001]. Mean TTR was higher in patients
with atrial fibrillation than in patients with prosthetic
heart valve (P<0.001). The abilities of TTR to predict
clinical and economic outcomes were comparable
between European and Japanese therapeutic
ranges. Patients with ideal TTR had fewer clinical complications and lower healthcare costs. Patients
with younger age exhibited worse TTR, as did those
with concurrent use of furosemide, famotidine, or
simvastatin. Mean OAK test score was 54.1%. Only
24 (13.8%) patients achieved a satisfactory overall
score of ≥75% in the test.
Conclusion: Warfarin use in Hong Kong patients
was poorly controlled, regardless of indication.
Patient knowledge concerning warfarin use was
suboptimal; thus, additional patient education is
warranted regarding warfarin.
New knowledge added by this study
- Warfarin control, in terms of time in therapeutic range (TTR), was suboptimal (40.2% with European therapeutic range and 49.1% with Japanese therapeutic range), regardless of indication.
- Abilities of TTR to predict clinical and economic outcomes were comparable between European and Japanese therapeutic ranges.
- Patients with younger age exhibited worse TTR, as did those with concurrent use of furosemide, famotidine, or simvastatin.
- Only 13.8% of interviewed patients achieved a satisfactory overall score on the Oral Anticoagulation Knowledge test.
- Warfarin is the most commonly prescribed anticoagulant in Hong Kong. However, warfarin control was suboptimal; this poor control was associated with worse clinical and economic outcomes. Poor anticoagulation control could increase healthcare expenses.
- Abilities to predict outcomes were similar between European and Japanese therapeutic ranges. Associations of suboptimal warfarin control with unfavourable outcomes were robust for both therapeutic ranges.
- Despite the establishment of a warfarin clinic and availability of educational materials and discussions regarding warfarin use, patient knowledge concerning warfarin therapy remains unsatisfactory, compared with prior studies in Hong Kong. Additional patient education concerning warfarin use is warranted. New approaches may be useful to deliver medication knowledge.
Introduction
Warfarin, an oral vitamin K antagonist, has been
widely used as anticoagulant therapy for the
treatment and prophylaxis of thromboembolic
disease. Patients with atrial fibrillation (AF) exhibit
elevated risks of mortality and morbidity, including
fivefold greater risk of stroke and threefold greater
risk of heart failure, compared with individuals
without AF.1 2 In patients with prosthetic heart valve
(PHV), the incidence of PHV thrombosis was 0.5%
to 6% per patient-year, depending on the prosthesis
site.3 Warfarin has been shown to significantly reduce
the risk of stroke in patients with non-valvular AF
and the risk of embolism in patients with PHV.4 5
To ensure the efficacy and safety of warfarin
therapy, strict control of the international normalised
ratio (INR) is required. One measurement of
INR does not indicate whether warfarin dose is
appropriate for a given patient. Instead, time in
therapeutic range (TTR) is commonly used in
clinical practice. According to the European Society
of Cardiology Guidelines for the management of
AF, the ideal TTR is regarded as 70%.6 However,
warfarin control in clinical practice is reportedly
unsatisfactory worldwide.7 8 Poor TTR has been
associated with elevated risks of major haemorrhage,
ischaemic stroke, and all-cause mortality.9
Hong Kong is currently following the
European Society of Cardiology Guidelines for the
Management of Atrial Fibrillation with respect to
warfarin; these guidelines recommend INR control between 2.0 and 3.0 in patients with normal heart
valve and between 2.5 and 3.5 in patients with
PHV.6 In contrast, the Japanese Guidelines for
Pharmacotherapy of Atrial Fibrillation (JCS 2013)
recommend INR control between 2.0 and 3.0 in
patients aged <70 years or patients with PHV, and
between 1.6 and 2.6 in patients aged ≥70 years.10 This
recommendation is based on the findings of a study
in which the incidence rate of major haemorrhagic
complications was determined to be lower at INR
between 1.6 and 2.6.11 It remains unknown whether
additional benefits would be obtained by application
of Japanese guidelines in Hong Kong.
There are extensive drug-drug interactions,
drug-herb interactions, and drug-food interactions
of warfarin, which may affect anticoagulation
control.12 13 To assure the efficacy and safety of
warfarin, patient education concerning warfarin is
needed.14 15 However, a study in 2008 showed that
only one in six patients with AF underwent regular
INR examinations in China; patients with AF also
commonly exhibited minimal knowledge concerning
the importance of regular INR examinations.16
The study aimed to investigate the adequacy
of warfarin control in clinical practice in Hong
Kong by means of the TTR; it compared warfarin
outcome prediction using European and Japanese
INR therapeutic ranges as concurrent primary
endpoints. Predictors for poor warfarin control were
analysed as secondary endpoints. The impacts of
TTR on both clinical and economic outcomes were
investigated, using the European therapeutic range.
Patient knowledge concerning warfarin therapy was
also assessed, as were predictors of this knowledge.
Methods
Patient recruitment
The single-centre cohort study was conducted in
the Prince of Wales Hospital, which is a regional
acute public hospital in Hong Kong. Patients who
received warfarin therapy in both the acute coronary
syndrome registry and warfarin clinic for at least
1 year and who had their last visit from 1 January
2010 to 31 August 2015 were included. One year
of warfarin therapy was presumed to be necessary
for patients to develop stable INR.8 Patients aged
<41 years and >90 years were excluded, due to the
infrequency of warfarin therapy in both age-groups
based on hospital records. Data for patient
recruitment and subsequent patient review were
retrieved through the Clinical Management System,
which is a computerised patient medical record
system.
Time in therapeutic range summary
Time in therapeutic range was defined as the fraction
of INRs in range, with the percentage derived by dividing number of INRs within the therapeutic
range by the total number of INRs recorded.17 Ideal
TTR was defined as 70%.6 Warfarin indications for
individual patients were categorised in four groups:
AF, PHV, both AF and PHV, and neither AF nor
PHV (eg, deep vein thrombosis and pulmonary
embolism). Associations of outcomes and adaptions
of either guidelines were subsequently determined.
Predictors of suboptimal time in therapeutic
range
Predictors of poor warfarin control, using the
European therapeutic range, were regarded as
secondary endpoints in our study. Patients were
stratified into four quartiles according to TTR.
Patients with TTR in Quartile 1 were considered to
have poor warfarin control. Patients were compared
across the four quartiles to identify predictors.
