Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
EDITORIAL
Kidney health for everyone everywhere—from prevention to detection and equitable
access to care
Philip KT Li, MD, FRCP1 #; Guillermo Garcia-Garcia, MD2 #; SF Lui, MB, ChB, FRCP3 #; Sharon Andreoli, MD4 #; Winston WS Fung, MB BChir, MRCP1; Anne Hradsky, MA5; Latha Kumaraswami, BA6 #; Vassilios Liakopoulos, MD, PhD7 #; Ziyoda Rakhimova, BSc5; Gamal Saadi, MD8 #; Luisa Strani, ISN5 #; Ifeoma Ulasi, MB, BS, FWACP9 #; Kamyar Kalantar-Zadeh, MD, PhD10 #; for the World Kidney Day Steering Committee
1 Department of Medicine and Therapeutics, Carol and Richard Yu Peritoneal Dialysis Research Centre, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
2 Nephrology Service, Hospital Civil de Guadalajara Fray Antonio Alcalde, University of Guadalajara Health Sciences Center, Guadalajara, Mexico
3 Division of Health System, Policy and Management, Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
4 James Whitcomb Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, United States
5 World Kidney Day Office, Brussels, Belgium
6 TANKER Foundation, Chennai, India
7 Division of Nephrology and Hypertension, First Department of Internal Medicine, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
8 Nephrology Unit, Department of Internal Medicine, Faculty of Medicine, Cairo University, Giza, Egypt
9 Renal Unit, Department of Medicine, College of Medicine, University of Nigeria, Ituku-Ozalla, Enugu, Nigeria
10 Division of Nephrology and Hypertension and Kidney Transplantation, University of California Irvine School of Medicine, Orange, California, United States
# Members of the World Kidney Day Steering Committee
Corresponding authors: Dr Philip KT Li; Dr Kamyar Kalantar-Zadeh (philipli@cuhk.edu.hk; kkz@uci.edu)
Introduction
Around 850 million people are currently affected
by different types of kidney disorders.1 Up to one
in 10 adults worldwide has chronic kidney disease
(CKD), which is invariably irreversible and mostly
progressive. The global burden of CKD is increasing,
and CKD is projected to become the fifth most
common cause of years of life lost globally by 2040.2
If CKD remains uncontrolled and if the affected
person survives the ravages of cardiovascular and
other complications of the disease, CKD progresses
to end-stage renal disease (ESRD), where life cannot
be sustained without dialysis therapy or kidney
transplantation. Hence, CKD is a major cause of
catastrophic health expenditure.3 The costs of
dialysis and transplantation consume 2% to 3% of the
annual healthcare budget in high-income countries,
and spent on <0.03% of the total population of these
countries.4
Importantly, however, kidney disease can be
prevented and progression to ESRD can be delayed
with appropriate access to basic diagnostics and
early treatment including lifestyle modifications and
nutritional interventions.4 5 6 7 8 Despite this, access to
effective and sustainable kidney care remains highly
inequitable across the world, and kidney disease a low health priority in many countries. Kidney disease is crucially missing from the international
agenda for global health. Notably absent from the
impact indicators for the Sustainable Development
Goal Goal 3. Target 3.4: “By 2030, reduce by one
third premature mortality from non-communicable
diseases (NCDs) through prevention and treatment
and promote mental health and well-being” and from
the latest iteration of the United Nations Political
Declaration on NCDs, kidney diseases urgently
need to be given political attention, priority, and
consideration.9 Current global political commitments
on NCDs focus largely on four main diseases:
cardiovascular disease (CVD), cancer, diabetes, and
chronic respiratory diseases. Yet, it is estimated
that 55% of the global NCD burden is attributed to
diseases outside of this group.10 Furthermore, kidney
disease frequently co-exists with one or more of the
above four major NCDs, leading to worse health
outcomes. Chronic kidney disease is a major risk
factor for heart disease and cardiac death, as well
as for infections such as tuberculosis, and is a major
complication of other preventable and treatable
conditions including diabetes, hypertension, human
immunodeficiency virus, and hepatitis.4 5 6 7 As the
Sustainable Development Goals and Universal Health Coverage agendas progress and provide a platform for raising awareness of NCD healthcare
and monitoring needs, targeted action on kidney
disease prevention should become integral to the
global policy response.1 The global kidney health
community calls for the recognition of kidney disease
and effective identification and management of its
risk factors as a key contributor to the global NCD
burden and the implementation of an integrated and
people-centred approach to care.
