Hong Kong Med J 2019 Dec;25(6):468–72 | Epub 4 Dec 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
In vitro fertilisation in Hong Kong: the situation in
2019
MW Lui, MB, BS; William SB Yeung, PhD; PC Ho, MB,
BS, MD; Ernest HY Ng, MB, BS, MD
Department of Obstetrics and Gynaecology, The
University of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Prof Ernest HY Ng (nghye@hku.hk)
Abstract
The popularity of in vitro fertilisation has
continuously increased throughout the past 40 years owing to an
increased incidence of infertility and delayed planning for pregnancy.
The aim of this paper is to review the current situation of in vitro
fertilisation in Hong Kong. In Hong Kong, in 2018, 7995 women underwent
5055 fresh and 5050 frozen-thawed embryo in vitro fertilisation cycles,
resulting in an ongoing pregnancy rate of 33.7% per transfer. However,
in vitro fertilisation is associated with several problems, including a
high rate of multiple pregnancies and risks associated with cross-border
reproductive care. Single embryo transfer is a simple strategy to reduce
multiple pregnancies without compromising the cumulative live birth
rate.
Introduction
The incidence of infertility has increased in
recent decades, and women are more often delaying marriage and pregnancy.
The mean age at which women deliver their first baby has increased from
27.9 years in 1995 to 30.5 years in 2017.1
Owing to the natural decline in fertility with age, more and more couples
seek help to conceive through IVF. The number of IVF cycles performed in
Europe increased from 203 225 cycles in 1997 to 776 556 in 2014, with 170
163 babies born.2 A similar trend
has been observed worldwide.3 The
aim of this paper is to review the current situation of IVF in 2019.
History of the in vitro fertilisation process
Last year in 2018, the first “test tube” baby
Louise Brown celebrated her 40th birthday. In vitro fertilisation was
pioneered by Patrick Steptoe and Robert Edwards. They first obtained an
oocyte in a natural cycle using a laparoscope. After it was fertilised in
the laboratory, they transferred the embryo back to the donor’s uterus.
The techniques of IVF have progressed rapidly since then. The success rate
has also greatly increased from lower than 1% per cycle to around 35% per
cycle. The success rate has increased owing to several factors, including
ovarian stimulation, assisted fertilisation using intracytoplasmic sperm
insemination, improvements in culture conditions, and more advanced embryo
transfer techniques.
Modern IVF cycles include ovarian stimulation,
oocyte retrieval, fertilisation and culture in vitro, and embryo transfer
to the uterus. Ovarian stimulation involves administration of
gonadotropin, coupled with suppression of endogenous gonadotropin release
by either gonadotropin-releasing hormone (GnRH) agonist or antagonist. The
super-physiological doses of gonadotropins enhance growth of multiple
follicles, in contrast to the development of a single follicle in a
natural cycle. The use of GnRH agonist or antagonist reduces the risk of
premature ovulation. Women undergoing ovarian stimulation need close
monitoring, with pelvic scanning to determine the number and size of the
follicles and hormonal tests to measure the serum oestradiol level. When
the leading follicles reach >17 to 18 mm, human chorionic gonadotropin
is usually given to the patient to trigger the final maturation of the
oocytes.
At 36 to 38 hours after the trigger, oocyte
retrieval is typically performed through the transvaginal route under
ultrasound guidance. An aspiration needle is used to puncture through the
vagina to aspirate the follicular fluid with the oocytes and the follicles
are aspirated till complete collapse of the follicles. Oocyte retrieval
involves a small risk of bleeding, infection, pain, and risk of injury to
visceral organs.
In vitro fertilisation refers to the overnight
co-culture of oocytes with sperm. For couples with severe male factor
infertility or fertilisation problems in previous IVF cycles,
intracytoplasmic sperm insemination involving injection of a single sperm
into an oocyte is advised.4 The
fertilised embryo is transferred back to the uterine cavity either at
cleavage (Day 2 or 3) or blastocyst stage (Day 5). Surplus high quality
fertilised embryos can be frozen at the cleavage or blastocyst stage for
subsequent transfer by slow freezing or more commonly by vitrification,
which involves rapid freezing in liquid nitrogen. Vitrification enables a
rapid transition of the liquid form of water to a “glass” status, which
avoids crystallisation that can damage the cells.
