Hong Kong Med J 2020 Jun;26(3):216–26 | Epub 28 May 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Preservation of fertility in premenopausal
patients with breast cancer
Samuel SY Wang, Bmed MD1; Herbert Loong, MB, BS, MRCP2; Jacqueline PW Chung, MB, ChB, MRCOG3; Winnie Yeo, MB, BS, FRCP (Lond)4
1 Prince of Wales Clinical School, Faculty of Medicine, University of New
South Wales, Sydney, Australia
2 Faculty of Medicine, Department of Clinical Oncology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
3 Assisted Reproductive Technology Unit, Department of Obstetrics and
Gynaecology, Prince of Wales Hospital, The Chinese University of Hong
Kong, Hong Kong
4 Department of Clinical Oncology, State Key Laboratory of Translational
Oncology, Faculty of Medicine, The Chinese University of Hong Kong,
Hong Kong
Corresponding author: Dr Samuel SY Wang (z3424197@unsw.edu.au)
Abstract
Introduction: Cancer survivorship is increasingly
important with advances in cancer therapeutics.
Minimisation of treatment-related morbidity is an
area that requires attention. This situation is most
pressing in premenopausal patients with breast
cancer, in whom advances in hormonal and targeted
therapies have improved mortality rates. However,
treatment-related infertility is still poorly addressed,
and in East Asia, there is limited discussion regarding
management of treatment-related infertility.
Methods: A search of the literature was conducted
using PubMed, Google Scholar, and Science Direct
using the terms “breast cancer”, “fertility preservation”,
“oocyte and embryonic cryopreservation”, “GnRH-a
co-administration”, “ovarian tissue cryopreservation
and transplantation”, “Japan”, “China”, “Korea”, and
‘Singapore”. Only studies published in English from
1980-2019 were included. The focus of the review
was on identifying the current fertility preservation
methods available to premenopausal women with
breast cancer and the barriers that impede access.
Results: Fertility preservation options include
GnRH-a co-administration to minimise treatment-associated
infertility, oocyte and embryonic
cryopreservation, and emerging treatments such as
ovarian tissue cryopreservation and transplantation.
In East Asia, the uptake of fertility preservation
options has been limited despite it being a major patient concern. A lack of awareness of fertility
preservation treatments hinders discussion between
patients and clinicians about fertility preservation.
Conclusion: Despite progress in fertility preservation technologies, their impact for patients
will be minimal if there is a lack of awareness/use of
the technology. This review aims to raise awareness
of such technologies among clinicians, enabling
discussion between patients and clinicians about
fertility preservation options.
Introduction
Recently, survival among patients with breast
cancer has significantly improved. With better
understanding of the disease’s diverse biology and
increased availability of treatments, the 5-year
survival rate for women diagnosed with breast cancer
has increased from 75.2% between 1975 and 1977 to
88.2% between 2001 and 2008, leading to a substantial
increase in breast cancer survivors.1 More strikingly, over 10% of breast cancer cases occur in women
under age 45 years. Among these premenopausal
survivors, 50% or more will live 20 years or longer
following diagnosis. Thus, there is a need to address
survivorship issues pertaining to long-term toxicity
associated with breast cancer treatment.1 A study
showed that 20 038 premenopausal women are
diagnosed with breast cancer annually in the
United States, with an estimated 96% (19 416) of these premenopausal patients at risk of infertility
because of chemotherapy or hormonal therapy.1
Following chemotherapy, the reported incidence
of amenorrhea varies between 40% and 68%.2 The
agents most responsible for inducing amenorrhoea
and premature ovarian failure (POF) are alkylating
agents such as cyclophosphamide, whereas
antimetabolites have a lesser effect.2 A brief summary
of the association between infertility risk and type of
chemotherapy can be seen in Table 1.3 4
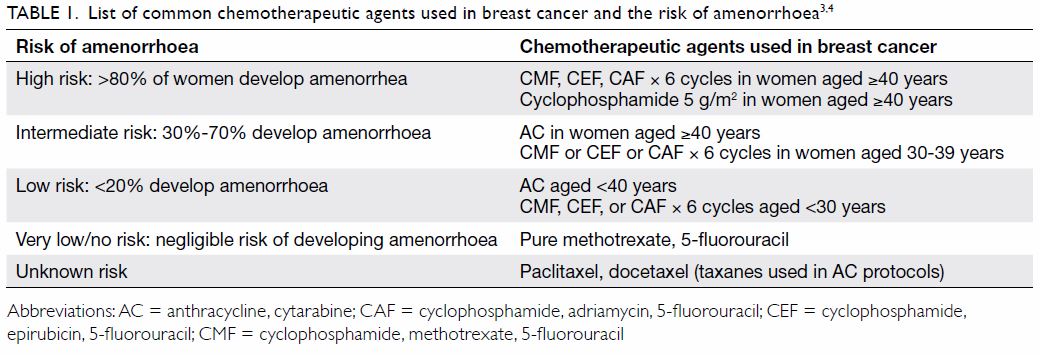
Table 1. List of common chemotherapeutic agents used in breast cancer and the risk of amenorrhoea3 4
Apart from chemotherapy, hormonal
modulation with tamoxifen is beneficial for hormone
receptor–positive disease, which accounts for 70%
of breast cancers. A 5-year course of tamoxifen
reduces recurrence by 47% and mortality by 26%.5
However, tamoxifen is teratogenic, and pregnancy
is contra-indicated during treatment; hence,
pregnancy is often postponed. Although tamoxifen may not directly damage the ovaries, several studies
have reported its association with higher rates of
treatment-related amenorrhea, particularly after age
40 years.5 It is likely that tamoxifen’s length of therapy
may indirectly contribute to infertility alongside age-related
fertility decline.5 Apart from difficulties with
conception, premenopausal patients with breast
cancer also experience high rates of spontaneous
abortion (29%) and premature deliveries with low
birth weight (40%).2
Minimising cancer treatment–associated
infertility is especially important for premenopausal
women who have not yet established a family. Female
infertility in premenopausal women can cause
these women great distress by preventing them
from achieving the important life and social goal of motherhood. A recent study ranks this among the top
five concerns among patients with premenopausal
breast cancer.6 Moreover, in addition to impacting patients’ mental health, the fear of infertility also
impacts treatment compliance.6 Of all patients, 29% do not comply with treatment because of treatment-associated
infertility fears, which impacts patients’
prognosis and life expectancy.7 8 9
While these fears are increasingly recognised
by doctors, recent literature suggests that 32% of
patients do not recall discussing fertility with their
doctors. This lack of communication could be caused
by a lack of awareness or confidence discussing
fertility management in the context of breast cancer.
