Hong Kong Med J 2019 Dec;25(6):438–43 | Epub 4 Dec 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Substance abuse effects on urinary tract:
methamphetamine and ketamine
CH Yee, MB, BS, FRCS (Edin)1; CF Ng,
MB, ChB, FRCS (Edin)1; YL Hong, MSc2; PT Lai, BN1;
YH Tam, MB, ChB, FRCS (Edin)2
1 Department of Surgery, SH Ho Urology
Centre, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Surgery, The Chinese
University of Hong Kong, Shatin, Hong Kong
Corresponding author: Dr CH Yee (yeechihang@surgery.cuhk.edu.hk)
Abstract
Introduction: Ketamine is known
to cause urinary tract dysfunction. Recently, methamphetamine (MA) abuse
has become a growing problem in Asia. We investigated the symptomatology
and voiding function in patients who abused MA and ketamine and compared
their urinary tract toxicity profiles.
Methods: In the period of 23
months from 1 October 2016, all consecutive new cases of patients
presenting with MA- or ketamine-related urological disorder were
recruited into a prospective cohort. Polysubstance abuse patients were
excluded. Data were analysed by comparison between patients with
ketamine abuse and MA abuse. Basic demographic data and initial
symptomatology were recorded, and questionnaires on urinary symptoms and
the Montreal Cognitive Assessment (MoCA) were used as assessment tools.
Results: Thirty-eight patients
were included for analysis. There was a statistically significant
difference in mean age between patients with MA and ketamine abuse (27.2
± 7.2 years and 31.6 ± 4.8 years, respectively, P=0.011). Urinary
frequency was the most common urological symptom in our cohort of
patients. There was a significant difference in the
prevalence of dysuria (ketamine 43.5%, MA 6.7%, P=0.026) and a
significant trend in the difference in hesitancy (ketamine 4.3%, MA
26.7%, P=0.069). Overall, questionnaires assessing urinary storage
symptoms and voiding symptoms did not find a statistically significant
difference between the two groups. The MoCA revealed that both groups
had cognitive impairment (ketamine 24.8 ± 2.5, MA 23.6 ± 2.9, P=0.298).
Conclusions: Abuse of MA caused
urinary tract dysfunction, predominantly storage symptoms. Compared with
ketamine abuse, MA abuse was not commonly associated with dysuria or pelvic
pain.
New knowledge added by this study
- Conventionally, methamphetamine has mainly been implicated for its neurological impact. Our study illustrated the impact of methamphetamine on the urinary tract, ie, an increase in storage symptoms.
- Cognitive impairment from ketamine abuse was also documented in our study with a valid assessment.
- Management of methamphetamine and ketamine abuse should involve multiple disciplines to improve the comprehensiveness of assessment and treatment.
Introduction
Both the range of available drugs and the scope of
drug markets are expanding and diversifying. Abuse of substances such as
amphetamine-type stimulants, cannabis, and cocaine are major global health
concerns. According to World Health Organization statistics, the number of
cannabis users increased from 183 million in 2015 to 192 million in 2016
worldwide, whereas 34 million people abuse amphetamines and prescription
stimulants.1
While the spectrum of substance abuse can be wide,
many forms of illicit drug use inevitably induce toxicity and detrimental
effects on the urinary tract. Smoking cannabis was found to have a
significant association with bladder cancer in a hospital-based
case-control study,2 attributed to
the common carcinogens present in cannabis and tobacco smoke.3 Acute renal infarction has been observed in patients
who used cocaine.4 Ketamine has
received particular attention in the past few years for its impacts on
both the upper and lower urinary tract.5
It has been one of the most commonly abused substances by teenagers since
2005 in Asian cities such as Hong Kong.6
In recent years, methamphetamine (MA) abuse has
also become a serious and growing problem in Asia.7 The proportion of people abusing MA increased from
28.8% to 75.1% over a span of 5 years in China.8
Japan has seen its third epidemic of MA abuse since 1995.9 South Korea has also had an increase in psychotropic
drug abuse, predominantly MA, from 7919 people in 2014 to 11 396 in 2016.10 In Hong Kong, 25.9% of people
who abused drugs had exposure to amphetamine-type psychotropic substances
in 2017.11 While the psychological
and neurological effects of MA have been widely discussed, the urological
aspects of the drug’s side-effects have not yet been well documented in
the literature. We investigated the symptomatology and voiding function in
a cohort of patients who abused the two most common psychotropic
substances in our locality, namely MA and ketamine, and compared their
urinary tract toxicity profiles.