Factors included were age, sex, co-morbidities,
medication profile, and patient knowledge
concerning warfarin therapy. Co-morbidities
comprised hypertension, heart failure, thyroid
disorder, liver dysfunction, and diabetes mellitus.
Ten commonly prescribed medications were chosen
for medication profile comparison, based on a pilot
study of the first 20 recruited patients. The pilot
study was conducted using the same recruitment
criteria and the 20 patients were selected at random.
All prescribed medications were recorded for these
20 patients. The 10 most commonly prescribed
medications included aspirin, hydrochlorothiazide,
metoprolol, diltiazem, diclofenac, famotidine, senna,
simvastatin, lisinopril, and pantoprazole. For other
cardiovascular medications, the potential impact
was suspected with their high-frequency use in the
cohort and further investigation was performed. The
potential impact was detected using ongoing data
collection based on low TTR and high thrombotic
and bleeding events of patients with certain
medications that were not included in the list of
10 medications previously. The investigators
evaluated each additional medication carefully and
its impact on the clinical outcomes.
Impact of time in therapeutic range on
clinical outcome
Impacts of TTR on clinical outcomes were
investigated; patient TTR values were stratified
into four quartiles. Thrombotic events, bleeding
complications, and overall incidences of
complications were assessed. Stroke, pulmonary
embolism, acute coronary syndrome, and arterial
embolism were included as thrombotic events in
our study. Severity of bleeding complications was
classified based on discussion at the Control of
Anticoagulation Subcommittee of the International
Society of Thrombosis and Haemostasis.18 Major bleeding included: (1) fatal bleeding; and/or
(2) symptomatic bleeding in a critical area or
organ (eg, intracranial, intraspinal, intraocular,
retroperitoneal, intraarticular or pericardial, or
intramuscular with compartment syndrome); and/or
(3) bleeding causing a decline in haemoglobin level
of ≥2 g/dL (1.24 mmol/L), or leading to transfusion
of ≥2 units of whole blood or red cells. Otherwise,
all non-major bleeds were regarded as minor bleeds.
Impact of time in therapeutic range on
economic outcome
Impacts of TTR, using the European therapeutic
range, on economic outcomes were investigated.
Costs were calculated per day of warfarin therapy,
such that patients’ direct healthcare costs could
be calculated regardless of the length of warfarin
therapy. Direct healthcare costs related to warfarin
(from the healthcare provider perspective) were
calculated using the Hong Kong government
gazette.19 Costs for INR examinations, procedures
(eg, surgery and diagnostic tests, excluding INR
examinations), hospitalisation, clinic visits, and
overall costs were compared separately.
Knowledge assessment
Patient knowledge concerning warfarin therapy was
assessed using the Oral Anticoagulation Knowledge
(OAK) test.20 Question 14 of the original test was
omitted from our study, because the frequencies
of INR tests and follow-up visits were determined
by local physicians in Hong Kong. A “Do not
know” option was included to minimise random
guessing. The assessment was translated into
Chinese and performed via phone interviews from
2 January 2016 to 1 April 2016. Patient knowledge
was considered satisfactory if a score of ≥75% was
achieved.21 Predictors for OAK score performance
were identified.
Statistical analysis
For descriptive statistics, frequencies and percentages
were used for categorical variables; means ± standard
deviations were used for continuous variables.
The Wilcoxon signed rank test, Chi squared test,
Fisher’s exact test, and one-way analysis of variance
(pairwise comparison with the Tukey method) were
used for comparisons of TTR with European and
Japanese therapeutic ranges. Fisher’s exact test and
Mann-Whitney U test were used to determine the
impacts of TTR on clinical and economic outcomes,
respectively. An ordinal regression model with
stepwise selection was used to identify independent
predictors for poor warfarin control. Multiple linear
regression with stepwise selection for variables
was used to determine predictors for OAK score.
Two-sided P values <0.05 were considered statistically significant. All statistical analysis was
performed by SPSS (Windows version 22.0; IBM
Corp, Armonk [NY], US) and R (version 3.5.3;
https://www.r-project.org/).
Results
Baseline characteristics
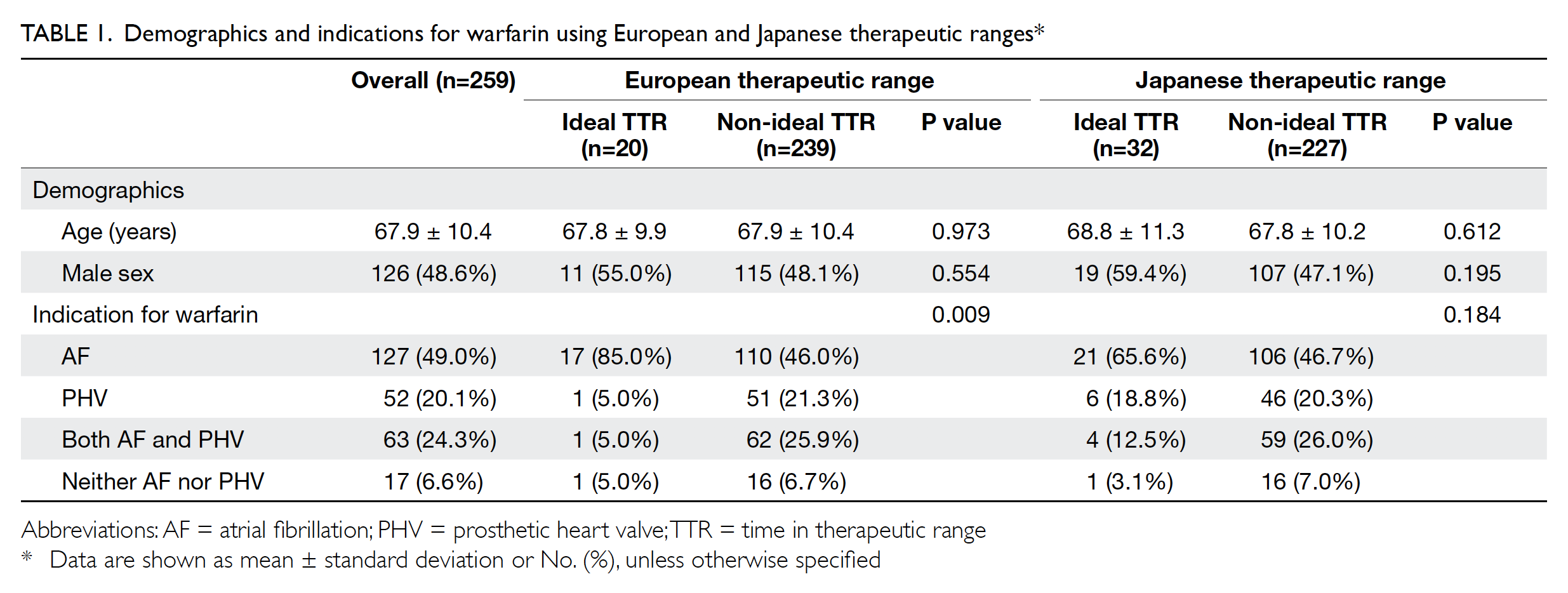
In total, 259 patients were included in the study;
among them, 126 (48.6%) were men. The mean
patient age was 67.9±10.4 years. The detailed
demographic characteristics of the patients are
shown in Table 1.