This phenomenon was highlighted by a finding that
37% of oncologists feared delaying chemotherapy for
fertility preservation, and 49% of oncologists were
concerned about pregnancy safety after breast cancer
treatment.8 10 A Hong Kong study showed that only
45.6% of clinicians from diverse fields such as clinical
oncology, haematology, obstetrics and gynaecology,
paediatrics, and surgery were familiar with fertility
preservation.11 Unfortunately, this lack of clinicians’
awareness is detrimental to premenopausal patients
with breast cancer. Modern reproductive medicine
can preserve female fertility in these patients.12
This review aims to summarise the existing fertility
preservation options for patients with breast cancer
to enable clinicians to have informed discussions
with their patients.
Methods
A literature search was conducted using PubMed,
Google Scholar, and Science Direct using the terms
“breast cancer”, “fertility preservation”, “oocyte
and embryonic cryopreservation”, “GnRH-a co-administration”,
and “ovarian tissue cryopreservation
and transplantation”. Only studies published in
English from 1980 to 2019 were included. The
focus of the literature review was on identifying the
current fertility preservation methods available to
premenopausal women with breast cancer and the
barriers that impede access.
Co-administration of gonadotropin-releasing
hormone agonist during chemotherapy
Chemotherapy accelerates follicular destruction,
which reduces synthesis of inhibins and oestrogens.
The use of adjuvant gonadotropin-releasing
hormone agonist (GnRH-a) to limit chemotherapy-induced
ovarian toxicity has been proposed by
Glode et al.13 Gonadotropin-releasing hormone
agonist has been shown to be protective against
ovarian follicular depletion in mice.14 15 A current
postulate on the protective mechanism of GnRH-a
is the creation of a hypogonadotropic state. Reduced
oestrogen and inhibin levels increase follicle-stimulating
hormone (FSH) secretion via negative
feedback. Consequently, supraphysiological FSH
levels accelerate preantral follicle maturation and
recruitment, which is vulnerable to chemotherapy.
Gonadotropin-releasing hormone agonist induces
pituitary desensitisation, preventing any increase
in FSH concentration and hence minimising
chemotherapy-induced follicle destruction.16 17
The evidence that GnRH-a reduces
chemotherapy-associated POF and improves
pregnancy rates is increasing.18 The use of GnRH-a
to preserve ovarian function and fertility has recently
been recommended as a reliable strategy for at least
breast cancer.19 A pilot study undertaken by Recchia
et al20 reported that patients <40 years with breast
cancer who received chemotherapy with GnRH-a
co-treatment of 3.6 mg goserelin every 28 days for
1 year resumed cyclic ovarian function. Following
a median follow-up of 79 months, Recchia et al20
observed amenorrhea in none of the patients aged
<40 years and 49% of patients aged >40 years. Four
pregnancies were observed, three ended at term, and
one was voluntarily terminated. Additionally, such
a procedure did not affect the clinical outcomes of
patients with breast cancer: after a median follow-up
of 55 months, the disease-free survival and overall
survival were 84% and 94%, respectively.20 The
limitation of that study was the absence of a parallel
control group. However, the observed excellent
survival rates lessen the theoretical risk of hormonal
manipulation in oestrogen-sensitive cancers. Other
studies have also shown reduced onset of POF
and no significant disruption of cyclical ovarian
function.21 22
Subsequent trials such as PROMISE-GIM6
have built upon the work by Del Mastro et al.23 In
the PROMISE-GIM6 trial, GnRH-a triptorelin
3.75 mg was administered intramuscularly at least
1 week prior to chemotherapy and subsequently
every 4 weeks for the duration of chemotherapy.
Patients were premenopausal women with stage I
to III breast cancer who were offered adjuvant or
neoadjuvant chemotherapy.23 The early menopause
rate was 25.9% in the control group and 8.9% in
the triptorelin group: an absolute difference of -17% (95% confidence interval [95% CI]=-26%
to -7.9%; P<0.001) was observed.23 Another trial
showed that in premenopausal women with either
hormone-receptor-positive or hormone-receptor-negative
breast cancer, concurrent administration
of triptorelin and chemotherapy was associated
with a higher long-term probability of recovery of
ovarian function compared with chemotherapy
alone, without a statistically significant difference
in pregnancy rate.24 Another recent prospective,
randomised, parallel group study using GnRH-a
goserelin administered prior and throughout
chemotherapy for patients with stage I-IIIB breast
cancer showed a reduction in the rate of POF
for women aged <40 years.7 Goserelin reduced
the prevalence of amenorrhoea between 12 and
24 months from 38% in the control group to 22% in
the treated group. The prevalence of POF was also
reduced to 18.5% from 34.8% in the control group.7 Finally, a meta-analysis of randomised control trials
involving GnRH-a during chemotherapy in patients
with premenopausal breast cancer showed a reduced
rate of POF and increased pregnancy rate without
negative prognostic consequences.24 The meta-analysis
included 12 trials involving 1231 breast
cancer patients, and the data showed that GnRH-a
was associated with a significantly reduced risk of
POF (odds ratio=0.36, 95% CI=0.23-0.57; P<0.001).25
Among the five trials that reported pregnancies,
more patients treated with GnRH-a achieved
pregnancy (33 vs 19 women; odds ratio=1.83, 95%
CI=1.02-3.28; P=0.041). In the three studies that
reported disease-free survival, no between-group
difference was observed (hazard ratio=1.00, 95%
CI=0.49-2.04; P=0.939).25 These results suggest that
GnRH-a provides improved fertility preservation
for female patients with breast cancer without
affecting cancer progression and survival rates.24
However, data regarding live births (which are the
primary goal of fertility preservation) following
GnRH-a administration have been relatively scant.
Currently, it seems that administration of GnRH-a to
patients with breast cancer is a potentially beneficial
fertility preservation strategy. The ease of GnRH-a
co-administration also has the potential to benefit
patients outside tertiary and university medical
centres.
Embryo and oocyte cryopreservation
Embryo cryopreservation is the most established
fertility preservation technique and has entered
routine clinical practice. Following oocyte
harvesting, oocytes can be fertilised in vitro by donor
or partner sperm and the embryos cryopreserved.
The benefit of this technique is that embryos tend
to survive cryopreservation better than oocytes. The
improvements in vitrification technology have led to
an even higher embryo survival rate.
An alternative to embryo cryopreservation
is mature oocyte cryopreservation. After embryo
cryopreservation, this is the second-most established
technique, and it has entered clinical practice.
An advantage to this technique over embryo
cryopreservation is that it does not require sperm
from a donor or partner, which is more suitable for
single women.