In vitro fertilisation is not without risks, even
in healthy couples. The most common complications of IVF are multiple
pregnancy followed by ovarian hyperstimulation syndrome, which can lead to
ascites, pleural effusion, venous thromboembolism, or even death. Although
severe ovarian hyperstimulation syndrome has a low incidence, it can be
potentially life-threatening and is avoidable. In cycles using GnRH
antagonists to prevent premature ovulation, agonist can be used instead of
human chorionic gonadotropin as a trigger for oocyte final maturation in
order to lower the risk of ovarian hyperstimulation syndrome.2 5
Overall, the success of IVF greatly depends on the
woman’s age. As shown by the latest data by ESHRE (European Society of
Human Reproduction and Embryology),2
in 2014, live birth rates after fresh transfer IVF in women aged <35,
35 to 39, and ≥40 years were 23.8%, 18.8%, and 8.1%, respectively. For
women aged <30 years, the cumulative live birth rate after completion
of a cycle can be nearly 70%, compared with 17.3% in women aged ≥40 years.4
In vitro fertilisation in Hong Kong
History and development
In Hong Kong, IVF programmes were initiated in the
Department of Obstetrics and Gynaecology, The Chinese University of Hong
Kong in December 1984, in the Hong Kong Sanatorium & Hospital in
January 1986, and in the Department of Obstetrics and Gynaecology, The
University of Hong Kong in July 1986.7
The Hong Kong Sanatorium & Hospital programme achieved the first
successful IVF pregnancy and delivery in Hong Kong.7
By August 1987, 522 IVF cycles had been initiated
in Hong Kong and the overall clinical pregnancy rate was 5.2% per cycle
initiated.6 From January 1992 to
December 1993, 912 IVF cycles, 158 cycles of gamete intrafallopian
transfer, 87 cycles of zygote intrafallopian transfer, and 233 cycles of
frozen-thawed embryo transfer (FET) were initiated, with delivery rates
per cycle started of 8.4%, 29.1%, 13.8%, and 11.2%, respectively.8
More recently, according to the Council on Human
Reproductive Technology (HRT Council) Annual Report,9 the number of women undergoing IVF increased from 2415
in 2009 to 7995 in 2018, with the number of fresh and FET IVF cycles
increasing from 2768 to 10 105 over the same period (Table
1). The ongoing pregnancy rate per transfer increased slightly from
29.9% in 2009 to 32.8% in 2015 and remained steady in 2015 to 2018 (Table
2). The ongoing pregnancy rate per transfer was 33.7% in 2018.
However, there was no significant increase in live birth rates throughout
the years. Both ongoing and live birth rates are markedly reduced in women
aged ≥41 years.