Time in therapeutic range summary
The overall mean INR was 2.3±0.3. The median
follow-up time for included patients was 2065 days
(interquartile range=1556-2065). The median
number of INR examinations was 46 (interquartile
range=33-73). Using the European therapeutic
range, 34.5% of all measured INR values were
within the therapeutic range. The overall TTR was 40.2±17.1%; 7.7% of patients had ideal TTR during
the study period. Using the Japanese therapeutic
range, 44.1% of all measured INR values were
within the therapeutic range. The overall TTR was
49.1±16.1%; this was significantly higher than the
TTR when using the European therapeutic range
(P<0.001). Notably, 12.4% of all patients had ideal
TTR during the study period.
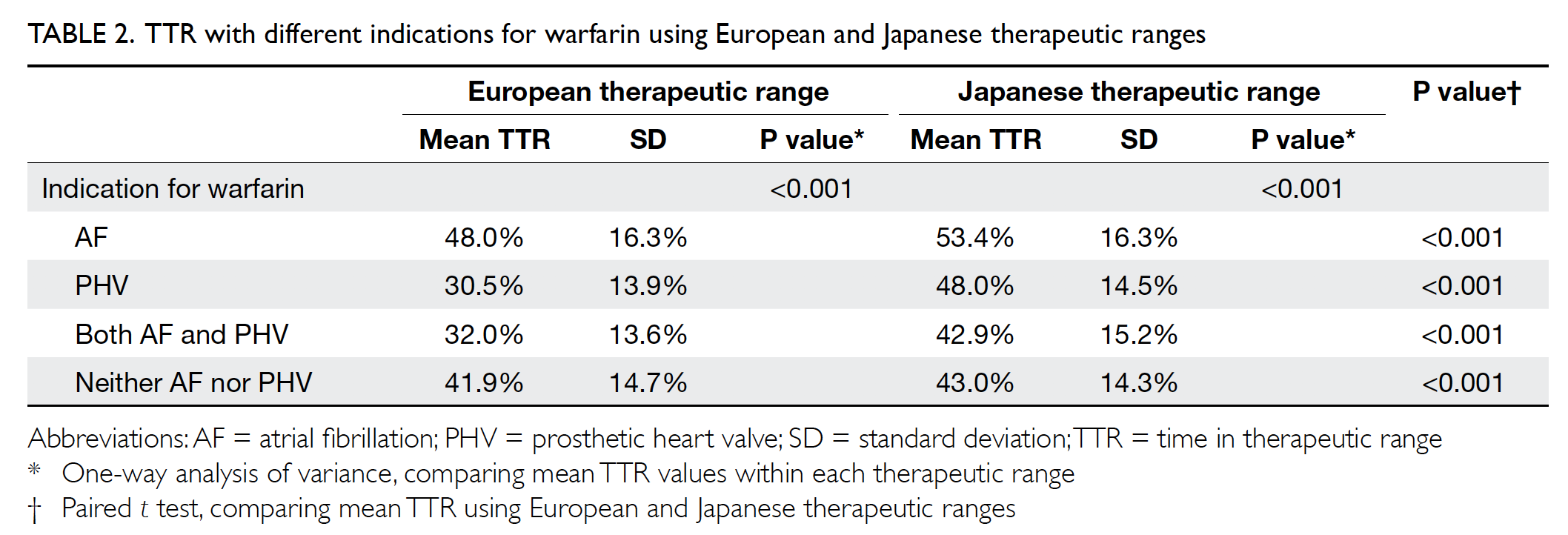
Mean TTR values for different indications
were compared, as shown in Table 2. When using
the European therapeutic range, the mean TTR with
an indication for AF was significantly higher than
both the mean TTR with an indication for PHV
(P<0.001) and the mean TTR with an indication for
AF and PHV (P<0.001). When using the Japanese
therapeutic range, the mean TTR with an indication
for AF was also significantly higher than the mean
TTR with an indication for both AF and PHV
(P<0.001). The mean TTR values were significantly
higher when using the Japanese therapeutic range
than when using the European therapeutic range
within each indication category.