Definition and classification of
chronic kidney disease prevention
According to the expert definitions including the Centers for Disease Control and Prevention,11
the term “prevention” refers to activities that
are typically categorised by the following three
definitions: (1) primary prevention, which implies
intervening before health effects occur in an effort
to prevent the onset of illness or injury before the
disease process begins; (2) secondary prevention,
which suggests preventive measures that lead to
early diagnosis and prompt treatment of a disease
to prevent more severe problems developing and
includes screening to identify diseases in the earliest
stages; and (3) tertiary prevention, which indicates
managing disease after it is well established in order
to control disease progression and the emergence of
more severe complications, which is often by means of targeted measures such as pharmacotherapy, rehabilitation, and screening for and management of
complications. These definitions have an important
bearing on the prevention and management of the
CKD, and accurate identification of risk factors that
cause CKD or lead to faster progression to renal
failure as shown in the Figure are relevant in health
policy decisions and health education and awareness
related to CKD.12
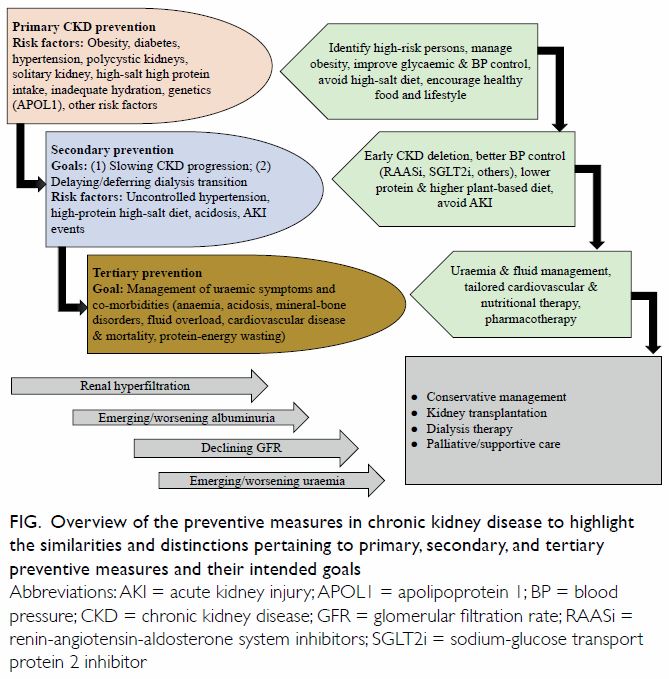
Figure. Overview of the preventive measures in chronic kidney disease to highlight the similarities and distinctions pertaining to primary, secondary, and tertiary preventive measures and their intended goals
Primary prevention of chronic kidney disease
The incidence and prevalence of CKD have been
rising worldwide.13 This primary level of prevention
requires awareness of modifiable CKD risk factors
and efforts to focus healthcare resources on those
patients who are at the highest risk of developing
new onset or de novo CKD.
Measures to achieve effective primary
prevention should focus on the two leading risk
factors for CKD including diabetes mellitus and
hypertension. Evidence suggests that an initial
mechanism of injury is renal hyperfiltration with
seemingly elevated glomerular filtration rate
(GFR), above normal ranges. This is often the
result of glomerular hypertension that is often
seen in patients with obesity or diabetes mellitus,
but it can also occur after high dietary protein
intake.8 Other CKD risk factors include polycystic
kidneys or other congenital or acquired structural
anomalies of the kidney and urinary tracts, primary
glomerulonephritis, exposure to nephrotoxic
substances or medications (such as nonsteroidal
anti-inflammatory drugs), having one single kidney,
eg, solitary kidney after cancer nephrectomy, high
dietary salt intake, inadequate hydration with
recurrent volume depletion, heat stress, exposure to
pesticides and heavy metals (as has been speculated
as the main cause of Mesoamerican nephropathy),
and possibly high protein intake in those at higher
risk of CKD.8 Among non-modifiable risk factors
are advancing age and genetic factors such as
apolipoprotein 1 gene that is mostly encountered in
those with sub-Saharan African ethnicity, especially
among African Americans. Certain disease states
may cause de novo CKD such as cardiovascular
and atheroembolic diseases (also known as
secondary cardiorenal syndrome) and liver diseases
(hepatorenal syndrome). Some of the risk factors of
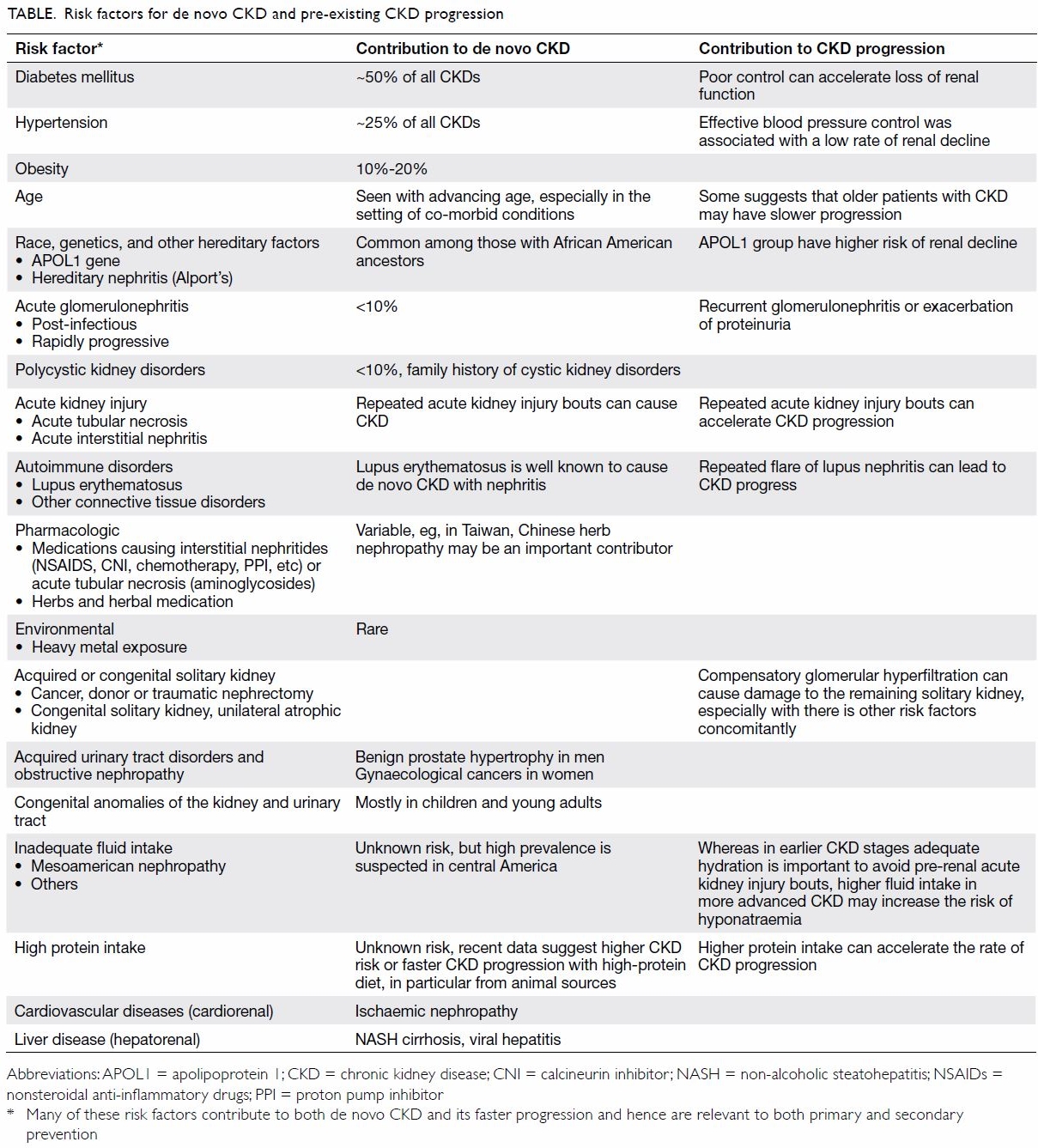
CKD are shown in the Table.