Methods
In the period of 23 months from 1 October 2016, all
consecutive new cases of patients who attended our centre for MA- or
ketamine-related urological disorders were seen in a dedicated clinic and
were recruited into a prospective cohort. Ethics committee approval was
granted for the study (CREC Ref CRE-2011.454). Written informed consent
was given by all participants before entering the study.
Basic demographic data were recorded before clinic
attendance, including age, sex, employment status, drinking habits, and
smoking history. Habits of substance abuse were characterised. Serum
creatinine levels, urine microscopy and culture, and uroflowmetry were
measured. Initial symptomatology enquiry included the presence and
characteristics of frequency, urgency, suprapubic pain, haematuria,
hesitancy, intermittency, and incomplete emptying. Functional bladder
capacity was calculated by adding the voided volume to post-void urine
residuals during the uroflowmetry assessment. Urological symptoms were
assessed with the International Prostate Symptom Score (IPSS) or the
Overactive Bladder Symptom Score (OABSS).12
The International Index of Erectile Function (IIEF) was used to assess
sexual function in male respondents who were sexually active in the
preceding 4 weeks. Another component of symptom assessment was the Pelvic
Pain and Urgency/Frequency (PUF) patient symptom scale. The Chinese
version of the PUF symptom scale is a validated assessment tool for
cystitis.13 For cognitive
dysfunction, we employed the Montreal Cognitive Assessment (MoCA) as an
assessment tool. Chu et al14
proved the validity and reliability of the Cantonese Chinese MoCA as a
brief screening tool for cognitive impairment.
Polysubstance abuse patients were excluded. Data
were analysed by comparison between two groups of patients, namely those
with ketamine abuse only and those with MA abuse only. Descriptive
statistics were used to characterise the clinical characteristics of the
study cohort. Chi squared tests were used for categorical data, and
Mann-Whitney U tests were used for continuous data. A P value of
<0.05 was considered to indicate statistical significance. The SPSS
(Windows version 24.0; IBM Corp, Armonk [NY], United States) was used for
all calculations.
Results
From October 2016 to August 2018, 66 new patients
attended our clinic for urological problems secondary to substance abuse.
After excluding patients with substance abuse other than ketamine and MA,
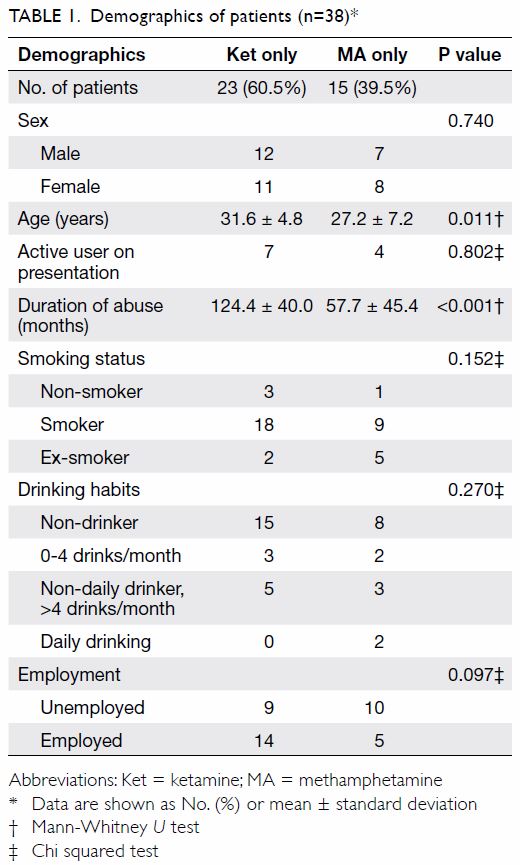
38 patients were included for analysis (Table 1). Both genders contributed 19 patients.
There was a statistically significant difference in mean age between the
two groups of patients with MA and ketamine abuse (27.2 ± 7.2 years and
31.6 ± 4.8 years, respectively, P=0.011). Most patients were not active
substance abusers upon presentation to the clinic. While all patients had
a history of substance abuse, only two (5.3%) patients were consuming
alcohol on a daily basis.