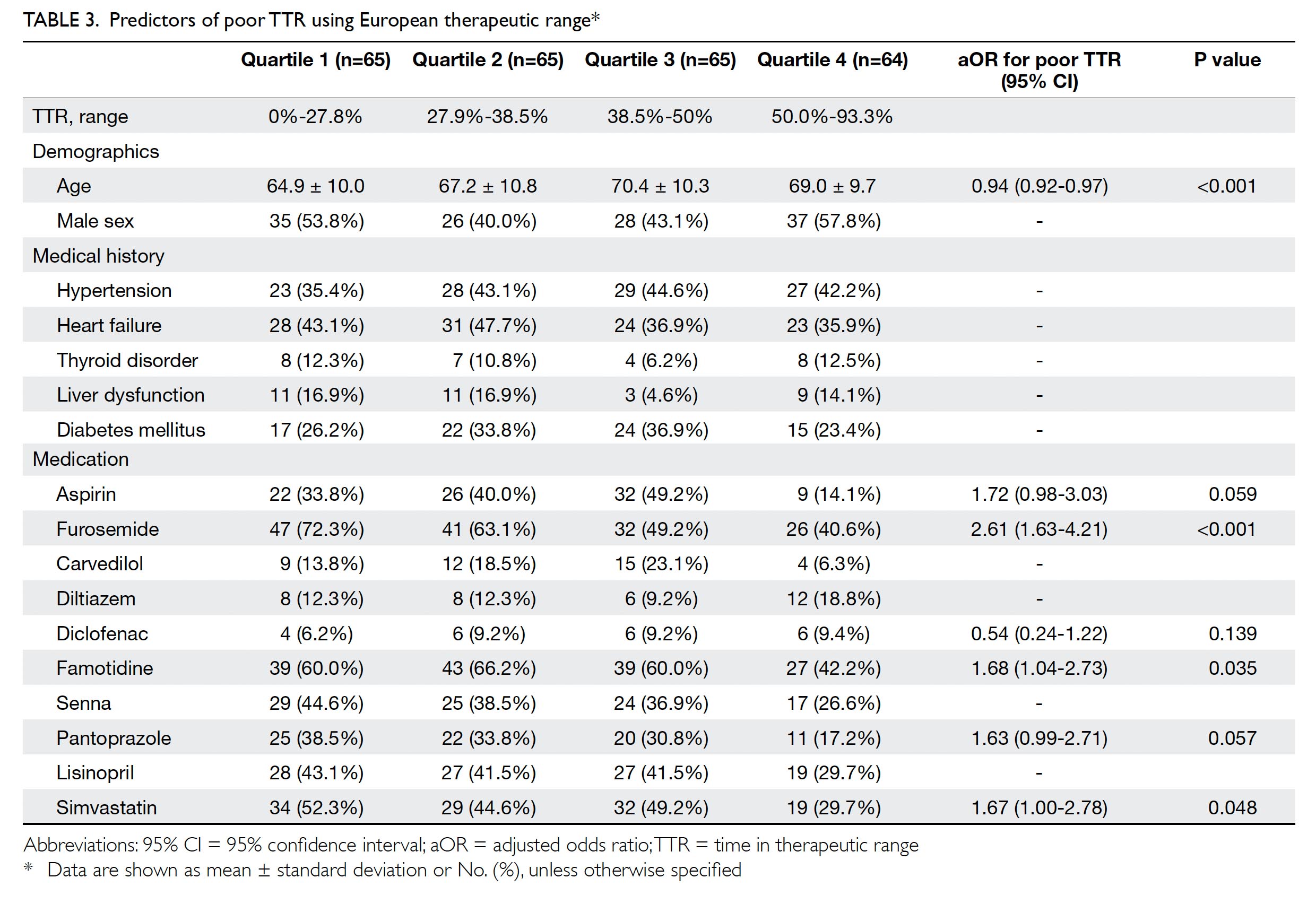
Predictors of suboptimal time in therapeutic
range
Patients were divided into four quartiles according
to their TTR, using the European therapeutic range
(Table 3). Predictors were determined by performing
regression across the four quartiles. Adjusted odds
ratios (ORs) for poor TTR were calculated. The
results showed that younger age was associated with
worse TTR, as were concurrent use of furosemide,
famotidine, or simvastatin.
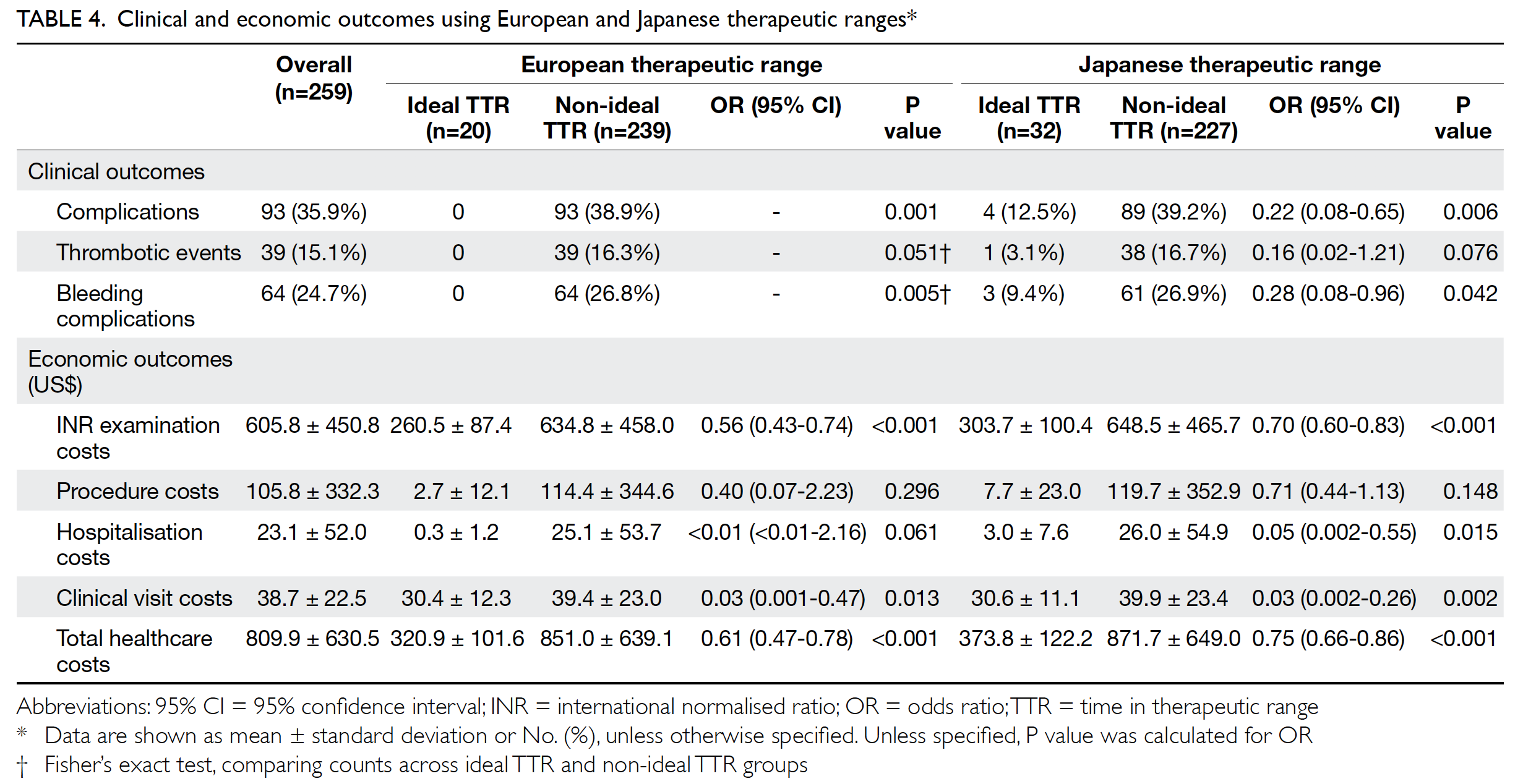
Impact of time in therapeutic range on
clinical outcome
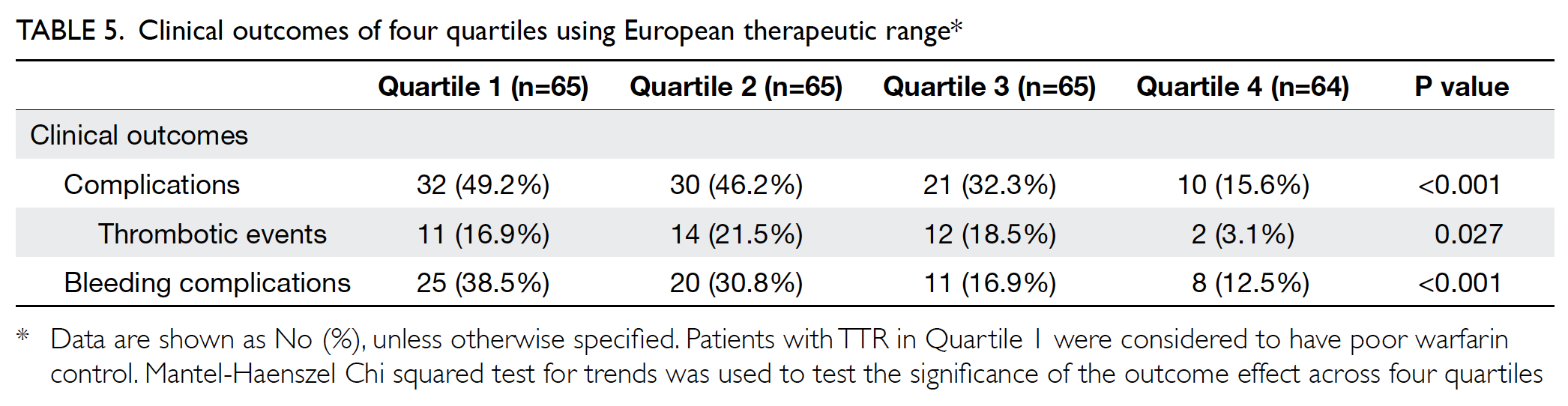
Clinical outcomes were compared between the
two therapeutic ranges (Table 4). Of the 259 patients,
35.9% experienced complications. Of the 39 patients
with thrombotic events, 41.0% had recurrent
non-ST-elevation myocardial infarction and
33.3% had stroke. Among patients with bleeding
complications, 68.8% experienced minor bleeding.
Patients with ideal TTR had significantly fewer
overall complications and bleeding complications,
compared with patients with non-ideal TTR, in
both European and Japanese therapeutic ranges.
All patients who had complications were those with non-ideal TTR, using the European therapeutic
range. When patients were further stratified
into quartiles based on TTR using the European
therapeutic range, TTR exhibited statistically
significant associations with each tested clinical
outcome (Table 5).
Impact of time in therapeutic range on
economic outcome
Healthcare costs are expressed in terms of US$ per year
(US$1=HK$7.8), as shown in Table 4. When including
all services related to warfarin, average patient
costs were US$809.9/year. In terms of economic
outcomes, the INR examination, clinical visit, and
total healthcare costs were significantly lower for
patients with ideal TTR when using either European
or Japanese therapeutic ranges. Using the Japanese
therapeutic range, patients with ideal TTR also
had lower hospitalisation costs. When using the
European therapeutic range, healthcare provider
costs increased by US$530.1/year for each patient
with non-ideal TTR.
Knowledge assessment
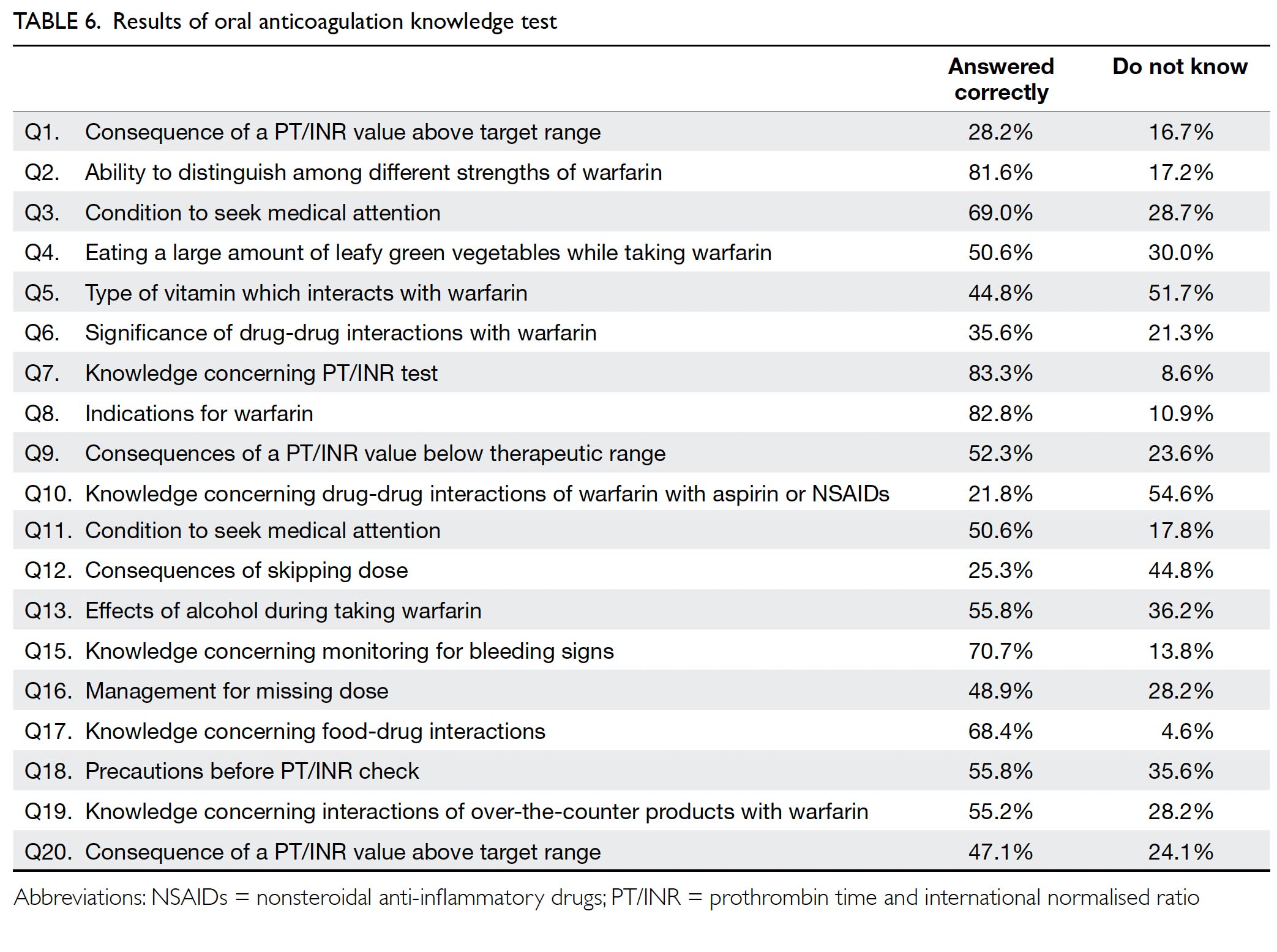
In total, 174 patients completed the OAK test, with a mean score of 54.1% correct for the 19 questions
used in our version of the test. The mean duration