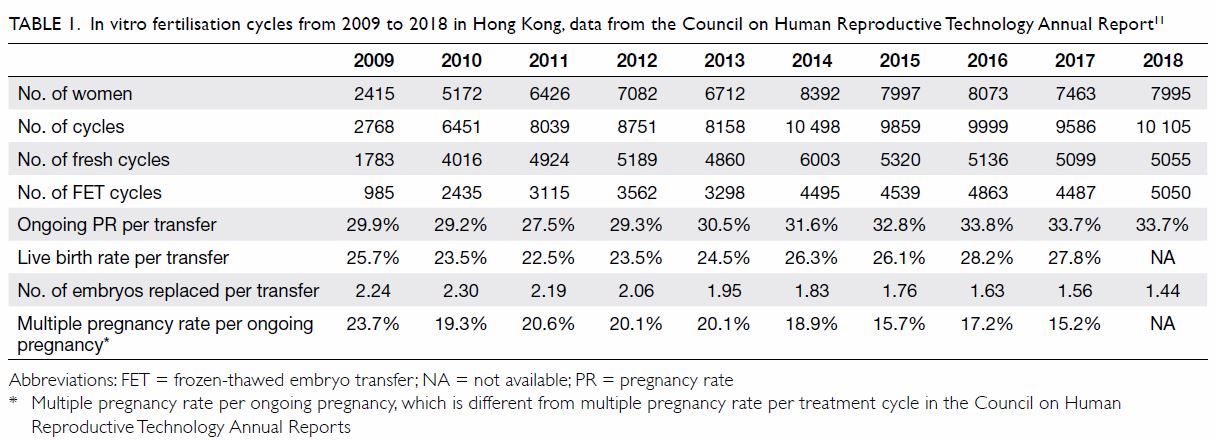
Table 1. In vitro fertilisation cycles from 2009 to 2018 in Hong Kong, data from the Council on Human Reproductive Technology Annual Report11
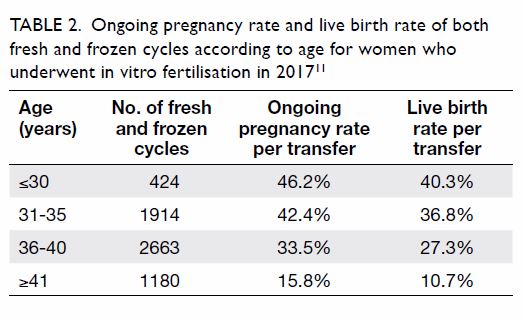
Table 2. Ongoing pregnancy rate and live birth rate of both fresh and frozen cycles according to age for women who underwent in vitro fertilisation in 201711
The number of embryos transferred has reduced
gradually over the years, though double embryo transfer still is the most
common. The single embryo transfer rate has increased from 12.9% (321
cycles) in 2009 to 49.2% (3043 cycles) in 2017. As a result, the multiple
pregnancy rate per ongoing pregnancy has decreased, but remained high at
15.2% in 2017.
Regulation of assisted reproduction
Assisted reproduction in Hong Kong is regulated by
the Human Reproductive Technology Ordinance (Cap. 561).10 Centres that provide IVF must obtain a treatment
licence from the HRT Council. At the time of writing, there are 18
licensed treatment centres in Hong Kong and of these, 13 provide IVF. Of
these 13 treatment centres, 10 are in the private sector and three are in
the public sector.11
As stated in the HRT Council Code of Practice,6 in Hong Kong, IVF can be provided only to legally
married couples in monogamous relationship. The maximum number of embryos
that can be transferred to the woman is three per cycle. Frozen embryos
can be stored for up to 10 years from the day of freezing, and gametes can
be stored until the patient is aged 55 years. All IVF data must be
documented clearly and reported promptly and accurately to the HRT
Council. Commercial oocyte donation is not allowed in Hong Kong.11 No payment to the oocyte donor is allowed apart from
reimbursing the loss of earnings and the expenses, such as transportation
and appointment fee. Sex selection, unless medically indicated, is also
prohibited in Hong Kong. Although surrogacy is legal in Hong Kong, none of
the centres in Hong Kong has a licence for surrogacy arrangements, and
surrogacy agreements have been found to be unenforceable under the law.11
Public in vitro fertilisation service
Currently, IVF service is provided in three public
hospitals in Hong Kong: Kwong Wah Hospital, Prince of Wales Hospital, and
Queen Mary Hospital. Eligible couples can receive partial subsidy funding
from the Hong Kong Hospital Authority for up to three IVF cycles. The
number of publicly funded cycles has increased to around 1000 cycles in
total in 2018. The criteria for funded IVF cycles include women who are
permanent Hong Kong residents, aged <40 years, and with no biological
children. The waiting time for IVF in public hospitals in Hong Kong is up
to 3 years. The provision of IVF in public hospitals is not entirely free
of charge. Couples pay approximately HK$20 000 for all medication,
procedures, and embryo storage for up to three cycles, but this is lower
than the HK$80 000 to HK$100 000 required for just one IVF cycle in the
private sector.