Vitrification has enabled mature oocyte
cryopreservation by improving oocyte survival rates
and clinical outcomes.26 A prospective, randomised
study conducted with healthy young oocyte donors
showed that a 97% survival rate was obtained through
this technology.26 Traditional cryopreservation
exposes cells to low temperatures for prolonged
periods causing, cytoplasmic ice crystal formation,
which compromises cell survival upon thawing.27
Vitrification is solidification that occurs without
ice crystallisation but through extreme elevation
in viscosity. This phenomenon is achieved using
high cooling rates from -15 000 to -30 000°C/min,
minimising ice crystal formation. Vitrification
has been optimised to reduce cryoprotectant
concentration and subsequent cytotoxicity.28
A study on mature oocyte cryopreservation via
vitrification for non-oncological patients yielded 693
oocytes, of which 666 (96.1%) survived.26 A total of 487 (73.1%) were then successfully fertilised, leading to 117 embryos transferred to 57 recipients. The
pregnancy rate and implantation rate per transfer
were 63.2% and 38.5%, respectively, resulting in 28
healthy babies born.26 An overview of the work of
several research groups shows that oocyte survival
rates using this method have ranged between 91% and
99%, fertilisation rates were between 87% and 91%,
and pregnancy rates were between 33% and 57%.29 30 31
Subsequent work was done in cancer patients: 357
cancer patients had their oocytes cryopreserved,
and 11 patients returned post-treatment for in
vitro fertilisation (IVF).32 The oocyte survival rate
was 92.3%, the fertilisation rate was 76.6%, and
average number of embryos transferred was 1.8 ±
0.7. Four live births at term were achieved with no
malformations.32 Table 2 summarises some outcomes
of the recent studies on oocyte cryopreservation for
fertility preservation in cancer.32 33
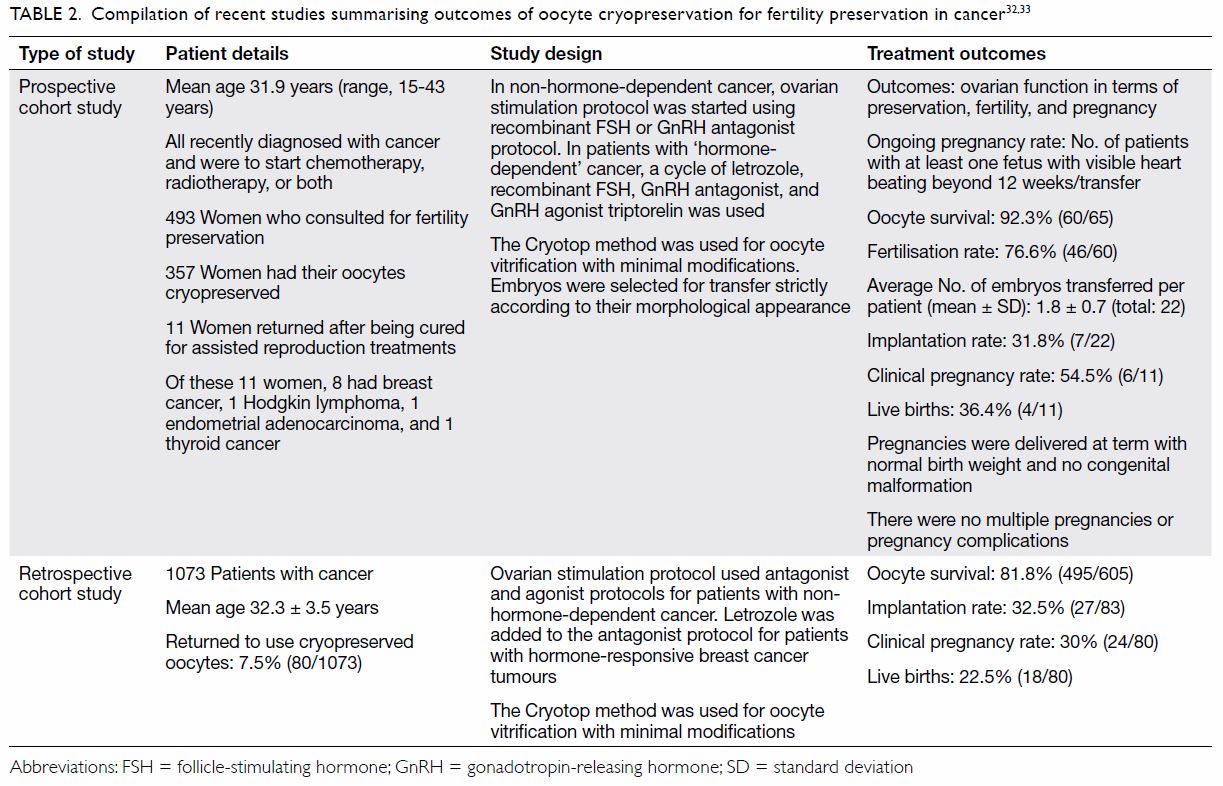
Table 2. Compilation of recent studies summarising outcomes of oocyte cryopreservation for fertility preservation in cancer32 33
Immature oocyte cryopreservation is
another extension of oocyte cryopreservation
technology. This technique is far less established,
but it enables immature eggs to be removed before
chemotherapy and have the eggs matured in vitro.
This technique is suitable for single female patients
who have oncological emergencies and cannot delay
chemotherapy for ovarian stimulation. The benefit of immature oocytes over mature oocytes is their
survivability: immature oocytes are relatively cryoresistant
because of their smaller size and lack of
meiotic spindle. The greatest challenge for clinical
translation of immature oocyte cryopreservation
technology is the difficulties encountered during in
vitro maturation of immature oocytes. Currently,
only a single live birth has been reported following
the slow freezing of immature oocytes.34
Protocols of ovarian stimulation
Embryonic or oocyte cryopreservation is a proven
approach in fertility preservation, especially in
chemotherapy.35 For both types of cryopreservation,
a period of 8 to 12 days is needed for ovarian
stimulation and subsequent oocyte harvesting.
Traditionally but now uncommonly performed, the
ovarian stimulation protocol begins with GnRH-a
administration during the preceding cycle’s luteal
phase to promote ovarian quiescence followed by
daily gonadotropin injections. Serial measurements
of oestradiol levels and follicular diameter are
taken for monitoring. When there are more than
three dominant follicles present, human chorionic
gonadotropin is administered to trigger ovulation
and oocytes collected.36 This protocol has many
disadvantages, chiefly delay of the commencement of
chemotherapy and high oestrogen exposure (which
leads to increased risk of breast cancer progression
and recurrence, particularly in hormone-sensitive
breast cancer). Therefore, various new stimulation
protocols have developed to enable either a shorter
stimulation period or a lower oestrogen level during
stimulation.