Among measures to prevent the emergence
of de novo CKD are screening efforts to identify
and treat patients at high risk of CKD, especially
those with diabetes mellitus and hypertension.
Hence, targeting primordial risk factors of these
two conditions including metabolic syndrome and
overnutrition is relevant to primary CKD prevention
as is correcting obesity.14 Promoting healthier
lifestyle is an important means to that end including physical activity and healthier diet. The latter should
be based on more plant-based foods with less meat,
less sodium intake, more complex carbohydrates
with higher fibre intake, and less saturated fat. In
those with hypertension and diabetes, optimising
blood pressure and glycaemic control has shown to be effective in preventing diabetic and hypertensive nephropathies. A recent expert panel suggested
that patients with solitary kidney should avoid high
protein intake >1 g/kg body weight per day.15 Obesity
should be avoided, and weight reduction strategies
should be considered.14
Secondary prevention in chronic kidney
disease
Evidence suggests that among patients with CKD,
the vast majority have an early stage of the disease, ie,
CKD stages 1 and 2 with microalbuminuria (30-300
mg/day) or CKD stage 3B (ie, estimated GFR between
45 and 60 mL/min/1.73 m2).16 In these patients with
pre-existing disease, the secondary prevention of
CKD has the highest priority. For these earlier stages
of CKD, the main goal of kidney health education
and clinical interventions is how to slow disease
progression. Uncontrolled or poorly controlled
hypertension is one of the most established risk
factors for faster CKD progression. The underlying
pathophysiology of faster CKD progression relates
to ongoing damage to the kidney structure and loss
of nephrons with worsening interstitial fibrosis as
it happens with sustained hypertension. A target
of blood pressure of <130/80 mmHg should be
recommended.
The cornerstone of the pharmacotherapy
in secondary prevention is the use of angiotensin
pathway modulators, also known as renin-angiotensin-
aldosterone system inhibitors. These
drugs reduce both systemic blood pressure and
intraglomerular pressure by opening efferent
arterioles of the glomeruli, hence, leading to
longevity of the remaining nephrons. Low-protein
diet appears to have a synergistic effect on renin-angiotensin-
aldosterone system inhibitor therapy.17
In terms of the potential effect of controlling
glycaemic status and correcting obesity on the
rate of CKD progression, there are mixed data.
However, recent data suggest that a new class
of antidiabetic medications known as sodium-glucose
cotransporter-2 inhibitors can slow CKD
progression, but this effect may not be related
to glycaemic modulation of the medication. The
CREDENCE study demonstrated that the risk of
renal failure is significantly lower in the canagliflozin
group than the placebo group.18 Another emerging
antidiabetic agent in delaying kidney injury is
the glucagon-like peptide-1 receptor agonist.19 A
glycated haemoglobin level of <7% should be the
goal. Whereas acute kidney injury (AKI) may or
may not cause de novo CKD, AKI events that are
superimposed on pre-existing CKD may accelerate
disease progression.20 In particular, judicious use of
nephrotoxic agents (such as iodine-based contrast,
chemotherapeutic agents and immunomodulatory
drugs) in patients with pre-existing CKD is
imperative in order to prevent new AKI. A relatively
recent case of successful secondary prevention
that highlights the significance of implementing
preventive strategies in CKD is the use of a
vasopressin V2-receptor antagonists in autosomal
dominant polycystic kidney disease.21
Tertiary prevention in chronic kidney disease
In patients with advanced CKD, management of
uraemia and related co-morbid conditions such as
anaemia, mineral and bone disorders, and CVD is
of high priority, so that these patients can continue
to achieve the highest longevity. These measures can
be collectively referred to as tertiary prevention of
CKD. In these patients, CVD burden is exceptionally
high, especially if they have underlying diabetes
or hypertension, while they often do not follow
other traditional profile of cardiovascular risk such
as obesity or hyperlipidaemia. Indeed, in these
patients, a so-called reverse epidemiology exists,
in which hyperlipidaemia and obesity appear to be
protective at this advanced stage of CKD. This could
be due to the overshadowing impact of the protein-energy
wasting that happens more frequently with
worsening uraemia and which is associated with
weight loss and poor outcomes including CVD and
death. Whereas many of these patients, if they survive
ravages of protein-energy wasting and CVD, will
eventually receive renal replacement therapy in form
of dialysis therapy or kidney transplantation, a new
trend is emerging to maintain them longer without
dialysis by implementing conservative management
of CKD. However, in some with additional co-morbidities
such as metastatic cancers, palliative
measures with supportive care can be considered.
