Hong
Kong Med J 2019 Dec;25(6):491.e1-2
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Giant perivascular spaces: an uncommon cause of
obstructive hydrocephalus
MH So, MB, BS FRCR; WK Lo, FRCR, FHKAM (Radiology)
Department of Diagnostic and Interventional
Radiology, Kwong Wah Hospital, Yaumatei, Hong Kong
Corresponding author: Dr MH So (manhon.so@gmail.com)
A 55-year-old man with good past health presented
to the emergency department with unsteady gait for 6 months with recent
mild left-sided weakness. Urgent computed tomography (CT) scan of the
brain showed a multiseptated cystic lesion in the right
mesencephalothalamic region with pressure effect on the third ventricle
causing obstructive hydrocephalus (Fig 1). Urgent magnetic resonance imaging (MRI) scan
(Fig 2) of the lesion showed no post-gadolinium
enhancement, no restricted diffusion, complete suppression of the cystic
areas on T2 fluid attenuation inversion recovery (FLAIR) sequence and no
abnormal parenchymal signal intensities compared with normal brain
parenchyma. These imaging findings are consistent with tumefactive
perivascular space. Invasive biopsy and surgical excision were avoided,
and the patient underwent surgery for ventricular drain insertion.
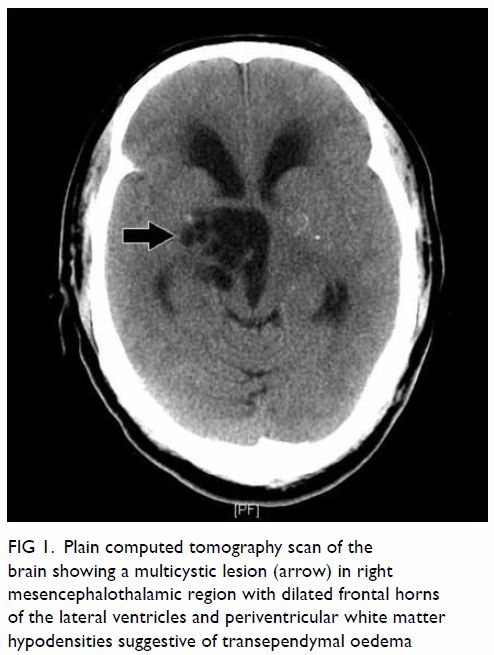
Figure 1. Plain computed tomography scan of the brain showing a multicystic lesion (arrow) in right mesencephalothalamic region with dilated frontal horns of the lateral ventricles and periventricular white matter hypodensities suggestive of transependymal oedema
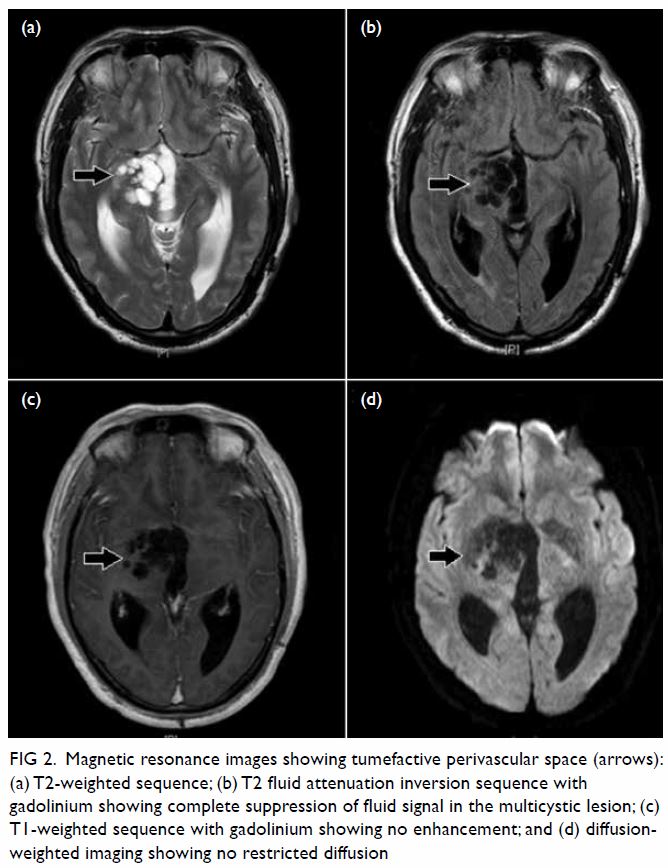
Figure 2. Magnetic resonance images showing tumefactive perivascular space (arrows): (a) T2-weighted sequence; (b) T2 fluid attenuation inversion sequence with gadolinium showing complete suppression of fluid signal in the multicystic lesion; (c) T1-weighted sequence with gadolinium showing no enhancement; and (d) diffusionweighted imaging showing no restricted diffusion
Dilated perivascular spaces (PVSs) in the brain are
interstitial fluid-filled structures lined by pia-mater that have
accompanying patent penetrating arteries within, most commonly seen along
the lenticulostriate arteries.1
They can be unilocular or multilocular and may have a radial pattern along
the course of the penetrating arteries. The PVSs occur across all
age-groups and are more frequent and larger with advancing age. The cause
of dilated PVS remains unknown though numerous theories have been
postulated including increased permeability of arterial wall and
obstruction/disturbance of interstitial fluid drainage/flow. Dilated PVSs
may be associated with microvascular diseases, trauma, non-vascular
dementia, multiple sclerosis, and the mucopolysaccharidoses.
Rarely PVSs are markedly expanded and are termed