Random start protocols have been proposed to
minimise the time for oocyte collection by decreasing
total duration of the IVF cycle and have been shown
to be equally effective as conventional start protocols
in terms of the total number of mature oocytes
retrieved, oocyte maturity rate, and fertilisation
rate.37 38 39 Other novel stimulation protocols with
tamoxifen or aromatase inhibitors have been
developed to increase the safety margin of ovarian
stimulation in patients with oestrogen-sensitive
tumours. Tamoxifen is a selective oestrogen receptor
modulator. In addition to its anti-oestrogenic action
in breast tissue, it acts as an antagonist in the central
nervous system and interferes with the negative
feedback exerted by oestrogen on the hypothalamic/pituitary axis, leading to an increase in GnRH secretion from the hypothalamus. A few studies
have explored tamoxifen use for ovarian stimulation
in patients with breast cancer.40 41 42 A higher number
of mature oocytes and subsequent embryos were
obtained from the tamoxifen group compared with
natural cycle, with at least one embryo generated
per tamoxifen patient. Two patients conceived,
one miscarried at 8 weeks of pregnancy, but her risk of spontaneous abortion was high at age 42 years. The other patient delivered a healthy set of
twins.40 In a more recent study, co-administration
of tamoxifen with ovarian stimulation for fertility
preservation did not interfere with IVF results.
Comparisons were made between those who did
and did not receive concurrent tamoxifen. The mean
percentages of oocytes retrieved were 12.65% and
10.2%, respectively, and the numbers of embryos
cryopreserved were 8.5 and 6.4, respectively.42
Patients co-treated with tamoxifen had marginally
higher oestradiol levels, but the difference was not
statistically significant. However, co-treatment with
tamoxifen was considered to be safe, as the long-term
recurrence risk at up to 10 years was not increased.42
Aromatase inhibitors (eg, letrozole)
significantly suppress plasma oestrogen levels
by competitively inhibiting aromatase enzyme
activity. Centrally, it releases the hypothalamic/pituitary axis from oestrogenic negative feedback,
increasing secretion of FSH by the pituitary gland
while increasing the FSH sensitivity of the ovarian
granulosa cells. Combined letrozole-FSH protocols
have resulted in oestradiol levels lower than those
seen in natural cycles alongside fertility outcomes
similar to standard IVF protocols.43 44 In a recent
trial, 131 women with stage ≤3 breast cancer
underwent ovarian stimulation with concurrent
daily letrozole 5 mg prior to cryopreserving embryos
and subsequent adjuvant chemotherapy. The overall
live birth rate per embryo transfer was similar to the
United States national mean among infertile women
of a similar age without cancer who underwent IVF–embryo transfer (45.0 vs 38.2; P=0.2).45 Another
trial highlighted the safety, feasibility, and utility of
two consecutive ovarian stimulation cycles with the
use of letrozole-gonadotropin protocol for fertility
preservation in patients with breast cancer.46 The
mean total number of oocytes harvested (16.1 ± 13.2 vs 9.1 ± 5.2) and embryos generated (6.4 ± 2.9 vs 3.7 ± 3.1) were significantly higher in patients
who underwent two cycles compared with those
who underwent one cycle.46 There was no significant
delay in time interval from surgery to chemotherapy
between the two-cycle and single-cycle groups
(63.7 ± 7.7 vs 58.0 ± 12.1 days, respectively). The
recurrence rate was similar between two-cycle (0 of
17) and single-cycle (2 of 49) patients.46
Ovarian tissue cryopreservation and
transplantation
Cryopreservation and autotransplantation of
ovarian tissue are emerging technologies, and
women considering such treatments should do so
judiciously under specialised expertise in the setting
of clinical trials. These techniques rely upon slow
freezing technology used to cryopreserve oocytes
and embryos, but it is more difficult to optimise the procedure because ovaries contain many different
cell types. Upon freezing and thawing, problems
occur with fertilisation of maturing oocytes.47
The best follicular survival rate is approximately
70% to 80%, with light microscopy revealing
normal follicles. However, electron microscopy
has detected ultrastructural changes.48 The benefits of autotransplantation of ovarian tissue
include restoration of endocrine and reproductive
function; however, greater clinical evaluation of
this technology is needed. Autotransplantation of
ovarian tissue can be orthotopic or heterotopic.
Orthotopic refers to transplantation of ovarian tissue
to the original ovary site, while heterotopic refers
to transplanting the ovarian tissue to a foreign site.
Currently, the most effective site for graft longevity
is still unknown. Orthotopic ovarian transplantation
allows for natural conception, while heterotopic
transplantation in accessible sites minimises
repeated invasive procedures and improves ease of
oocyte recovery.49
Oktay et al50 reported restoration of hormonal
function, follicular growth, and oocyte retrieval
after heterotopic transplantation in a patient with
breast cancer. They retrieved 20 oocytes from
subcutaneously implanted ovarian tissue 6 years
after chemotherapy resulting in one fertilisation, but
no pregnancy ensued.50 There have since been case
reports of spontaneous pregnancy and live births
after autotransplantation of cryopreserved human
ovarian tissue in patients with cancer.51 52 An early
case report described a patient with triple negative
medullary breast cancer undergoing ovarian tissue
cryopreservation. Following cancer treatment, she
underwent an ovarian tissue transplant, and menses
occurred 63 days after transplantation. Sixteen mature oocytes were harvested following four
sessions of ovarian stimulation. All vitrified oocytes
survived thawing, and 77.7% were fertilised. Two day
3 embryos were implanted, and two healthy boys
were born at 34 weeks.53
As of 2017, there have been an estimated 86
successful births and nine ongoing pregnancies
using cryopreserved ovarian tissue internationally.54
The majority of the patient population that has
undergone this procedure has a cancer diagnosis, of
which many are haematological malignancies, which
require urgent chemotherapy. Furthermore, of the
singleton pregnancies, the mean gestational age was
39 weeks, and the mean birth weight was 3168 g,
which are within normal standards.54 These early results suggest that ovarian tissue preservation and
subsequent transplantation might become a suitable
fertility preservation therapy in premenopausal
women. A summary of a large case series of live
births from ovarian tissue transplantation is shown
in Table 3. It focuses on the perinatal outcomes of
40 live births from 32 women.54 The reference also briefly compiles the 86 live births and nine ongoing
pregnancies after transplantation of frozen-thawed
ovarian cortex.54

Table 3. Summary of ovarian tissue cryopreservation live births published in peer-reviewed papers 54
The advantage of ovarian cryopreservation and
transplantation is that it does not require an ovarian
stimulation protocol, which delays cancer treatment.