Identification of chronic kidney
disease
The lack of awareness of CKD around the world
is one of the reasons for late presentation of CKD
in both developed and developing economies.22 23 24
The overall CKD awareness among the general
population and even high cardiovascular risk groups
across 12 low-income and middle-income countries
(LMIC) was <10%.24
Given its asymptomatic nature, screening
of CKD plays an important role in early detection.
Consensus and positional statements have
been published by the International Society of
Nephrology,25 National Kidney Foundation,26
Kidney Disease Improving Global Outcomes,27
NICE Guidelines,28 and Asian Forum for Chronic
Kidney Disease Initiatives.29 There was lack of trials
to evaluate screening and monitoring of CKD.30
Currently, most will promote a targeted screening
approach to early detection of CKD. Some of the
major groups at risk for targeted screening include
patients with diabetes; hypertension; patients with
a family history of CKD; patients taking potentially
nephrotoxic drugs, herbs, substances, or indigenous
medicines; patients with a history of AKI; and
patients aged >65 years.29 31 Chronic kidney disease
can be detected with two simple tests: a urine test for the detection of proteinuria and a blood test to
estimate the GFR.26 29
Given that currently a population screening
for CKD is not recommended and it was claimed
that it might add unintended harm to the general
population being screened,30 there is no speciality
society or preventive services group which
recommends general screening.32 Low-to-middle-income
countries are ill-equipped to deal with the
devastating consequences of CKD, particularly the
late stages of the disease. There are suggestions
that screening should primarily include high-risk
patients, but also extend to those with suboptimal
levels of risk, eg, pre-diabetes and prehypertension.33
Cost-effectiveness of early
detection programmes
Universal screening of the general population
would be time-consuming, expensive, and has been
shown to be not cost-effective. Unless selectively
directed towards high-risk groups, such as the
case of unknown cause of CKD in disadvantaged
populations,34 according to a cost-effectiveness
analysis using a Markov decision analytic model,
population-based dipstick screening for proteinuria
has an unfavourable cost-effectiveness ratio.35 A
more recent Korean study confirmed that their
National Health Screening Program for CKD is
more cost-effective for patients with diabetes or
hypertension than the general population.36 From an
economic perspective, screening CKD by detection
of proteinuria was shown to be cost-effective in
patients with hypertension or diabetes in a systematic
review.37 The incidence of CKD, rate of progression,
and effectiveness of drug therapy were major drivers
of cost-effectiveness and thus CKD screening may
be more cost-effective in populations with higher
incidences of CKD, rapid rates of progression, and
more effective drug therapy.
Rational approach to chronic
kidney disease early detection
The approach towards CKD early detection will
include the decision for frequency of screening,
who should perform the screening and intervention
after screening.23 Screening frequency for targeted
patients should be yearly if no abnormality is
detected on initial evaluation. This is in line with
the Kidney Disease Improving Global Outcomes
resolution that the frequency of testing should
be according to the target group to be tested and
generally needs not be more frequent than once
per year.27 Who should perform the screening is
always a question especially when the healthcare
professional availability is a challenge in lower-income
economies. Physicians, nurses, paramedical staff, and other trained healthcare professionals are eligible to do the screening tests. Intervention after screening is also important and patients detected to
have CKD should be referred to primary care and
general physicians with experience in management
of kidney disease for follow-up. A management
protocol should be provided to primary care and
general physicians. Further referral to nephrologists
for management will be based on the well-defined
protocols.24 27 29
Integration of chronic kidney
disease prevention into national non-communicable disease programmes
Given the close links between CKD and other
NCDs, it is critical that CKD advocacy efforts be
aligned with existing initiatives concerning diabetes,
hypertension, and CVD, particularly in LMIC. Some
countries and regions have successfully introduced
CKD prevention strategies as part of their NCD
programmes. As an example, in 2003, a kidney
health promotion programme was introduced in
Taiwan, with its key components including a ban on
herbs containing aristolochic acid, public awareness
campaigns, patient education, funding for CKD
research, and the setting up of teams to provide
integrated care.38 In Cuba, the Ministry of Public
Health has implemented a national programme for
the prevention of CKD. Since 1996 the programme
has followed several steps: (1) analysis of the
resources and health situation in the country; (2)
epidemiological research to define the burden of
CKD; and (3) continuing education for nephrologists,
family doctors, and other health professionals. The
main goal has been to bring nephrology care closer
to the community through a regional redistribution
of nephrology services and joint treatment of
patients with CKD by primary healthcare physicians
and nephrologists.39 The integration of CKD
prevention into NCD programme has resulted in
the reduction of renal and cardiovascular risks in the
general population. Main outcomes have been the
reduction in the prevalence of risk factors, such as
low birth weight, smoking, and infectious diseases.