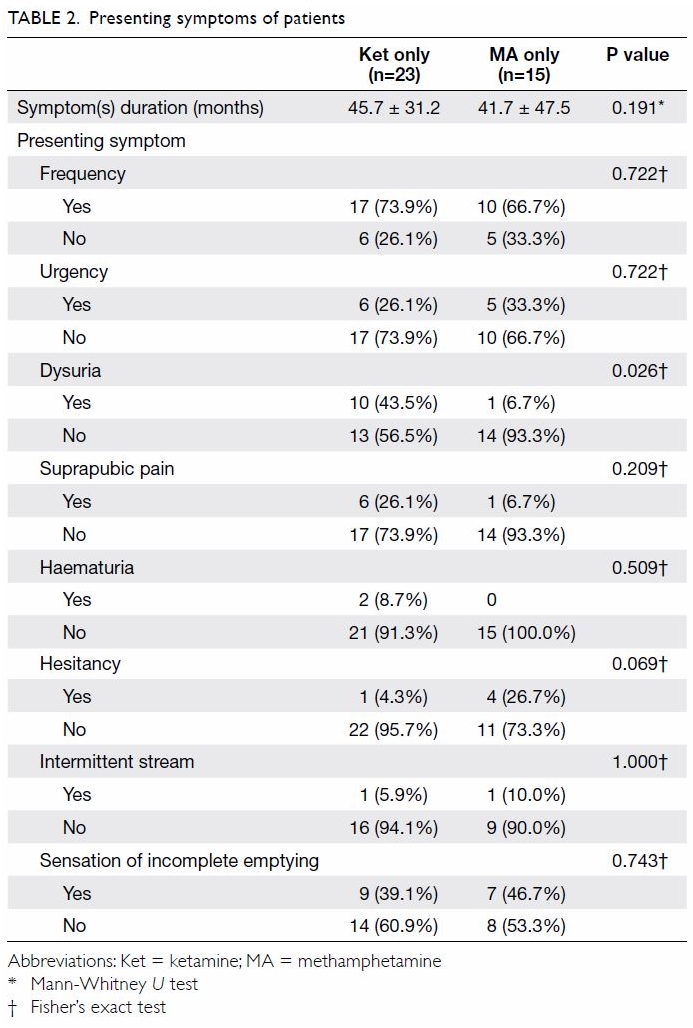
Urinary frequency was the single most common
urological symptom in our patient cohort. Regardless of whether the
patient was consuming ketamine alone, MA alone, or a combination of
ketamine and MA, urinary frequency was found in 71.1% of the patients,
with no statistically significant differences between these groups (Table
2). Other symptoms that shared similar distributions between both
groups were urgency, suprapubic pain, intermittent stream, and sensation
of incomplete emptying. There was a statistically significant difference
in the prevalence of dysuria between the two groups (ketamine 43.5%, MA
6.7%, P=0.026). A trend was observed in the difference in prevalence of
hesitancy (ketamine 4.3%, MA 26.7%, P=0.069).
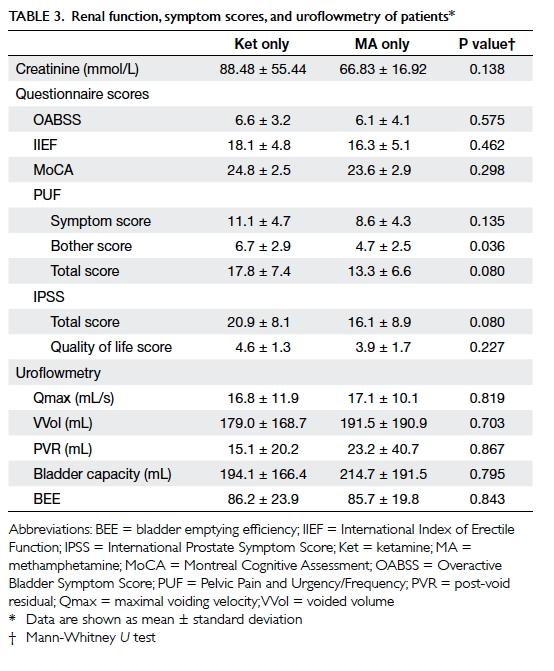
To summarise the results of questionnaires that
assess urinary storage symptoms and voiding symptoms as a whole, neither
OABSS nor IPSS revealed a statistically significant difference between the
two patient groups (Table 3). The mean IPSS score of the ketamine only
group was 20.9 ± 8.1, falling into the severe symptom category, whereas
that of the MA only group was 16.1 ± 8.9, falling into the moderate
symptom group. No significant differences in maximal voiding velocity,
voided volume, post-void residual, or bladder capacity were observed
between the two groups. Similarly, no significant difference was observed
in sexual function between the male patients of these three groups, as
assessed by IIEF.