Additionally, this procedure is especially suitable
for prepubescent cancer patients, in whom ovarian
stimulation is contra-indicated. Moreover, ovarian
cryopreservation and transplantation not only
restores fertility but also restores gonadal/endocrine
function. Finally, because hundreds of immature
oocytes can be harvested at once, a huge ovarian
reserve can be preserved. The disadvantage of ovarian tissue cryopreservation and transplantation
is that it requires at least two surgical operations: one
for removal and another for future re-implantation.
Following implantation, there is the challenge of
minimising ischaemia, which could lead to follicle
loss or initiate maturation of the immature oocytes.
To minimise follicle loss post-transplant, the entire
ovarian cortex is often cryopreserved. Another
potential concern of autotransplantation is the risk
of cancer cell transmission, which has the highest
probability in cases of haematological cancers.
Shaw et al55 reported lymphoma in mice with fresh
or cryobanked grafted ovarian tissue from donor
mice with lymphoma. Clinically, however, ovarian
metastases are uncommon in cancers affecting young
people.56 Kim et al57 reported in a mouse model
that ovarian tissue from patients with high-grade
lymphoma appears safe for autotransplantation,
as none of the grafted mice tested positive for
lymphoma. Further research is required to assess
the optimal site for transplantation, improve
methods of detecting residual disease in harvested
tissue, ascertain the optimal size of ovarian grafts,
optimise freezing/thawing techniques, and promote
re-vascularisation of the transplanted tissue.
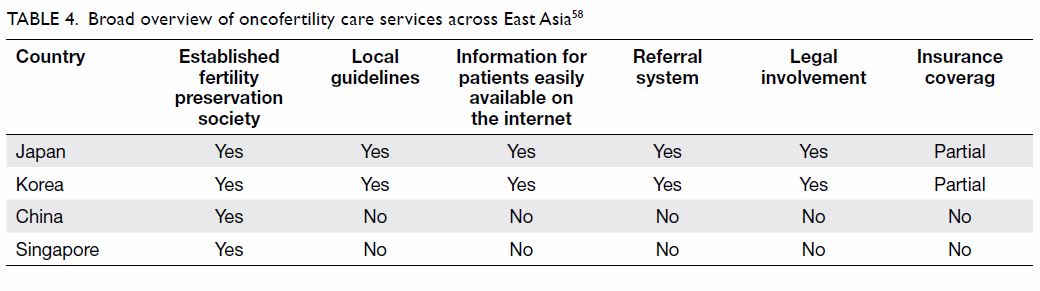
Oncofertility in East Asia
In the context of Asia, the Asian Society for Fertility
Preservation (ASFP) was established to promote
the science and clinical application of fertility
preservation techniques. The members of the ASFP
include China, Hong Kong, Taiwan, Singapore,
Korea, Japan, Vietnam, India, Thailand, Indonesia,
the Philippines, and Pakistan. This review will focus
broadly on fertility preservation care services in East
Asia (ie, China, Singapore, Korea, and Japan), as seen
in Table 4.
In Japan, oocyte, embryo, and ovarian tissue
cryopreservation are available. However, despite
the availability of the technology, uptake of fertility
preservation techniques has been limited, as the
majority of cancer hospitals do not provide fertility
preservation services.58 This problem is further
compounded by the lack of robust coordinated care
networks to assist in delivering oncofertility care.58 To overcome this problem, the Japanese have been
aggressively experimenting with various referral
service models: (1) a reproductive care centre-led
model wherein reproductive care centres reach out
to cancer centres, and (2) a cancer centre model
in which the cancer centre serves as the basis of
the referral network.59 To facilitate the running of
the referral network, the Japanese have focused on
harnessing allied health to drive a psychosocial-based
care delivery system.59 They aim to train oncofertility navigators that are able to provide psychosocial care
whilst also guiding patients on the technical aspects
of their fertility preservation journey.59
The efforts of the Korean Society for Fertility
Preservation (KSFP) have produced a well-established
referral network that even covers
regional healthcare centres, rendering high-quality
fertility preservation treatments accessible at various
institutions.58 60 Fertility preservation treatments have
a multidisciplinary focus incorporating physicians,
nurses, mental health professionals, office staff,
and laboratory personnel.58 60 To facilitate patient
communication regarding fertility preservation
under the time pressure of cancer treatment, print
material and web resources are distributed to patients
during the fertility preservation consultation.58 60
However, despite this concerted effort, uptake of
fertility preservation treatments has been limited.60 The main issue raised by the KSFP was access to
care: the oncologists noted that there was a lack
of discussion of fertility preservation options and
referrals to fertility specialists.60 This may be for several reasons: exclusive focus on cancer treatment
and its perceived urgency, the perception of limited
options for fertility preservation, the perception that
fertility is unimportant to patients, and not knowing
the referral pathway for patients interested in fertility
preservation.60
Like many East Asian countries, China has
the technology to perform fertility preservation
techniques. However, similarly, the limiting factor for
uptake of these technologies is barriers to access. Like
in Korea, there is a lack of knowledge and awareness
of fertility preservation techniques among healthcare
professionals: among obstetrics and gynaecology
specialists, despite knowing about cancer therapy being gonadotoxic, about 20% of them were not
familiar with fertility preservation techniques.61 Only
50% of obstetrics and gynaecology specialists who
were familiar with fertility preservation techniques
had been consulted by oncologists about managing
a patient’s infertility risk.61 Despite this, 96.6% of obstetrics and gynaecology specialists reported
being keen on collaborating with oncologists to
preserve the fertility of female patients with cancer.61
This suggests that despite a willingness to collaborate
on fertility preservation, there has been limited
communication between oncologists and obstetrics
and gynaecology specialists.