There has been an increased rate of the diagnosis
of diabetes and of glycaemic control, as well as an
increased diagnosis of patients with hypertension,
higher prescription use of renoprotective treatment
with angiotensin-converting enzyme inhibitor, and
higher rates of blood pressure control.40 Recently,
the United States Department of Health and Human
Services has introduced an ambitious programme to
reduce the number of Americans developing ESRD
by 25% by 2030. The programme, known as the
Advancing American Kidney Health initiative, has set goals with metrics to measure its success; among
them is to put more efforts to prevent, detect, and
slow the progression of kidney disease, in part by
addressing traditional risk factors such as diabetes
and hypertension. To reduce the risk of kidney
failure, the programme contemplates advancing
public health surveillance and research to identify
populations at risk and those in early stages of kidney
disease, and to encourage adoption of evidence-based
interventions to delay or stop progression to
kidney failure.41 Ongoing programmes, such as the
Special Diabetes Program for Indians, represent
an important part of this approach by providing
team-based care and care management. After
implementation of that programme, the incidence
of diabetes-related kidney failure among Indian
populations decreased by over 40% between 2000
and 2015.42
Involvement of primary care
physicians and other health professionals
Detection and prevention of CKD programmes
require considerable resources both in terms
of manpower and funds. Availability of such
resources will depend primarily on the leadership
of nephrologists.43 However, the number of
nephrologists is not sufficient to provide renal
care to the growing number of patients with CKD
worldwide. It has been suggested that most cases
of non-progressive CKD can be managed without
referral to a nephrologist, and specialist referral
can be reserved for patients with an estimated
GFR <30 mL/min/1.73 m2, rapidly declining kidney
function, persistent proteinuria, or uncontrolled
hypertension or diabetes.44 It has been demonstrated
that with an educational intervention the clinical
competence of family physicians increases, resulting
in preserved renal function in diabetic patients with
early renal disease.45 The practitioners who received
the educational intervention used significantly
more angiotensin-converting enzyme inhibitors,
angiotensin-receptor blockers, and statins than did
practitioners who did not receive it. The results
were similar to those found in patients treated by
nephrologists.46 The role of primary healthcare
professionals in the implementation of CKD
prevention strategies in LMIC has been recently
illustrated.47
The e-Learning has become an increasingly
popular approach to medical education. Online
learning programmes for NCD prevention and
treatment, including CKD, have been successfully
implemented in Mexico. By 2015, over 5000 health
professionals (including non-nephrologists) had
been trained using an electronic health education
platform.48
Shortage of nephrology
manpower—implications for prevention
The resources for nephrology care remain at critical levels in many parts of the world. Even in Western
developed countries, nephrologists are frequently in
short supply. In a selection of European countries with
similar, predominantly public, healthcare systems,
there was a substantial variation in the nephrology
workforce. Countries such as Italy, Greece, and Spain
reported the highest ratios, whereas countries such
as Ireland, Turkey, and the United Kingdom had the
lowest ratios.49 In the United States, the number of
nephrologists per 1000 ESRD patients has declined
from 18 in 1997 to 14 in 2010.50 The situation in the
developing world is even worse. With the exception
of Nigeria, Sudan, Kenya and South Africa, in many
countries of sub-Saharan Africa there are <10
nephrologists. The number of nephrology nurses
and dialysis technicians is also insufficient.51 In Latin
America the average number of nephrologists is
13.4 per million population (pmp). However, there
is unequal distribution between countries; those
with <10 nephrologists pmp (Honduras, 2.1 pmp;
Guatemala, 3.3 pmp; and Nicaragua, 4.6 pmp), and
those >25 pmp (Cuba, 45.2 pmp; Uruguay, 44.2 pmp;
and Argentina, 26.8 pmp).52
The causes of this shortage are multiple.
Potential contributors to this variation include the
increasing burden of CKD, erosion of nephrology
practice scope by other specialists, lack of workforce
planning in some countries relative to others, and
the development of new care delivery models.50 A
novel strategy has been the successful International
Society of Nephrology’s Fellowship programme.
Since its implementation in 1985, over 600 fellows
from >83 LMIC have been trained. A significant
number of fellowships were undertaken in selected
developed centres within the fellow’s own region. In
a recent survey, 85% of responding fellows were re-employed
by their home institutions.53 54
Interdisciplinary prevention
approach
Since 1994, a National Institute of Health consensus
advocated for early medical intervention in
predialysis patients. Owing to the complexity of care
of CKD, it was recommended that patients should
be referred to a multidisciplinary team consisting
of nephrologist, dietitian, nurse, social worker, and
health psychologist, with the aim to reduce predialysis
and dialysis morbidity and mortality.55 In Mexico,
a nurse-led protocol-driven multidisciplinary
programme reported better preservation in estimated
GFR and a trend in the improvement of quality of
care of patients with CKD similar to those reported by other multidisciplinary clinic programmes in
the developed world. Additionally, more patients
started dialysis non-emergently, and some obtained
a pre-emptive kidney transplant. For those unable
to obtain dialysis or who choose not to, a palliative
care programme is now being implemented.56 Care
models supporting primary care providers or allied
health workers achieved better effectiveness in
slowing kidney function decline when compared to
those providing speciality care. Future models should
address region-specific causes of CKD, increase the
quality of diagnostic capabilities, establish referral
pathways, and provide better assessments of clinical
effectiveness and cost-effectiveness.57
Online educational programmes
for chronic kidney disease
prevention and treatment
Whereas it is important to enhance the promotion
and implementation of prevention of kidney disease
and kidney failure among healthcare professionals,
it is equally important to promote prevention with
education programmes for those at risk of kidney
disease and kidney failure, and with the general
population at large. It is a stepwise process, from
awareness, through engagement, participation,
and empowerment, to partnership. As highlighted
above, in general, the health literacy of the general
population is low. Awareness and understanding of
kidney disease are inadequate. Education is key to
engaging patients with kidney disease. It is the path to
self-management and patient-centred care. Narva et
al58 found patient education is associated with better
patient outcomes. Obstacles include the complex
nature of kidney disease information, low baseline
awareness, limited health literacy and numeracy,
limited availability of CKD information, and lack
of readiness to learn. New education approaches
should be developed through research and quality
improvement efforts. Schatell59 found that web-based
kidney education is helpful in supporting patient self-management. The internet offers a
wealth of resources on education. Understanding
the types of internet sources that patients with
CKD use today can help renal professionals to point
patients in the right direction. It is important that
reputable healthcare organisations, preferably at a
national level, facilitate users to have easier access
to health information on their websites (as shown
in online supplementary Appendix). The mode of
communication currently used by patients and the
population at large is through the internet—websites,
portals, and other social media, such as Facebook and
Twitter. There are also free apps on popular mobile
devices providing education on kidney disease.