Pelvic pain assessment with the PUF symptom scale
revealed higher scores in the ketamine group, especially in the Bother
score domain (ketamine 6.7 ± 2.9, MA 4.7 ± 2.5, P=0.036). Cognitive
assessment using MoCA revealed that both groups had impairment, but there
was no significant difference between the MA group and the ketamine group
(ketamine 24.8 ± 2.5, MA 23.6 ± 2.9, P=0.298). Serum creatinine did not
differ significantly between the groups (ketamine 88.48 ± 55.44, MA 66.83
± 16.92, P=0.138).
Discussion
Substance abuse is a significant public health
problem, with approximately 5.2% of the world population aged between 15
and 64 years having used illicit drugs at least once in the previous year.1 Southeast and East Asia have been
a global hub for MA production and trafficking over the past decades, and
its abuse is common in areas of South Korea, China, Taiwan, Japan, the
Golden Triangle, and Iran.10
Psychotropic substance abuse is the most common form of drug abuse in Hong
Kong, and since 2015, MA has taken over ketamine’s spot as the leading
drug of abuse among all psychotropic substances.11
As MA has become the new trendy drug of abuse, and most drug abusers are
young and had their first drug exposure at an early age, this can account
for our finding that patients who used MA had a lower mean age than
patients who used ketamine in the cohort (Table 1).
Methamphetamine belongs to the class of
amphetamines that also includes other drugs such as MDMA
(3,4-methylenedioxy-N-methylamphetamine). The stimulant, euphoric,
anorectic, empathogenic, entactogenic, and hallucinogenic properties of MA
drive its popularity for abuse. Kolbrich et al15
demonstrated the fast, widespread, and long-lasting distribution of MA in
the human brain, paralleling the long-lasting behavioural and neurological
effects of the drug. Our data on cognitive impairment in MA users echoed
this finding, demonstrating impaired function in this group by MoCA
assessment.
Much of the focus on MA in the literature has been
placed on its neurological and behavioural aspects. Unlike ketamine, whose
effects on the urinary tract and treatment modalities have been more
commonly discussed,16 similar
research endeavours have not been undertaken in the area of MA, even
though it is a more widely abused drug. Thus, our study was an effort to
investigate the clinical presentation of MA abuse on the urinary tract and
compare it with ketamine abuse, another common drug of illicit use. As
illustrated by our findings in the cohort, patients who used MA reported
at least moderate severity of urinary symptoms by IPSS assessment. Because
we studied a group of young patients with mean age 27.2 years, we conclude
that the urological impact of MA abuse cannot be neglected.
In the current study, storage symptoms
(particularly urinary frequency) had similar prevalence between patients
who used MA and ketamine (Table 2). On assessment of storage symptoms by
OABSS, patients in both groups attained similar scores to patients with
overactive bladder syndrome.17 In
the case of ketamine abuse, storage symptoms can be attributed to denuded
mucosa and infiltration of inflammatory cells into the lamina propria of
the bladder, eventually leading to chronic inflammation and fibrosis.5 It has been postulated that the storage symptoms from
MA abuse can be the result of a dysfunctional dopamine pathway in detrusor
control. The β-phenylethylamine core structure of MA allows it to cross
the blood-brain barrier easily and to resist brain biotransformation.
Furthermore, its structural similarity with monoamine neurotransmitters
allows amphetamines to act as competitive substrates at dopamine’s
membrane transporters. It also promotes dopamine release from storage
vesicles. All these effects increase cytoplasmic dopamine concentrations
and enhance reverse transport.18
However, long-term exposure to amphetamines may result in dopamine neuron
terminal damage or loss. A post-mortem study of people who used MA
chronically showed a mean 50% to 60% reduction in dopamine levels
throughout the striatum.19 Another
study on dopamine dysregulation reported a 25% to 30% decrease in the
maximal extent of dopamine-induced stimulation of adenylyl cyclase
activity in the striatum.20 These
results suggested that dopamine signalling in the striatum of people who
use MA chronically was impaired both presynaptically and postsynaptically.
An animal study showed that a selective D1 antagonist decreased bladder
capacity in rats.21 Taking
Parkinson’s disease, which is the result of dopaminergic neuron
degeneration with the basal ganglia failing to suppress micturition,22 as a reference, the pathological dopaminergic pathway
could be one of the aetiologies behind MA-related urinary symptoms.
