© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Purple glove syndrome caused by intravenous phenytoin:
two case reports
CY Tsang, MB, ChB, MRCSEd1; Joanne YW
Ng, MB, BS1; SH Yuen, FRCSEd, FHKAM (Orthopaedic Surgery)1; IT
Lau, FRCP, HKAM (Medicine)2; YF Leung, FRCSEd, FHKAM
(Orthopaedic Surgery)1
1 Department of Orthopaedics and
Traumatology, Tseung Kwan O Hospital, Hong Kong
2 Department of Medicine, Tseung Kwan O
Hospital, Hong Kong
Corresponding author: Dr CY Tsang (tcy634@ha.org.hk)
Case 1
An 81-year-old woman was admitted in June 2014 to
Tseung Kwan O Hospital with aspiration pneumonia. She had temporal lobe
necrosis and absence seizure after radiotherapy for nasopharyngeal
carcinoma. She was prescribed lifelong oral phenytoin with no adverse
reaction reported to date. Oral phenytoin was changed to intravenous, 100
mg every 8 hours, via a 22G catheter in her right wrist due to
oropharyngeal dysphagia. The catheter had been changed several times due
to blockage with no apparent extravasation. Erythematous swelling of the
skin around previous cannula sites was treated as cellulitis by
intravenous ciprofloxacin.
The following day the patient’s right hand
deteriorated and showed marked circumferential purplish discolouration,
swelling, and gangrenous changes to the skin (Fig 1). Similar minor changes over the left hand
were noted. She was afebrile and not septic. White cell count (12.8 × 109/L)
was mildly elevated and C-reactive protein was elevated at 196 mg/L.
Nonetheless, these results could be interpreted as being due to concurrent
aspiration pneumonia.
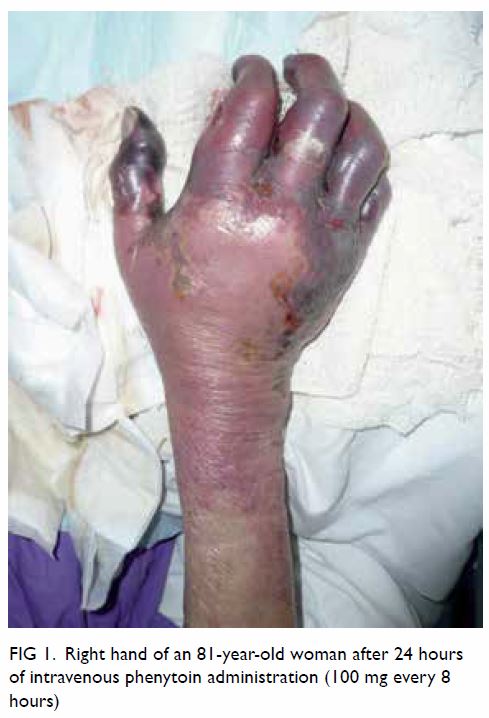
Figure 1. Right hand of an 81-year-old woman after 24 hours of intravenous phenytoin administration (100 mg every 8 hours)
Computed tomographic angiography revealed fluid
collection in the patient’s right hand up to the right forearm with
reasonable arterial supply. Intravenous penicillin G and ciprofloxacin
were started. Serial clinical assessments prior to surgery showed no
further deterioration. Emergency incision, drainage, and carpal tunnel
release were performed. Findings were skin and fat necrosis, venous
thrombosis and a large amount of clear fluid over the fascial layers of
the palm and fingers. There was no turbid or dishwater discharge. The
transverse carpal retinaculum was thickened and tight. Mild necrosis of
the right-hand small muscles was noted. Cultures of intra-operative tissue
samples were negative for micro-organisms.
On day 15 of intravenous phenytoin injection, the
patient was diagnosed with purple glove syndrome (PGS). Intravenous
phenytoin was stopped immediately and changed to valproate. The condition
of the patient’s left-hand improved rapidly but that of her right hand
continued to deteriorate (Fig 2) and necessitated below-elbow amputation. The
amputation wound healed and she was referred for rehabilitation.
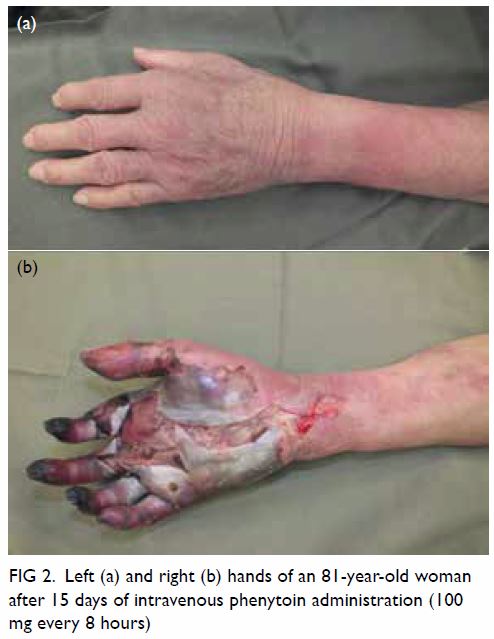
Figure 2. Left (a) and right (b) hands of an 81-year-old woman after 15 days of intravenous phenytoin administration (100 mg every 8 hours)
Case 2
An 87-year-old woman with brain metastasis from
lung carcinoma was prescribed oral phenytoin for epilepsy. She was
admitted to Tseung Kwan O Hospital in April 2016 with fever. She was
prescribed 100 mg phenytoin every 8 hours via a 22G catheter in her left
hand due to potential dysphagia. She complained of painful swelling of her
left hand after several doses of phenytoin. There was no extravasation of
drug. The patient was afebrile and not septic. Diffuse circumferential
purplish, tender swelling of the distal left forearm and left hand was
noted. The patient’s hand was in intrinsic minus posture. There was no
crepitation or fluctuance. The patient’s left wrist and finger movements
were limited by pain. Passive stretching of the fingers exacerbated her
pain. Radial pulse was palpable with good capillary refill. White cell
count (12.4 × 109/L) and C-reactive protein were slightly
elevated (17.2 mg/L).