There is no shortage of information on the internet. The challenge is how to effectively push important
healthcare information in a targeted manner, and to
facilitate users seeking information in their efforts
to pull relevant and reliable information from the
internet. It is important that health information is
relevant for the condition (primary, secondary or
tertiary prevention), and offered at the right time
to the right recipient. It is possible, with the use of
information technology and informatics, to provide
relevant and targeted information for patients at high
risk, coupling the information based on diagnosis
and drugs prescribed. Engagement of professional
society resources and patient groups is a crucial
step to promote community partnership and patient
empowerment on prevention. Additional resources
may be available from charitable and philanthropic
organisations.
Renewed focus on prevention,
awareness-raising, and education
Given the urgency for increased education and
awareness on the importance of the preventive
measures, we suggest the following goals to redirect
the focus on plans and actions:
1. Empowerment through health literacy in order
to develop and support national campaigns that
bring public awareness to prevention of kidney
disease.
2. Population-based approaches to manage key known risks for kidney disease such as blood pressure control, and effective management of obesity and diabetes.
3. Implementation of the World Health Organization ‘Best Buys’ approach including screening of at-risk populations for CKD, universal access to essential diagnostics of early CKD, availability of affordable basic technologies and essential medicines and task shifting from doctors to frontline healthcare workers to more effectively target the progression of CKD and other secondary preventative approaches.
2. Population-based approaches to manage key known risks for kidney disease such as blood pressure control, and effective management of obesity and diabetes.
3. Implementation of the World Health Organization ‘Best Buys’ approach including screening of at-risk populations for CKD, universal access to essential diagnostics of early CKD, availability of affordable basic technologies and essential medicines and task shifting from doctors to frontline healthcare workers to more effectively target the progression of CKD and other secondary preventative approaches.
To that end, the motto “Kidney Health for
Everyone, Everywhere” is more than a tagline or
wishful thinking. It is a policy imperative which
can be successfully achieved if policy-makers,
nephrologists, and healthcare professionals place
prevention and primary care for kidney disease
within the context of their Universal Health Coverage
programmes.
Author contributions
PKT Li and K Kalantar-Zadeh serve as the corresponding authors. All the authors contributed equally otherwise.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This editorial received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration
This article was originally published in Kidney International, volume 97, pages 226-232, Copyright World Kidney Day
Steering Committee (2020), and reprinted concurrently in
several journals. The articles cover identical concepts and
wording but vary in minor stylistic and spelling changes,
detail, and length of manuscript in keeping with each journal’s
style. Any of these versions may be used in citing this article.
References
1. International Society of Nephrology. 2019 United Nations
high level meeting on universal health coverage: moving
together to build kidney health worldwide. Available from:
https://www.theisn.org/images/Advocacy_4_pager_2019_
Final_WEB_pagebypage.pdf. Accessed 20 Jul 2019.
2. Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific
mortality for 250 causes of death: reference and alternative
scenarios for 2016-40 for 195 countries and territories. Lancet
2018;392:2052-90. Crossref
3. Essue BM, Laba TL, Knaul F, et al. Economic burden of
chronic ill health and injuries for households in low- and
middle-income countries. In: Jamison DT, Gelband H, Horton
S, et al, editors. Disease Control Priorities Improving Health
and Reducing Poverty, 3rd ed. Washington, DC: World Bank;
2018: 121-43. Crossref
4. Vanholder R, Annemans L, Brown E, et al. Reducing the costs
of chronic kidney disease while delivering quality health care:
a call to action. Nat Rev Nephrol 2017;13:393-409. Crossref
5. Luyckx VA, Tuttle KR, Garcia-Garcia G, et al. Reducing major
risk factors for chronic kidney disease. Kidney Int Suppl
(2011) 2017;7:71-87. Crossref
6. Luyckx VA, Tonelli M, Stanifer JW. The global burden of
kidney disease and the sustainable development goals. Bull
World Health Organ 2018;96:414-22D. Crossref
7. Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events
in people with chronic kidney disease compared with those
with diabetes: a population-level cohort study. Lancet
2012;380:807-14. Crossref
8. Kalantar-Zadeh K, Fouque D. Nutritional management of
chronic kidney disease. N Engl J Med 2017;377:1765-76. Crossref
9. United Nations General Assembly. Political declaration of
the third high-level meeting of the General Assembly on
the prevention and control of non-communicable diseases.
Available from: https://www.un.org/ga/search/view_doc.
asp?symbol=A/73/L.2&Lang=E. Accessed 19 Nov 2019.