Finally, Singapore, being a small city-state,
has limited resources for fertility preservation care
delivery services: in fact, it does not have a fertility
preservation society.58 All fertility preservation
services are concentrated in one large tertiary hospital
in Singapore.58 Reproductive preservation techniques
exist in Singapore; however, access to them is
again limited by both the patient’s and oncologist’s
knowledge and awareness of the technology.58
Discussion
Fertility preservation is a major concern for
premenopausal patients. Hence, at the outset
of chemotherapy, its gonadotoxic effects must
be discussed by a multidisciplinary team. When
discussing fertility preservation options, their
nature, success rate, risk, cost, and potential
ethical implications should be discussed with
the patient.62 Additionally, it is important to set realistic expectations about the fertility preservation treatment with the patient: factors such as patient
age, ovarian reserve, presence of a partner, presence
of previous live births, financial status, and religious
background should be considered.62 Finally, medical
factors that may influence the feasibility of fertility
preservation, such as severity of the gonadotoxic
chemotherapy, time available before commencing
chemotherapy, and available expertise and facilities,
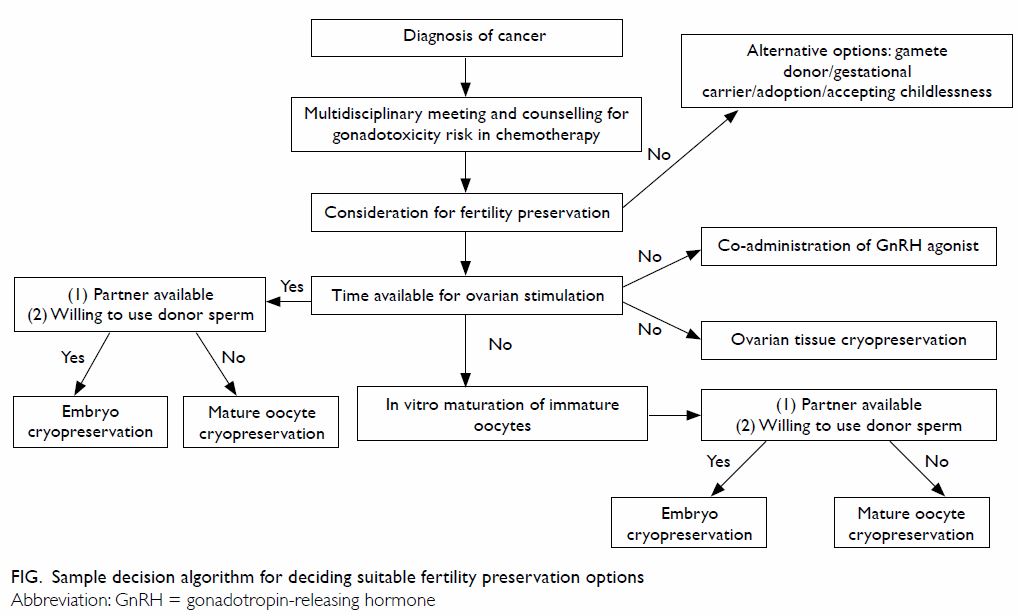
should also be explained to the patient. A sample
decision algorithm for deciding about the suitability
of fertility preservation options in both emerging
and routine clinical practice is shown in the Figure.
Another common patient concern is cancer
recurrence or disease progression due to future
pregnancy. This fear may cause patients to delay
or abandon future pregnancies. A meta-analysis
showed that women who become pregnant following
breast cancer treatment have an improved prognosis,
reflected by significantly increased overall survival
compared with those who did not become pregnant
(pooled hazard ratio=0.63, 95% CI=0.51-0.79).63
The meta-analysis also revealed a non-significant
increase in disease-free survival for pregnant
women.63 The meta-analysis findings, however,
may be due to a selection bias termed the “healthy
mother” effect, where healthier women are more
likely to conceive than those who have relapsed hence
skewing the true effect.64 Nonetheless a subsequent multicentre case-control study has supported the
conclusion of the meta-analysis regarding pregnancy
safety following breast cancer treatment.65 At 7.2
years after pregnancy, no difference in disease-free
survival was observed between pregnant and
nonpregnant patients.65 Although there was no difference in overall survival for oestrogen receptor–positive pregnant patients, there was an increased
overall survival for oestrogen receptor–negative
patients.65 Therefore, pregnancy is being considered
safe following previous breast cancer diagnosis and
maybe associated with an improved prognosis for
oestrogen receptor–negative patients.
All of the patient’s fears and concerns
regarding fertility preservation should be discussed
with a dedicated oncofertility unit. This allows for
informed discussion between patient and healthcare
practitioner, which can help to allay patients’ fears.
Such communication can be facilitated via printouts,
brochures, and decision aids, which can help to
avoid miscommunication and allow patients to be
fully informed of their potential choices in fertility
preservation. Research has shown that a dedicated
oncofertility unit can improve the frequency and
thoroughness of fertility preservation discussions.66 This research is particularly relevant, as oncofertility
units allow focus on young cancer patients, including
those with breast cancer, who will benefit the most
from fertility preservation options.
Conclusions
Fertility preservation is still a major issue for
premenopausal patients with breast cancer. Several
treatment modalities can now be considered and
combined.67 A potential fertility preservation
protocol for premenopausal patients with breast
cancer could involve an initial oocyte and ovarian
tissue harvest and subsequent cryopreservation of
oocytes, embryos, and ovarian tissue. Gonadotropin-releasing
hormone agonist can be co-administrated
alongside chemotherapy to minimise POF.
This review aimed to summarise and evaluate
the current clinical status of fertility preservation
techniques available to premenopausal patients with
breast cancer, thereby raising awareness of fertility
preservation techniques among oncologists, fertility
specialists, surgeons, nurses, and psychologists
who care for premenopausal patients with breast
cancer. Hopefully, a multidisciplinary and holistic
approach to fertility preservation treatments for
premenopausal patients with breast cancer will be
possible in East Asia.
Author contributions
Concept or design: SSY Wang.
Acquisition of data: SSY Wang.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: SSY Wang.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
As editors of the journal, H Loong and JPW Chung were not involved in the peer review process. Other authors have no
conflicts of interest to declare.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Trivers KF, Fink AK, Partridge AH, et al. Estimates of
young breast cancer survivors at risk for infertility in the
U.S. Oncologist 2014;19:814-22. Crossref
2. Kasum M, Beketić-Orešković L, Peddi PF, Orešković S,
Johnson RH. Fertility after breast cancer treatment. Eur J
Obstet Gynecol Reprod Biol 2014;173:13-8. Crossref
3. Levine J. Gonadotoxicity of cancer therapies in pediatric
and reproductive-age females. In: Gracia C, Woodruff
TK, editors. Oncofertility Medical Practice. New York:
Springer; 2012: 3-14. Crossref
4. Yuksel A, Bildik G, Senbabaoglu F, et al. The magnitude of
gonadotoxicity of chemotherapy drugs on ovarian follicles
and granulosa cells varies depending upon the category of
the drugs and the type of granulosa cells. Human Reprod
2015;30:2926-35. Crossref
5. Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of
fertility concerns on tamoxifen initiation and persistence.