tumefactive or giant PVSs. Some authors define tumefactive PVSs as those
>1.5 cm. Tumefactive PVSs are most commonly located in
mesencephalothalamic region.2 They
are also seen in cerebral white matter and in the cerebellar dentate
nuclei. They can exhibit mass effect and cause obstructive hydrocephalus
when occurring in the mesencephalothalamic region as in our case. The MRI
signal intensities of typical PVSs should follow cerebrospinal fluid in
all sequences including FLAIR imaging with no post-gadolinium enhancement.
There is no restricted diffusion as the compartments are communicating.
Tumefactive PVSs in cerebral white matter may have perilesional abnormal
T2 and FLAIR hyperintensities in up to 50% of cases. The mass effect of
the tumefactive PVS may cause chronic ischaemic change in adjacent white
matter.3 Histopathological results
typically show a pial-lined cyst with no evidence of neoplasm or
infection.
Differential diagnoses include cystic infarction,
tumour, and infection.4 Cystic
infarctions assume a slit-like or ovoid shape whereas PVSs are more
rounded or linear. The cystic content of tumours is usually not isointense
to cerebrospinal fluid on MRI. Solid components are often present, which
may enhance after contrast and are surrounded by oedema. Parasitic
infections have a range of appearances on CT or MRI scans with contrast
enhancement and oedema during the active phase and calcifications in the
quiescent phase.
Asymptomatic tumefactive PVSs can be managed by
follow-up imaging for stability in size. Spontaneous regression of
tumefactive PVSs without surgical intervention is rare. Tumefactive PVSs
with mass effect and obstructive hydrocephalus can be treated surgically
with ventriculostomy, cyst fenestration, ventriculoperitoneal shunting, or
cystoperitoneal shunting. When the appearance is typical, surgical biopsy
or excision should be avoided.
Author contributions
All authors contributed to the concept, acquisition
and interpretation of data, drafting of the manuscript, and revision for
important intellectual content. All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the
Declaration of Helsinki. The patient provided informed consent for all
procedures.
References
1. AI Abdulsalam H, Alatar AA, Elwatidy S.
Giant tumefactive perivascular spaces: a case report and literature
review. World Neurosurg 2018;112:201-4. Crossref
2. Choh NA, Shaheen F, Robbani I, Singh M,
Gojwari T. Tumefactive Virchow–Robin spaces: a rare cause of obstructive
hydrocephalus. Ann Indian Acad Neurol 2014;17:345-6. Crossref
3. Salzman KL, Osborn AG, House P, et al.
Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol 2005;26:298-
305.
4. Kwee RM, Kwee TC. Virchow-Robin spaces
at MR imaging. Radiographics 2007;27:1071-86. Crossref