10. Lopez AD, Williams TN, Levin A, et al. Remembering
the forgotten non-communicable diseases. BMC Med
2014;12:200.Crossref
11. Centers for Disease Control and Prevention. At-a-glance:
Executive Summary, Picture of America. Available from:
https://www.cdc.gov/pictureofamerica. Accessed 19 Nov
2019.
12. Levey AS, Schoolwerth AC, Burrows NR, Williams DE,
Stith KR, McClellan W; Centers for Disease Control and Prevention Expert Panel. Comprehensive public health
strategies for preventing the development, progression, and
complications of CKD: report of an expert panel convened by
the Centers for Disease Control and Prevention. Am J Kidney
Dis 2009;53:522-35. Crossref
13. Saran R, Robinson B, Abbott KC, et al. US Renal Data System
2018 Annual Data Report: Epidemiology of Kidney Disease in
the United States. Am J Kidney Dis 2019;73(3S1):A7-A8. Crossref
14. Kovesdy CP, Furth SL, Zoccali C, World Kidney Day Steering
Committee. Obesity and kidney disease: hidden consequences
of the epidemic. J Ren Nutr 2017;27:75-7. Crossref
15. Tantisattamo E, Dafoe DC, Reddy UG, et al. Current
management of patients with acquired solitary kidney. Kidney
Int Rep 2019;4:1205-18. Crossref
16. Webster AC, Nagler EV, Morton RL, Masson P. Chronic
kidney disease. Lancet 2017;389:1238-52. Crossref
17. Koppe L, Fouque D. The role for protein restriction in
addition to renin-angiotensin-aldosterone system inhibitors
in the management of CKD. Am J Kidney Dis 2019;73:248-57. Crossref
18. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal
outcomes in type 2 diabetes and nephropathy. N Engl J Med
2019;380:2295-306. Crossref
19. Greco EV, Russo G, Giandalia A, Viazzi F, Pontremoli R, De
Cosmo S. GLP-1 receptor agonists and kidney protection.
Medicina (Kaunas) 2019;55(6).pii: E233. Crossref
20. Rifkin DE, Coca SG, Kalantar-Zadeh K. Does AKI truly lead
to CKD? J Am Soc Nephrol 2012;23:979-84. Crossref
21. Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in
patients with autosomal dominant polycystic kidney disease.
N Engl J Med 2012;367:2407-18. Crossref
22. Verhave JC, Troyanov S, Mongeau F, et al. Prevalence,
awareness, and management of CKD and cardiovascular risk
factors in publicly funded health care. Clin J Am Soc Nephrol
2014;9:713-9. Crossref
23. Chow KM, Szeto CC, Kwan B, Leung CB, Li PK. Public lacks
knowledge on chronic kidney disease: telephone survey.
Hong Kong Med J 2014;20:139-44. Crossref
24. Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney
disease and cardiovascular risk in six regions of the world
(ISN-KDDC): a cross-sectional study. Lancet Glob Health
2016;4:e307-19. Crossref
25. Li PK, Weening JJ, Dirks J, et al. A report with consensus
statements of the International Society of Nephrology 2004
Consensus Workshop on Prevention of Progression of
Renal Disease, Hong Kong, June 29, 2004. Kidney Int Suppl
2005;(94):S2-7. Crossref
26. Vassalotti JA, Stevens LA, Levey AS. Testing for chronic
kidney disease: A position statement from the National
Kidney Foundation. Am J Kidney Dis 2007;50:169-80. Crossref
27. Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as
a global public health problem: Approaches and initiatives—a
position statement from Kidney Disease Improving Global
Outcomes. Kidney Int 2007;72:247-59. Crossref
28. Crowe E, Halpin D, Stevens P; Guideline Development Group.
Early identification and management of chronic kidney
disease: summary of NICE guidance. BMJ 2008;337:a1530. Crossref
29. Li PK, Chow KM, Matsuo S, et al. Asian chronic kidney
disease best practice recommendations: positional statements
for early detection of chronic kidney disease from Asian
Forum for Chronic Kidney Disease Initiatives (AFCKDI).
Nephrology (Carlton) 2011;16:633-41. Crossref
30. Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: A
systematic review for the U.S. Preventive Services Task Force
and for an American College of Physicians Clinical Practice
Guideline. Ann Intern Med 2012;156:570-81.Crossref
31. Li PK, Ng JK, Cheng YL, et al. Relatives in silent kidney disease
screening (RISKS) study: a Chinese cohort study. Nephrology
(Carlton) 2017;22 Suppl 4:35-42. Crossref
32. Samal L, Linder JA. The primary care perspective on routine
urine dipstick screening to identify patients with albuminuria.
Clin J Am Soc Nephrol 2013;8:131-5.Crossref
33. George C, Mogueo A, Okpechi I, Echouffo-Tcheugui JB,
Kengne AP. Chronic kidney disease in low-income to middle-income
countries: the case for increased screening. BMJ Glob
Health 2017;2:e000256. Crossref
34. Gonzalez-Quiroz M, Nitsch D, Hamilton S, et al. Rationale
and population-based prospective cohort protocol for the
disadvantaged populations at risk of decline in eGFR (CO-DEGREE).