of warfarin therapy for this subgroup of patients
during the study period was 4.8±1.4 years. Only
24 (13.8%) patients achieved the satisfactory overall
test score of ≥75%. Of the 19 questions in the test,
only four were answered correctly by ≥70% of
respondents (Table 6).
Multiple linear regression revealed that
respondents with older age (adjusted β=-0.17;
95% confidence interval [CI]=-0.23 to -0.11;
P=0.001) or co-morbid diabetes (adjusted β=-1.21;
95% CI=-2.29 to -0.12; P=0.03) were more likely
to have low scores on the OAK test. In contrast,
respondents with co-morbid hypertension
(adjusted β=1.68; 95% CI=0.56-2.80; P=0.004) or
co-morbid thyroid dysfunction (adjusted β=2.38;
95% CI=0.80-3.97; P=0.003) were more likely to
have high scores on the OAK test. Respondents with better TTR tended to be more likely to have high
scores on the OAK test, although this difference
was not statistically significant (adjusted β=2.73;
95% CI=-0.21-5.68; P=0.069).
Discussion
Status of warfarin control in Hong Kong
The mean TTR observed in our study was lower
than that observed in studies performed in Western
nations. A meta-analysis of 40 studies using the
European therapeutic range identified a mean
TTR of 75.2% after 4 to 12 months of warfarin
management.22 A study focusing on warfarin use
in Japanese patients using the Japanese therapeutic
range showed an overall TTR of 69.7% in patients
with non-valvular AF.23 Studies in Hong Kong
showed that the mean TTR for target INR of 2.0
to 3.0 in patients with AF improved from 24.2% to 39.7% in the past decade.24 25 Our study showed
better warfarin control in patients with AF (mean
TTR=48.0%), compared with past local data;
however, the rate of control remains unsatisfactory.
A prior retrospective study demonstrated a mean
TTR of 72.5% in Swedish patients with mechanical
heart valve prosthesis; another study showed that
the mean TTR was 47.48% in Malaysian patients
with mechanical heart valve(s) replacement.26 27 The
mean TTR in patients with PHV in this study was
30.5%, which was lower than the previously reported
rate. Our study also demonstrated that warfarin
control was worse in patients with PHV than in
patients with AF.
The lower TTR in Hong Kong, compared with that in
Western nations, could be attributed to ethnicity.
Geographical differences in the genetic polymorphism