J Natl Cancer Inst 2015;107(10):djv202. Crossref
6. Howard-Anderson J, Ganz PA, Bower JE, Stanton AL.
Quality of life, fertility concerns, and behavioral health
outcomes in younger breast cancer survivors: a systematic
review. J Natl Cancer Inst 2012;104:386-405. Crossref
7. Paluch-Shimon S, Pagani O, Partridge AH, et al. ESO-ESMO
3rd international consensus guidelines for breast
cancer in young women (BCY3). Breast 2017;35:203-17. Crossref
8. Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study
of fertility concerns and preservation strategies in young
women with breast cancer. J Clin Oncol 2014;32:1151-6. Crossref
9. Partridge AH, Gelber S, Peppercorn J, et al. Web-based
survey of fertility issues in young women with breast
cancer. J Clin Oncol 2004;22:4174-83. Crossref
10. Biglia N, Torrisi R, D’Alonzo M, Codacci Pisanelli G, Rota
S, Peccatori FA. Attitudes on fertility issues in breast
cancer patients: an Italian survey. Gynecol Endocrinol
2015;31:458-64. Crossref
11. Chung JP, Lao T, Li TC. Evaluation of the awareness of,
attitude to, and knowledge about fertility preservation in
cancer patients among clinical practitioners in Hong Kong.
Hong Kong Med J 2017;23:556-61. Crossref
12. Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR,
Tucker MJ. Successful elective and medically indicated
oocyte vitrification and warming for autologous in vitro
fertilization, with predicted birth probabilities for fertility
preservation according to number of cryopreserved
oocytes and age at retrieval. Fertil Steril 2016;105:459-66.
e2. Crossref
13. Glode LM, Robinson J, Gould SF. Protection from
cyclophosphamide-induced testicular damage with an
analogue of gonadotropin-releasing hormone. Lancet
1981;317:1132-4. Crossref
14. Bohlmann MK, von Wolff M, Strowitzki T. Comment
on the symposium article “Fertility after treatment for Hodgkin’s disease”, by Z. Blumenfeld, E. Dann, I. Avivi
et al. (Ann Oncol 2002;13 Suppl 1:138-147). Ann Oncol
2003;14:499. Crossref
15. Oktay K, Sönmezer M, Öktem Ö, Fox K, Emons G, Bang H.
Absence of conclusive evidence for the safety and efficacy
of gonadotropin-releasing hormone analogue treatment in
protecting against chemotherapy-induced gonadal injury.
Oncologist 2007;12:1055-66. Crossref
16. Blumenfeld Z. How to preserve fertility in young women
exposed to chemotherapy? The role of GnRH agonist
cotreatment in addition to cryopreservation of embrya,
oocytes, or ovaries. Oncologist 2007;12:1044-54. Crossref
17. Lobo RA. Potential options for preservation of fertility in
women. N Engl J Med 2005;353:64-73. Crossref
18. Blumenfeld Z, von Wolff M. GnRH-analogues and oral
contraceptives for fertility preservation in women during
chemotherapy. Hum Reprod Update 2008;14:543-52. Crossref
19. Lambertini M, Del Mastro L, Pescio MC, et al. Cancer
and fertility preservation: international recommendations
from an expert meeting. BMC Med 2016;14:1. Crossref
20. Recchia F, Sica G, De Filippis S, Saggio G, Rosselli M, Rea S.
Goserelin as ovarian protection in the adjuvant treatment
of premenopausal breast cancer: a phase II pilot study.
Anticancer Drugs 2002;13:417-24. Crossref
21. Huser M, Crha I, Ventruba P, et al. Prevention of ovarian
function damage by a GnRH analogue during chemotherapy
in Hodgkin lymphoma patients. Hum Reprod 2008;23:863-8. Crossref
22. Blumenfeld Z, Avivi I, Eckman A, Epelbaum R, Rowe
JM, Dann EJ. Gonadotropin-releasing hormone agonist
decreases chemotherapy-induced gonadotoxicity and
premature ovarian failure in young female patients with
Hodgkin lymphoma. Fertil Steril 2008;89:166-73. Crossref
23. Del Mastro L, Boni L, Michelotti A, et al. Effect of the
gonadotropin-releasing hormone analogue triptorelin
on the occurrence of chemotherapy-induced early
menopause in premenopausal women with breast cancer:
A randomized trial. JAMA 2011;306:269-76. Crossref
24. Lambertini M, Boni L, Michelotti A, et al. Ovarian
suppression with triptorelin during adjuvant breast
cancer chemotherapy and long-term ovarian function,
pregnancies, and disease-free survival: A randomized
clinical trial. JAMA 2015;314:2632-40. Crossref
25. Lambertini M, Ceppi M, Poggio F, et al. Ovarian
suppression using luteinizing hormone-releasing hormone
agonists during chemotherapy to preserve ovarian function
and fertility of breast cancer patients: a meta-analysis of
randomized studies. Ann Oncol 2015;26:2408-19. Crossref
26. Cobo A, Domingo J, Pérez S, Crespo J, Remohí J, Pellicer A.
Vitrification: an effective new approach to oocyte banking
and preserving fertility in cancer patients. Clin Transl
Oncol 2008;10:268-73. Crossref
27. Edgar DH, Gook DA. A critical appraisal of cryopreservation
(slow cooling versus vitrification) of human oocytes and
embryos. Hum Reprod Update 2012;18:536-54. Crossref
28. Kuwayama M. Highly efficient vitrification for
cryopreservation of human oocytes and embryos: The
Cryotop method. Theriogenology 2007;67:73-80. Crossref
29. Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient
vitrification method for cryopreservation of human
oocytes. Reprod Biomed Online 2005;11:300-8. Crossref
30. Lucena E, Bernal DP, Lucena C, Rojas A, Moran A, Lucena
A. Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril 2006;85:108-11. Crossref
31. Antinori M, Licata E, Dani G, Cerusico F, Versaci C,
Antinori S. Cryotop vitrification of human oocytes results
in high survival rate and healthy deliveries. Reprod Biomed
Online 2007;14:72-9. Crossref
32. Martinez M, Rabadan S, Domingo J, Cobo A, Pellicer
A, Garcia-Velasco JA. Obstetric outcome after oocyte
vitrification and warming for fertility preservation in
women with cancer. Reprod Biomed Online 2014;29:722-8. Crossref
33. Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí
J. Elective and onco-fertility preservation: factors related to
IVF outcomes. Hum Reprod 2018;33:2222-31. Crossref
34. Tucker MJ, Wright G, Morton PC, Massey JB. Birth after
cryopreservation of immature oocytes with subsequent in
vitro maturation. Fertil Steril 1998;70:578-9. Crossref
35. Kim SS. Fertility preservation in female cancer patients:
current developments and future directions. Fertil Steril
2006;85:1-11. Crossref
36. Hacker NF, Gambone JC, Hobel CJ. Hacker & Moore’s
essentials of obstetrics and gynecology. 6th ed. Philadelphia:
Elsevier Health Sciences; 2015.