Recent in vitro fertilisation technology developments
Chromosome aneuploidy
Chromosome aneuploidy is an error in cell division
that results in the “daughter” cells having the wrong number of
chromosomes. Chromosome aneuploidy is a major reason for failure of
conception, pregnancy loss, and congenital anomalies following both
natural conception and IVF pregnancies and its prevalence increases
exponentially with maternal age. The need to assess embryo quality and
select those with the highest potential for implantation on the basis of
morphology has led to preimplantation genetic testing for aneuploidy
(PGT-A).9 This involves biopsy of a
few cells from an embryo at the blastocyst stage and assessment of the
comprehensive chromosome copy numbers. Although PGT-A cannot create a
healthy embryo or improve the health of an embryo, it can provide an
accurate method of selecting of embryos with a normal number of
chromosomes for transfer. This in turn has the potential to increase the
chance of having a healthy live birth per each transfer and to reduce the
risk of miscarriage or abnormal fetus caused by an abnormal number of
chromosomes.12 However, the
potential damage of trophectoderm biopsy on the developing embryos remains
unknown. In addition, the concordance of trophectoderm biopsy and
blastocyst is questionable especially in those with segmental aneuploidy13 and mosaicism.14 Gene editing may provide a new perspective in future,
but there is still a long way ahead. Gene editing technologies are
immature and imprecise, and involve unknown long-term risks.
Oocyte freezing
Oocyte freezing by vitrification is no longer
considered to be experimental and is offered to women who desire
preservation of their fertility potential before chemotherapy or
radiotherapy for cancer treatment. In Hong Kong, the indication of oocyte
freezing has been extended to single women who wish to delay parenthood
for education or career purposes. In general, fertility preservation for
cancer patients is underutilised, partly owing to lack of funding in the
public sector and inadequate information for patients. Fertility
preservation for cancer patients requires a close cooperation between
fertility specialists, oncologists, paediatricians, and surgeons. Oocyte
or embryo freezing provides more promising results in fertility
preservation, but it involves a delay of treatment for at least 2 weeks
and it is possible only in postpubertal women. Owing to the lack of
funding, patients often need to pay for their own treatment.
Problems facing in vitro fertilisation service in Hong
Kong
Multiple pregnancy
There is an international goal aiming to reduce the
incidence of multiple pregnancy as a result of IVF to <10%. Multiple
pregnancies after IVF create a huge burden on the healthcare system. The
chance of multiple pregnancy increases exponentially with the number of
embryos transferred. Multiple pregnancies are associated with high risk of
prematurity, low birth rate, and tremendous support from neonatal
intensive care unit. It has been estimated that one premature baby at 29
weeks can cost up to US$122 000 and prematurity cost a total of US$4567
billion in United Kingdom in 2006.15
Owing to improvements in embryo culture and cryopreservation techniques,
with the use of vitrification, the cumulative pregnancy rate is similar in
single or double embryo transfer.16
In Kwong Wah Hospital and Queen Mary Hospital, women can have only one
embryo transferred at a time unless they are aged ≥38 years, have failed
two IVF cycles, and have had no live births. Although single embryo
transfer results in a slightly longer interval to pregnancy, it can
significantly lower the multiple pregnancy rate to <2%.16
Cross-border reproductive care
The reasons for Hong Kong residents seeking
cross-border reproductive care are multifactorial. Reasons may include the
long waiting times for publicly funded IVF, the high cost of private IVF,
lack of oocyte donors, and ineligibility for IVF in Hong Kong. Those
ineligible for IVF in Hong Kong include unmarried couples, same-sex
couples, and those seeking IVF for surrogacy, sex selection, or social
reasons. Women seeking IVF overseas face risks including lack of
regulations in some centres and lack of medical care or insurance coverage
in case of complications.
Other problems
Other problems include the risks of ovarian
hyperstimulation syndrome, the high cost of IVF, and difficulty in getting
gamete and embryo donation.