In our cohort, 26.7% of patients who used MA
reported hesitancy during voiding, and 46.7% reported a sensation of
incomplete emptying (Table 2). Case reports in the literature have drawn
an association between amphetamines and urinary retention.23 24 These
findings underlined the possibility that MA-related urological pathology
might have multiple facets rather than purely concerning the dopaminergic
axis and storage symptoms. The possible aetiology may be the increased
release of norepinephrine from MA abuse, which may cause increased
α-adrenergic stimulation of the bladder neck.25
In a study of medical treatment for urinary symptoms in people who used
MA, Koo et al26 reported that
α-blockers resulted in a 33% treatment success rate in terms of IPSS
reduction for patients with predominant voiding symptoms. This observation
could be employed as a reference for treatment options.
Patients with ketamine abuse more commonly
experienced dysuria and pelvic pain. In our previous report of 319
patients with ketamine abuse, the mean PUF score was 22.2.16 In contrast, symptoms of dysuria and pelvic pain were
not commonly observed in patients with MA abuse. This could be because the
effects of MA on the urinary tract were more on neurology rather than
local tissue destruction, resulting in less local nociceptor stimulation.
Currently, there are no reports on histological assessment of urinary
tract tissues in people who use MA. This would be useful to facilitate a
more comprehensive investigation on the impact on MA on the urinary tract.
The relatively small sample size of our cohort
limited our statistical analysis. As MA abuse has only gained popularity
in recent years in our locality, and the urological sequelae of such abuse
might take years before it becomes prominent and severe, the current study
could act as an initial assessment highlighting the early observation of
urological presentation. One of the potential limitations of our study is
that the majority of the patients presenting to our clinic were not active
substance abusers. At the time point of assessment, the use of a
heterogeneous group of active and former abusers may introduce bias into
our evaluation. However, our cohort included mostly patients with long
abuse duration. Studies have demonstrated the persistent effects of drug
abuse even after a period of abstinence, namely dysfunctional dopamine
metabolism in patients who had used MA27
and urinary tract damage in patients who had used ketamine.5 Thus, the clinical picture captured by our study may
still reflect the impact of drug abuse on the urinary tract.
In conclusion, MA is a common drug of choice for
abuse in Asia. It causes urinary tract dysfunction, predominantly in the
form of storage symptoms. Compared with ketamine, MA abuse was not
commonly associated with dysuria or pelvic pain. In addition to the
behavioural impacts of MA abuse, its urinary tract implications should not
be neglected.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: CH Yee, CF Ng, YH Tam.
Acquisition of data: YL Hong, PT Lai.
Analysis or interpretation of data: CH Yee, YL Hong.
Drafting of the article: CH Yee, YL Hong.
Critical revision for important intellectual content: CH Yee, CF Ng, YH Tam.
Acquisition of data: YL Hong, PT Lai.
Analysis or interpretation of data: CH Yee, YL Hong.
Drafting of the article: CH Yee, YL Hong.
Critical revision for important intellectual content: CH Yee, CF Ng, YH Tam.
Conflicts of interest
As an editor of the journal, CF Ng was not involved
in the peer review process of the article. Other authors have no conflicts
of interest to disclose.
Funding/support
This research project was funded by the Beat Drugs
Fund, The Government of the Hong Kong Special Administrative Region.
Ethics approval
Ethics committee approval was granted for the study
(CREC Ref CRE-2011.454). Written informed consent was given by all
participants before entering the study.
References
1. United Nations Office on Drugs and
Crime. World drug report. 2018. Available from:
https://www.unodc.org/wdr2018/. Accessed 10 May 2019.
2. Chacko JA, Heiner JG, Siu W, Macy M,
Terris MK. Association between marijuana use and transitional cell
carcinoma. Urology 2006;67:100-4. Crossref
3. Bowles DW, O’Bryant CL, Camidge DR,
Jimeno A. The intersection between cannabis and cancer in the United
States. Crit Rev Oncol Hematol 2012;83:1-10. Crossref
4. Madhrira MM, Mohan S, Markowitz GS,
Pogue VA, Cheng JT. Acute bilateral renal infarction secondary to
cocaine-induced vasospasm. Kidney Int 2009;76:576-80. Crossref
5. Yee C, Ma WK, Ng CF, Chu SK.
Ketamine-associated uropathy: from presentation to management. Curr
Bladder Dysfunct Rep 2016;11:266-71. Crossref
6. Yiu-Cheung C. Acute and chronic toxicity
pattern in ketamine abusers in Hong Kong. J Med Toxicol 2012;8:267-70. Crossref
7. Ren Q, Ma M, Hashimoto K. Current status
of substance abuse in East Asia and therapeutic prospects. East Asian Arch
Psychiatry 2016;26:45-51.