37. Cakmak H, Rosen MP. Random-start ovarian stimulation
in patients with cancer. Curr Opin Obstet Gynecol
2015;27:215-21. Crossref
38. Cakmak H, Katz A, Cedars MI, Rosen MP. Effective
method for emergency fertility preservation: random-start
controlled ovarian stimulation. Fertil Steril 2013;100:1673-
80. Crossref
39. Kuang Y, Hong Q, Chen Q, et al. Luteal-phase ovarian
stimulation is feasible for producing competent oocytes in
women undergoing in vitro fertilization/intracytoplasmic
sperm injection treatment, with optimal pregnancy
outcomes in frozen-thawed embryo transfer cycles. Fertil
Steril 2014;101:105-11. Crossref
40. Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L,
Rosenwaks Z. Fertility preservation in breast cancer
patients: IVF and embryo cryopreservation after ovarian
stimulation with tamoxifen. Hum Reprod 2003;18:90-5. Crossref
41. Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks
Z. Fertility preservation in breast cancer patients: a
prospective controlled comparison of ovarian stimulation
with tamoxifen and letrozole for embryo cryopreservation.
J Clin Oncol 2005;23:4347-53. Crossref
42. Meirow D, Raanani H, Maman E, et al. Tamoxifen co-administration
during controlled ovarian hyperstimulation
for in vitro fertilization in breast cancer patients increases
the safety of fertility-preservation treatment strategies.
Fertil Steril 2014;102:488-95.e3. Crossref
43. Oktay K, Hourvitz A, Sahin G, et al. Letrozole reduces
estrogen and gonadotropin exposure in women with
breast cancer undergoing ovarian stimulation before
chemotherapy. J Clin Endocrinol Metab 2006;91:3885-90. Crossref
44. Azim AA, Costantini-Ferrando M, Oktay K. Safety of
fertility preservation by ovarian stimulation with letrozole
and gonadotropins in patients with breast cancer: a
prospective controlled study. J Clin Oncol 2008;26:2630-5. Crossref
45. Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility
preservation success subsequent to concurrent aromatase
inhibitor treatment and ovarian stimulation in women
with breast cancer. J Clin Oncol 2015;33:2424-9. Crossref
46. Turan V, Bedoschi G, Moy F, Oktay K. Safety and feasibility
of performing two consecutive ovarian stimulation cycles
with the use of letrozole-gonadotropin protocol for fertility preservation in breast cancer patients. Fertil Steril 2013;100:1681-5.e1.Crossref
47. Oktay K, Karlikaya G. Ovarian function after
transplantation of frozen, banked autologous ovarian
tissue. N Engl J Med 2000;342:1919. Crossref
48. Kim SS, Battaglia DE, Soules MR. The future of human ovarian cryopreservation and transplantation: fertility and
beyond. Fertil Steril 2001;75:1049-56. Crossref
49. Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt
A, Demylle D, Dolmans MM. Ovarian tissue
cryopreservation and transplantation: a review. Hum
Reprod Update 2006;12:519-35. Crossref
50. Oktay K, Buyuk E, Veeck L, et al. Embryo development
after heterotopic transplantation of cryopreserved ovarian
tissue. Lancet 2004;363:837-40. Crossref
51. Demeestere I, Simon P, Emiliani S, Delbaere A, Englert
Y. Fertility preservation: successful transplantation of
cryopreserved ovarian tissue in a young patient previously
treated for Hodgkin’s disease. Oncologist 2007;12:1437-42. Crossref
52. Donnez J, Dolmans MM, Demylle D, et al. Livebirth after
orthotopic transplantation of cryopreserved ovarian tissue.
Lancet 2004;364:1405-10. Crossref
53. Sánchez-Serrano M, Crespo J, Mirabet V, et al. Twins born
after transplantation of ovarian cortical tissue and oocyte
vitrification. Fertil Steril 2010;93:268.e11-3. Crossref
54. Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan
P, Andersen CY. 86 successful births and 9 ongoing
pregnancies worldwide in women transplanted with
frozen-thawed ovarian tissue: focus on birth and perinatal
outcome in 40 of these children. J Assist Reprod Genet
2017;34:325-36. Crossref
55. Shaw J, Bowles J, Koopman P, Wood E, Trounson A. Fresh
and cryopreserved ovarian tissue samples from donors
with lymphoma transmit the cancer to graft recipients.
Hum Reprod 1996;11:1668-73. Crossref
56. Lee SJ, Bae JH, Lee AW, Tong SY, Park YG, Park JS. Clinical
characteristics of metastatic tumors to the ovaries. J
Korean Med Sci 2009;24:114-9. Crossref
57. Kim SS, Radford J, Harris M, et al. Ovarian tissue harvested
from lymphoma patients to preserve fertility may be safe
for autotransplantation. Hum Reprod 2001;16:2056-60. Crossref
58. Harzif AK, Santawi VPA, Maidarti M, Wiweko B.
Investigation of each society for fertility preservation in
Asia. Front Endocrinol (Lausanne) 2019;10:151. Crossref
59. Takai Y. Recent advances in oncofertility care worldwide
and in Japan. Reprod Med Biol 2018;17:356-68. Crossref
60. Kim J, Kim SK, Hwang KJ, Kim SH. Fertility preservation
during cancer treatment: The Korean Society for Fertility
Preservation clinical guidelines. Clin Exp Reprod Med
2017;44:171-4. Crossref
61. Ju K, Kopp M, Wang Y, et al. A survey study of attitude
and knowledge regarding female fertility preservation
among reproductive health professionals in Fujian, China.
J Adolesc Young Adult Oncol 2018;8:67-73. Crossref
62. Tomasi-Cont N, Lambertini M, Hulsbosch S, Peccatori AF,
Amant F. Strategies for fertility preservation in young early
breast cancer patients. Breast 2014;23:503-10. Crossref
63. Hartman EK, Eslick GD. The prognosis of women diagnosed
with breast cancer before, during and after pregnancy: a
meta-analysis. Breast Cancer Res Treat 2016;160:347-60. Crossref
64. Sankila R, Heinävaara S, Hakulinen T. Survival of breast
cancer patients after subsequent term pregnancy: “healthy
mother effect”. Am J Obstet Gynecol 1994;170:818-23. Crossref
65. Lambertini M, Kroman N, Ameye L, et al. Long-term
safety of pregnancy following breast cancer according to
estrogen receptor status. J Natl Cancer Inst 2018;110:426-
9. Crossref
66. Lewin J, Ma JMZ, Mitchell L, et al. The positive effect of
a dedicated adolescent and young adult fertility program
on the rates of documentation of therapy-associated
infertility risk and fertility preservation options. Support
Care Cancer 2017;25:1915-22. Crossref
67. Blumenfeld Z, Katz G, Evron A. ‘An ounce of prevention
is worth a pound of cure’: the case for and against GnRHagonist
for fertility preservation. Ann Oncol 2014;25:1719-
28. Crossref