profile between Hong Kong and Western nations
could lead to differences in warfarin metabolism
and warfarin dosing.28 Moreover, previous evidence
suggests that individuals of East Asian ethnicity are
more likely to experience intracranial haemorrhage,
compared with individuals of Caucasian ethnicity
(in that study, “white race/ethnicity”) who exhibit
comparable levels of warfarin control.29 Notably,
the possibility that physicians targeted a lower INR
range in Hong Kong could not be ruled out in this
study.
European versus Japanese therapeutic range
The overall predictive abilities of European and
Japanese therapeutic ranges were similar. The
calculated ORs for each economic outcome across
European and Japanese therapeutic ranges were
similar, with the exception of procedural and
hospitalisation costs. For clinical outcomes, ORs
could not be calculated to compare ideal TTR
with non-ideal TTR, given that there were no
complications in the ideal TTR group. However,
there were complications in the group with ideal
TTR based on the Japanese therapeutic range. The
ORs calculated showed that the Japanese therapeutic
range could be used to predict clinical outcomes.
Notably, a lower INR target can be established
in Hong Kong. However, a larger, well-designed
randomised controlled trial is needed to establish
non-inferiority in terms of clinical outcomes, as well
as superiority in terms of economic outcomes, when
using the Japanese therapeutic range.
Impacts of time in therapeutic range on
outcomes
The level of warfarin control has been associated
with clinical outcomes. A systematic review of
47 studies revealed that TTR was negatively correlated
with major bleeding and thromboembolic events.30 Our results were consistent in demonstrating an
association of TTR with clinical outcome, which
indicated that patients with worse TTR were
more likely to experience overall complications,
thrombotic events, and bleeding complications.
Moreover, TTR has been associated with economic
outcomes. A previous study in the US showed that
patients with AF whose TTR was <60% had higher
total healthcare and stroke-related costs.31 Our study
demonstrated similar results, using a TTR cut-off of
70%. With better warfarin control, corresponding
healthcare expenses can be reduced; many such
expenses are borne by the government.