Conclusion
In vitro fertilisation provides hope for infertile
couples. However, there are many unresolved issues, especially the high
rate of multiple pregnancies and potential risks associated with
cross-border reproductive care.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: EHY Ng.
Acquisition of data: MW Lui.
Analysis or interpretation of data: MW Lui.
Drafting of the article: MW Lui.
Critical revision for important intellectual content: WSB Yeung, PC Ho, EHY Ng.
Acquisition of data: MW Lui.
Analysis or interpretation of data: MW Lui.
Drafting of the article: MW Lui.
Critical revision for important intellectual content: WSB Yeung, PC Ho, EHY Ng.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit.
References
1. Organisation for Economic Co-operation
and Development. OECD Family Database. SF2.3.B. Age of mothers at
childbirth and age-specific fertility. Available from:
http://www.oecd.org/els/family/database.htm. Accessed 26 Jul 2019.
2. De Geyter C, Calhaz-Jorge C, Kupka MS,
et al. ART in Europe, 2014: results generated from European registries by
ESHRE: The European IVF-monitoring Consortium (EIM) for the European
Society of Human Reproduction and Embryology (ESHRE). Hum Reprod
2018;33:1586-601. Crossref
3. Chambers GM, Wand H, Macaldowie A,
Chapman MG, Farquhar CM, Bowman M, Molloy, D, Ledger W. Population trends
and live birth rates associated with common ART treatment strategies. Hum
Reprod 2016;31:2632-41. Crossref
4. Babayev SN, Park CW, Bukulmez O.
Intracytoplasmic sperm injection indications: how rigorous? Semin Reprod
Med 2014;32:283-90. Crossref
5. McLernon DJ, Maheshwari A, Lee AJ,
Bhattacharya S. Cumulative live birth rates after one or more complete
cycles of IVF: a population-based study of linked cycle data from 178,898
women. Hum Reprod 2016;31:572-81. Crossref
6. Council on Human Reproductive
Technology. Code of Practice on Reproductive Technology and Embryo
Research. January 2013. Available from:
https://www.chrt.org.hk/english/service/service_cod.html. Accessed 26 Jul
2019.
7. Mao KR, Loong EP, Lee HC, et al. Current
status of in-vitro fertilization (IVF) in Hong Kong. J Hong Kong Med Assoc
1987;39:144-6.
8. Hong Kong IVF Study Group. Assisted
reproduction in Hong Kong: status in the 1990s. Hong Kong Med J
1996;2:253-6.
9. Lehmann L, El-Haddad A, Barr RD. Global
approach to hematologic malignancies. Hematol Oncol Clin North Am
2016;30:417-32. Crossref
10. Cap. 561 Human reproductive technology
ordinance 2000 (Hong Kong SAR). Available from:
https://www.elegislation.gov.hk/hk/cap561. Accessed 26 Jul 2019.
11. Council on Human Reproductive
Technology. Available from: https://www.chrt.org.hk/. Accessed 26 Jul
2019.
12. Maxwell SM, Grifo JA. Should every
embryo undergo preimplantation genetic testing for aneuploidy? A review of
the modern approach to in vitro fertilization. Best Pract Res Clin Obstet
Gynaecol 2018;53:38-47. Crossref
13. Victor AR, Griffin DK, Brake AJ, et
al. Assessment of aneuploidy concordance between clinical trophectoderm
biopsy and blastocyst. Hum Reprod 2019;34:181-92. Crossref
14. Capalbo A, Ubaldi FM, Rienzi L, Scott
R, Treff N. Detecting mosaicism in trophectoderm biopsies: current
challenges and future possibilities. Hum Reprod 2017;32:492-8. Crossref
15. Saha S, Gerdtham UG. Cost of illness
studies on reproductive, maternal, newborn, and child health: a systematic
literature review. Health Econ Rev 2013;3:24. Crossref
16. Pandian Z, Marjoribanks J, Ozturk O,
Serour G, Bhattacharya S. Number of embryos for transfer following in
vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane
Database Syst Rev 2013;(7):CD003416. Crossref