8. Sun HQ, Bao YP, Zhou SJ, Meng SQ, Lu L.
The new pattern of drug abuse in China. Curr Opin Psychiatry
2014;27:251-5. Crossref
9. Wada K, Funada M, Shimane T. Current
status of substance abuse and HIV infection in Japan. J Food Drug Anal
2013;21:S33-6. Crossref
10. Kwon NJ, Han E. A commentary on the
effects of methamphetamine and the status of methamphetamine abuse among
youths in South Korea, Japan, and China. Forensic Sci Int 2018;286:81-5. Crossref
11. Narcotics Division, Security Bureau,
Hong Kong SAR Government. Central Registry of Drug Abuse Sixty-Seventh
Report. Available from: https://www.nd.gov.hk/en/crda_report.htm. Accessed
10 May 2019.
12. Homma Y, Yoshida M, Seki N, et al.
Symptom assessment tool for overactive bladder syndrome–overactive bladder
symptom score. Urology 2006;68:318-23. Crossref
13. Ng CM, Ma WK, To KC, Yiu MK. The
Chinese version of the Pelvic Pain and Urgency/Frequency Symptom Scale: a
useful assessment tool for street-ketamine abusers with lower urinary
tract symptoms. Hong Kong Med J 2012;18:123-30.
14. Chu LW, Ng KH, Law AC, Lee AM, Kwan F.
Validity of the Cantonese Chinese Montreal Cognitive Assessment in
Southern Chinese. Geriatr Gerontol Int 2015;15:96-103. Crossref
15. Kolbrich EA, Goodwin RS, Gorelick DA,
Hayes RJ, Stein EA, Huestis MA. Plasma pharmacokinetics of
3,4-methylenedioxymethamphetamine after controlled oral administration to
young adults. Ther Drug Monit 2008;30:320-32. Crossref
16. Yee CH, Lai PT, Lee WM, Tam YH, Ng CF.
Clinical outcome of a prospective case series of patients with ketamine
cystitis who underwent standardized treatment protocol. Urology
2015;86:236-43. Crossref
17. Hung MJ, Chou CL, Yen TW, et al.
Development and validation of the Chinese Overactive Bladder Symptom Score
for assessing overactive bladder syndrome in a RESORT study. J Formos Med
Assoc 2013;112:276-82. Crossref
18. Carvalho M, Carmo H, Costa VM, et al.
Toxicity of amphetamines: an update. Arch Toxicol 2012;86:1167-231. Crossref
19. Wilson JM, Kalasinsky KS, Levey AI, et
al. Striatal dopamine nerve terminal markers in human, chronic
methamphetamine users. Nat Med 1996;2:699-703. Crossref
20. Tong J, Ross BM, Schmunk GA, et al.
Decreased striatal dopamine D1 receptor-stimulated adenylyl cyclase
activity in human methamphetamine users. Am J Psychiatry 2003;160:896-903.
Crossref
21. Seki S, Igawa Y, Kaidoh K, Ishizuka O,
Nishizawa O, Andersson KE. Role of dopamine D1 and D2 receptors in the
micturition reflex in conscious rats. Neurourol Urodyn 2001;20:105-13. Crossref
22. McDonald C, Winge K, Burn DJ. Lower
urinary tract symptoms in Parkinson’s disease: prevalence, aetiology and
management. Parkinsonism Relat Disord 2017;35:8-16. Crossref
23. Worsey J, Goble NM, Stott M, Smith PJ.
Bladder outflow obstruction secondary to intravenous amphetamine abuse. Br
J Urol 1989;64:320-1. Crossref
24. Delgado JH, Caruso MJ, Waksman JC,
Honigman B, Stillman D. Acute, transient urinary retention from combined
ecstasy and methamphetamine use. J Emerg Med 2004;26:173-5. Crossref
25. Skeldon SC, Goldenberg SL. Urological
complications of illicit drug use. Nat Rev Urol 2014;11:169-77. Crossref
26. Koo KC, Lee DH, Kim JH, et al.
Prevalence and management of lower urinary tract symptoms in
methamphetamine abusers: an under-recognized clinical identity. J Urol
2014;191:722-6. Crossref
27. Ares-Santos S, Granado N, Moratalla R.
The role of dopamine receptors in the neurotoxicity of methamphetamine. J
Intern Med 2013;273:437-53. Crossref