The patient was suspected to have PGS of her left
hand complicated by compartment syndrome. Intravenous phenytoin was
stopped 1 day after injection and switched to valproate. Serial clinical
assessments prior to surgery revealed no further deterioration. Emergency
fasciotomy and carpal tunnel release were performed. Intra-operative
findings were consistent with compartment syndrome and PGS. There was no
evidence of necrotising fasciitis. Cultures of intra-operative tissue
samples were negative for micro-organisms. Debridement and primary wound
closure were performed 12 days after fasciotomy and the patient was
transferred for rehabilitation.
Subsequently, the patient developed a breakthrough
seizure and was prescribed intravenous phenytoin via her right hand
because the physician did not notice that the patient had previously
developed PGS as a complication of this treatment. She developed PGS of
her right hand complicated by compartment syndrome. Emergency fasciotomy
and carpal tunnel release of the right forearm and right hand were
performed. Debridement and primary closure of wounds were performed 16
days after fasciotomy. In order to avoid future complications, a note was
made in her medical records that she had an adverse reaction to phenytoin.
Discussion
Purple glove syndrome is an adverse reaction to
intravenous phenytoin. Its incidence has been reported to range from 1.5%
to 5.9%.1 Its manifestation
includes pain, skin discolouration, blister formation, sloughing,
ulceration, necrosis, and compartment syndrome at the injection site
within hours of drug administration. Differential diagnoses include
extravasation of the medication, cellulitis, peripheral vascular disease,
venous thrombosis, and collagen vascular disease.2
The pathogenesis of PGS is poorly understood. It
has previously been considered to be a result of extravasation of highly
alkaline phenytoin (pH 12).3 4 However, PGS can occur in the apparent absence of
extravasation. Histological examination has suggested thrombosis might
play a role in pathogenesis.3 To
further complicate the matter, PGS has also been reported to be possible
following oral phenytoin administration.4
There is no literature to date to correlate any intravenous complication
with previous use of oral medication.
Risk factors for PGS after intravenous phenytoin
administration include older age, female sex, peripheral vascular disease,
sepsis, history of chronic debilitating disease, number of doses, higher
dosage (>15-20 mg/kg body weight), higher infusion rate (>25
mg/min), and use of an intravenous catheter <20G.5
Initial management includes analgesia, elevation of
the affected limb, compression, massage, and gentle heat. Phenytoin should
be stopped and changed to an alternative antiepileptic. Most cases of PGS
are mild and can be resolved with no long-term sequelae. Management is
mainly supportive. However, PGS may progress to limb ischaemia and
compartment syndrome. Frequent monitoring of neurovascular status is
required. Vascular study should be performed if perfusion is compromised.5 Anticoagulation, thrombectomy or
thrombolysis should be considered when arterial thrombosis is identified.
Emergency fasciotomy should be performed for compartment syndrome. An
unsalvageable limb should be amputated. Amputation has been reported to be
necessary in 10.1% of cases.5 The
use of regional blockade and hyaluronidase remains experimental.5
Purple glove syndrome may be prevented by avoiding
phenytoin if there is an alternative agent with similar efficacy, for
example valproate. Otherwise, oral phenytoin is preferred. If intravenous
phenytoin is used, it should be administered via a larger catheter into a
larger vein, at the correct dosage and infusion rate, and with a
post-infusion saline flush.5
Author contributions
All authors contributed to the concept of study,
acquisition and analysis of data, drafting of the article, and critical
revision for important intellectual content. All authors had full access
to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to
disclose.
Funding/support
This case report received no specific grant from
any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the
Declaration of Helsinki. The patient provided informed consent for all
procedures.
References
1. Rajabally H, Nageshwaran S, Russell S.
An atypical case of purple glove syndrome: an avoidable adverse event. BMJ
Case Rep 2012;2012. pii: bcr0120125653. Crossref
2. Lalla R, Malhotra HS, Garg RK, Sahu R.
Purple glove syndrome: a dreadful complication of intravenous phenytoin
administration. BMJ Case Rep 2012;2012. pii: bcr2012006653. Crossref
3. Okogbaa JI, Onor IO, Arije OA, Harris
MB, Lillis RA. Phenytoin-induced purple glove syndrome: a case report and
review of the literature. Hosp Pharm 2015;50:391-5. Crossref
4. Bhattacharjee P, Glusac EJ. Early
histopathologic changes in purple glove syndrome. J Cutan Pathol
2004;31:513-5. Crossref
5. Garbovsky LA, Drumheller BC, Perrone J.
Purple glove syndrome after phenytoin or fosphenytoin administration:
review of reported cases and recommendations for prevention. J Med Toxicol
2015;11:445-59. Crossref