BMJ Open 2019;9:e031169. Crossref
35. Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: A cost-effectiveness
analysis. JAMA 2003;290:3101-14. Crossref
36. Go DS, Kim SH, Park J, Ryu DR, Lee HJ, Jo MW. Cost-utility
analysis of the National Health Screening Program for chronic
kidney disease in Korea. Nephrology (Carlton) 2019;24:56-64. Crossref
37. Komenda P, Ferguson TW, Macdonald K, et al. Cost-effectiveness of primary screening for CKD: A systematic
review. Am J Kidney Dis 2014;63:789-97. Crossref
38. Hwang SJ, Tsai JC, Chen HC. Epidemiology, impact and
preventive care of chronic kidney disease in Taiwan.
Nephrology (Carlton) 2010;15 Suppl 2:3-9. Crossref
39. Almaguer M, Herrera R, Alfonso J, Magrans C, Mañalich R,
Martínez A. Primary health care strategies for the prevention
of end-stage renal disease in Cuba. Kidney Int Suppl
2005;97:S4-10. Crossref
40. Alamaguer-Lopez M, Herrera-Valdez R, Diaz J, Rodriguez
O. Integration of chronic kidney disease prevention into
noncommunicable disease programs in Cuba. In: Garcia-Garcia G, Agodoa LY, Norris KC, editors. Chronic Kidney
Disease in Disadvantaged Populations. London: Elsevier Inc;
2017: 357-65. Crossref
41. US Department of Health and Human Services. Advancing
American Kidney Health. Available from: https://aspe.
hhs.gov/pdf-report/advancing-american-kidney-health.
Accessed 26 Sep 2019.
42. US Department of Health and Human Services. The Special
Diabetes Program for Indians. Estimates of Medicare savings.
Available from: https://aspe.hhs.gov/pdf-report/specialdiabetes-
program-indians-estimates-medicare-savings.
Accessed 26 Sep 2019.
43. Bello AK, Nwankwo E, El Nahas AM. Prevention of
chronic kidney disease: a global challenge. Kidney Int Suppl
2005;(98):S11-7. Crossref
44. James MT, Hemmelgarn BR, Tonelli M. Early recognition and
prevention of chronic kidney disease. Lancet 2010;375:1296-
309. Crossref
45. Cortés-Sanabria L, Cabrera-Pivaral CE, Cueto-Manzano AM, et al. Improving care of patients with diabetes and CKD:
a pilot study for a cluster-randomized trial. Am J Kidney Dis
2008;51:777-88. Crossref
46. Martínez-Ramírez HR, Jalomo-Martínez B, Cortés-Sanabria
L, et al. Renal function preservation in type 2 diabetes mellitus
patients with early nephropathy: a comparative prospective
cohort study between primary health care doctors and a
nephrologist. Am J Kidney Dis 2006;47:78-87. Crossref
47. Cueto-Manzano AM, Martínez-Ramírez HR, Cortes-
Sanabria L, Rojas-Campos E. CKD screening and prevention
strategies in disadvantaged populations. The role of primary
health care professionals. In: Garcia-Garcia G, Agodoa LY,
Norrris KC, editors. Chronic Kidney Disease in Disadvantaged
Populations. London: Elsevier, Inc; 2017: 329-35. Crossref
48. Tapia-Conyer R, Gallardo-Rincon H, Betancourt-Cravioto M.
Chronic kidney disease in disadvantaged populations: Online
educational programs for NCD prevention and treatment.
In: Garcia-Garcia G, Agodoa LY, Norris KC, editors. Chronic
Kidney Disease in Disadvantaged Populations. London:
Elsevier, Inc; 2017: 337-45. Crossref
49. Bello AK, Levin A, Manns BJ, et al. Effective CKD care in
European countries: challenges and opportunities for health
policy. Am J Kidney Dis 2015;65:15-25. Crossref
50. Sharif MU, Elsayed ME, Stack AG. The global nephrology
workforce: emerging threats and potential solutions! Clin
Kidney J 2016;9:11-22. Crossref
51. Naicker S, Eastwood JB, Plange-Rhule J, Tutt RC. Shortage
of healthcare workers in sub-Saharan Africa: a nephrological
perspective. Clin Nephrol 2010;74 Suppl 1:S129-33. Crossref
52. Cusumano AM, Rosa-Diez GJ, Gonzalez-Bedat MC. Latin
American Dialysis and Transplant Registry: Experience and
contributions to end-stage renal disease epidemiology. World
J Nephrol 2016;5:389-97. Crossref
53. Feehally J, Brusselmans A, Finkelstein FO, et al. Improving
global health: measuring the success of capacity building
outreach programs: a view from the International Society of
Nephrology. Kidney Int Suppl (2011) 2016;6:42-51. Crossref
54. Harris DC, Dupuis S, Couser WG, Feehally J. Training
nephrologists from developing countries: does it have a
positive impact? Kidney Int Suppl (2011) 2012;2:275-8. Crossref
55. Morbidity and mortality of renal dialysis: an NIH consensus
conference statement. Consensus Development Conference
Panel. Ann Intern Med 1994;121:62-70. Crossref
56. Garcia-Garcia G, Martinez-Castellanos Y, Renoirte-Lopez
K, et al. Multidisciplinary care for poor patients with chronic
kidney disease in Mexico. Kidney Int Suppl (2011) 2013;3:178-
83. Crossref
57. Stanifer JW, Von Isenburg M, Chertow GM, Anand S.
Chronic kidney disease care models in low- and middleincome
countries: a systematic review. BMJ Glob Health
2018;3:e000728. Crossref
58. Narva AS, Norton JM, Boulware LE. Educating patients about
CKD: the path to self-management and patient-centered care.
Clin J Am Soc Nephrol 2016;11:694-703. Crossref
59. Schatell D. Web-based kidney education: supporting patient
self-management. Semin Dial 2013;26:154-8. Crossref