Predictors for suboptimal time in therapeutic
range
Predictors for suboptimal TTR have been
investigated in previous studies. Notably, heart
failure has been highly associated with poor
warfarin control8 23; however, this association was
not supported by our findings. In contrast, our study
showed that younger patients were more likely to
have poor TTR. This association might be related to
improved medication adherence in older patients,
because of better health consciousness among
those individuals.32 33 Concurrent use of furosemide,
famotidine, or simvastatin (in combination with
warfarin) was associated with poor TTR. Despite
common concurrent use of simvastatin and warfarin,
the anticoagulant effect of warfarin is reportedly
8% to 15% stronger in simvastatin-treated patients,
due to the CYP 2C9*3 polymorphism.34 Regarding
concurrent use of warfarin and pantoprazole,
altered warfarin absorption and metabolism have
been observed during in vitro studies of proton
pump inhibitor treatment35; however, there is a
lack of supporting clinical evidence.36 Our study
showed a tendency for enhanced likelihood of
poor TTR control in patients with concurrent use
of pantoprazole, although this association was not
statistically significant. Thus, the influence of proton
pump inhibitor use on warfarin control remains
unclear. Patients with concurrent use of aspirin and
warfarin exhibited a tendency for enhanced risk of
poor TTR; this association was also not statistically
significant. We noted a considerable reduction in
the number of patients in the fourth TTR quartile
(14.1%), compared with the other three groups
(range, 33.8-49.2%). Concurrent use of aspirin and
warfarin is known to enhance the risk of major
bleeding, which could cause physicians to approach
anticoagulation control more conservatively.37
Furthermore, the use of aspirin and poor TTR have
both been independently associated with higher
bleeding risk, while poor TTR has been regarded as
an independent contributor to all-cause mortality.38
Therefore, regardless of the concurrent use of
aspirin, optimal TTR should be achieved with regard to the appropriate INR therapeutic range to reduce
complications in patients receiving warfarin therapy.
Patient knowledge concerning warfarin
therapy
According to validation studies performed by
Zeolla et al,20 the mean OAK score among long-term
warfarin users was 72%. A study in Malaysia revealed
that only 11.2% of patients achieved a satisfactory
score, with a mean OAK score of 48% for the
cohort.39 Similar results were achieved in our study;
the mean score was 54.1% and 13.8% of patients
achieved a score of ≥75%. Poor OAK score could
be attributed to restricted medical consultation
time, leading to a lack of knowledge concerning
respective diseases and medications.40 Patients with
older age were more likely to have low OAK scores,
which was consistent with the findings of a previous
study that demonstrated a negative correlation
between age and warfarin knowledge.41 Nonetheless,
the observed relationships of co-morbidities with
warfarin knowledge require further analyses to
establish underlying explanations.
Study limitations
This study had several important limitations. This
was a single-centre study with limited sample size
and study population distribution skewed towards
AF patients concerning warfarin indications. The
target INR range for included patients was unknown.
Notably, some physicians may have set a lower goal
of 1.5 to 2.5 in patients with higher risk of bleeding.
Patient TTR could have been affected by medication
delay or refusal due to medical procedures. The
impacts of TTR on medication costs were not
investigated because differences in available
strengths of warfarin led to various combinations
of warfarin prescriptions. Moreover, we could not
adjust for diet, use of traditional Chinese medicine
or complementary alternative medications, and
medication non-compliance as factors that may
influence warfarin control. The OAK test was
amended to fit our local practices and was not
administered to some of the recruited patients in
this study. Further validation is needed concerning
the Chinese version of the amended OAK test.
Conclusion
Warfarin use in Hong Kong patients was poorly
controlled, regardless of indication. Patients with
indications for AF had better warfarin control. Using
the Japanese therapeutic range, the level of warfarin
control remained unsatisfactory. Our study showed
that TTR could be a predictor for both economic
and clinical outcomes. Younger age was found to be
an independent predictor of poor warfarin control,
as were concurrent use of aspirin or simvastatin.
Patients had poor knowledge concerning INR
value and interpretation. More education is needed
regarding drug-drug interactions of warfarin and
consequences of missed doses.
Author contributions
Concept or design: VWY Lee, BPY Yan.
Acquisition of data: IMH Lee, SKS Mak.
Analysis or interpretation of data: ASM Lam, VWY Lee, BPY Yan.
Drafting of the manuscript: ASM Lam, VWY Lee, BPY Yan.
Critical revision for important intellectual content: All authors.
Acquisition of data: IMH Lee, SKS Mak.
Analysis or interpretation of data: ASM Lam, VWY Lee, BPY Yan.
Drafting of the manuscript: ASM Lam, VWY Lee, BPY Yan.
Critical revision for important intellectual content: All authors.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, BPY Yan was not involved in the
peer review process. Other authors have disclosed no conflicts
of interest.
Declaration
This manuscript was posted on Research Square as a registered
online preprint (https://doi.org/10.21203/rs.2.15276/v1).
Funding/support
This research received no specific grant from any funding
agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Joint Chinese University
of Hong Kong–New Territories East Cluster Clinical
Research Ethics Committee (Ref CRE 2013.667). Informed
verbal consent was obtained from patients participating
in knowledge assessment, which was conducted via phone
interview. The need for patient consent was waived by the
Ethics Committee for the retrospective cohort study because
no personal identifiers or related information were obtained
during the data collection process.
References
1. Jabre P, Roger VL, Murad MH, et al. Mortality associated
with atrial fibrillation in patients with myocardial infarction
a systematic review and meta-analysis. Circulation
2011;123:1587-93. Crossref
2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an
independent risk factor for stroke: the Framingham Study.
Stroke 1991;22:983-8. Crossref
3. Cáceres-Lóriga FM, Pérez-López H, Santos-Gracia J,
Morlans-Hernandez K. Prosthetic heart valve thrombosis:
pathogenesis, diagnosis and management. Int J Cardiol
2006;110:1-6. Crossref
4. Aguilar MI, Hart R. Oral anticoagulants for preventing
stroke in patients with non-valvular atrial fibrillation and
no previous history of stroke or transient ischemic attacks.
Cochrane Database Syst Rev 2005;(3):CD001927. Crossref
5. Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic
and bleeding complications in patients with mechanical
heart valve prostheses. Circulation 1994;89:635-41. Crossref
6. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines
for the management of atrial fibrillation developed in
collaboration with EACTS. Eur Heart J 2016;37:2893-962. Crossref
7. Asarcıklı LD, Şen T, İpek EG, et al. Time in Therapeutic
Range (TTR) value of patients who use warfarin and factors
which influence TTR. J Am Coll Cardiol 2013;62:C127-8. Crossref
8. Nelson WW, Choi JC, Vanderpoel J, et al. Impact of
co-morbidities and patient characteristics on international
normalized ratio control over time in patients with
nonvalvular atrial fibrillation. Am J Cardiol 2013;112:509-
12. Crossref
9. Cancino RS, Hylek EM, Reisman JI, Rose AJ. Comparing
patient-level and site-level anticoagulation control as
predictors of adverse events. Thromb Res 2014;133:652-6. Crossref
10. JCS Joint Working Group. Guidelines for Pharmacotherapy
of Atrial Fibrillation (JCS 2013). Circ J 2014;78:1997-2021. Crossref
11. Yasaka M, Minematsu K, Yamaguchi T. Optimal intensity
of international normalized ratio in warfarin therapy for secondary prevention of stroke in patients with non-valvular atrial fibrillation. Intern Med 2001;40:1183-8. Crossref
12.Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005;165:1095-106. Crossref
13.Leite PM, Martins MAP, Castilho RO. Review on mechanisms and interactions in concomitant use of herbs and warfarin therapy. Biomed Pharmacother 2016;83:14- 21. Crossref
14. Wofford JL, Wells MD, Singh S. Best strategies for patient education about anticoagulation with warfarin: a systematic review. BMC Health Serv Res 2008;8:40. Crossref
15. Kagansky N, Knobler H, Rimon E, Ozer Z, Levy S. Safety of anticoagulation therapy in well-informed older patients. Arch Intern Med 2004;164:2044-50. Crossref
16. Zhou Z, Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol 2008;18:209-16. Crossref
17. Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. J Thromb Thrombolysis 2003;15:213-6. Crossref
18. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-4. Crossref
19.Government Logistics Department, Hong Kong SAR Government. Hospital Authority Ordinance (Chapter 113). Revision to list of public charges. Available from: http://www.gld.gov.hk/egazette/pdf/20172124/ egn201721243884.pdf. Accessed 6 Dec 2017.
20. Zeolla MM, Brodeur MR, Dominelli A, Haines ST, Allie ND. Development and validation of an instrument to determine patient knowledge: the Oral Anticoagulation Knowledge Test. Ann Pharmacother 2006;40:633-8. Crossref
21.Rahmani P, Guzman CL, Blostein MD, Tabah A, Muladzanov A, Kahn SR. Patients’ knowledge of anticoagulation and its association with clinical characteristics, INR Control and warfarin-related adverse events. Blood 2013;122:1738. Crossref
22. Erkens PM, ten Cate H, Büller HR, Prins MH. Benchmark for time in therapeutic range in venous thromboembolism: a systematic review and meta-analysis. PLoS One 2012;7:e42269. Crossref
23. Tomita H, Kadokami T, Momii H, et al. Patient factors against stable control of warfarin therapy for Japanese non-valvular atrial fibrillation patients. Thromb Res 2013;132:537-42. Crossref
24. Leung CS, Tam KM. Antithrombotic treatment of atrial fibrillation in a regional hospital in Hong Kong. Hong Kong Med J 2003;99:179-85.
25. Li WH, Huang D, Chiang CE, et al. Efficacy and safety of dabigatran, rivaroxaban, and warfarin for stroke prevention in Chinese patients with atrial fibrillation: the Hong Kong Atrial Fibrillation Project. Clin Cardiol 2017;40:222-9. Crossref
26. Grzymala-Lubanski B, Svensson PJ, Renlund H, Jeppsson A, Själander A. Warfarin treatment quality and prognosis in patients with mechanical heart valve prosthesis. Heart 2017;103:198-203. Crossref
27. Tan CS, Fong AY, Jong YH, Ong TK. INR control of patients with mechanical heart valve on long-term warfarin therapy. Glob Heart 2018;13:241-4. Crossref
28. Gaikwad T, Ghosh K, Shetty S. VKORC1 and CYP2C9 genotype distribution in Asian countries. Thromb Res 2014;134:537-44. Crossref
29. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007;50:309-15. Crossref
30. Wan Y, Heneghan C, Perera R, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes 2008;1:84-91. Crossref
31. Deitelzweig S, Evans M, Hillson E, et al. Warfarin time in therapeutic range and its impact on healthcare resource utilization and costs among patients with nonvalvular atrial fibrillation. Curr Med Res Opin 2016;32:87-94. Crossref
32. Skeppholm M, Friberg L. Adherence to warfarin treatment among patients with atrial fibrillation. Clin Res Cardiol 2014;103:998-1005. Crossref
33. Kang CD, Tsang PP, Li WT, et al. Determinants of medication adherence and blood pressure control among hypertensive patients in Hong Kong: a cross-sectional study. Int J Cardiol 2015;182:250-7. Crossref
34. Andersson ML, Mannheimer B, Lindh JD. The effect of simvastatin on warfarin anticoagulation: a Swedish register-based nationwide cohort study. Eur J Clin Pharmacol 2019;75:1387-92. Crossref
35. Li XQ, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 2004;32:821-7. Crossref
36. Henriksen DP, Stage TB, Hansen MR, Rasmussen L, Damkier P, Pottegård A. The potential drug-drug interaction between proton pump inhibitors and warfarin. Pharmacoepidemiol Drug Saf 2015;24:1337-40. Crossref
37. Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 2013;127:634-40. Crossref
38. Proietti M, Lip GY. Impact of quality of anticoagulation control on outcomes in patients with atrial fibrillation taking aspirin: an analysis from the SPORTIF trials. Int J Cardiol 2018;252:96-100. Crossref
39. Matalqah LM, Radaideh K, Sulaiman SA, Hassali MA, Kader MA. An instrument to measure anticoagulation knowledge among Malaysian community: a translation and validation study of the Oral Anticoagulation Knowledge (OAK) Test. Asian J Biomed Pharm Sci 2013;3:30-7.
40. Lee VW, Tam CS, Yan BP, Yu CM, Lam YY. Barriers to warfarin use for stroke prevention in patients with atrial fibrillation in Hong Kong. Clin Cardiol 2013;36:166-71. Crossref
41. Hasan SS, Shamala R, Syed IA, et al. Factors affecting warfarin-related knowledge and INR control of patients attending physician- and pharmacist-managed anticoagulation clinics. J Pharm Pract 2011;24:485-93. Crossref