Hong Kong Med J 2019 Dec;25(6):429–37 | Epub 4 Dec 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Risk of post-contrast acute kidney injury in emergency
department patients with sepsis
YC Hsu, MD1; HY Su, MD1; CK
Sun, MD, PhD1,2; CY Liang, MD3,4; TB Chen, PhD5;
CW Hsu, MD, PhD1,2
1 Department of Emergency Medicine, E-Da
Hospital, I-Shou University, Kaohsiung, Taiwan
2 School of Medicine for International
Students, College of Medicine, I-Shou University, Kaohsiung, Taiwan
3 Department of Emergency Medicine, E-Da
Cancer Hospital, I-Shou University, Kaohsiung, Taiwan
4 Department of Information Engineering,
I-Shou University, Kaohsiung, Taiwan
5 Department of Medical Imaging and
Radiological Sciences, I-Shou University, Kaohsiung, Taiwan
Corresponding author: Dr CW Hsu (saab30002000@gmail.com)
Abstract
Introduction: Although computed
tomography (CT) is a useful tool for exploring occult infection in
patients with sepsis in the emergency department, the potential
nephrotoxicity of contrast media is a major concern. Our study aimed to
investigate the association between use of contrast-enhanced CT and the
risks of acute kidney injury and other adverse outcomes in patients with
sepsis.
Methods: In total, 587 patients
with sepsis who underwent CT scan (enhanced CT group: 105, non-enhanced
CT group: 482) from January 2012 to December 2016 at a tertiary referral
centre were enrolled in this retrospective analysis, and propensity
score matching was performed to minimise the selection bias. The length
of stay, incidences of acute kidney injury and emergent dialysis, and
short-term mortality were compared between the two groups.
Results: Compared with patients
in the non-enhanced CT group, patients in the contrast-enhanced CT group
did not have increased risks of acute kidney injury (odds ratio
[OR]=1.38, 95% confidence interval [CI]=0.55-3.43; P=0.489), emergent
dialysis (OR=1.31, 95% CI=0.47-3.68; P=0.602), or short-term mortality
(OR=0.90, 95% CI=0.48-1.69; P=0.751). In addition, there was no
significant difference in the median length of hospital stay between
survivors in the two groups (20 vs 19 days, P=0.742).
Conclusions: Intravenous
contrast administration during CT scanning was not associated with
prolonged length of hospital stay in patients with sepsis in an
emergency setting. Moreover, the use of contrast-enhanced CT was not
associated with increased risks of acute kidney injury, emergent
dialysis, or short-term mortality.
New knowledge added by this study
- The risks of nephrotoxicity and other adverse outcomes (ie, emergent dialysis, short-term mortality, and increased length of stay) were not increased after intravenous contrast administration during computed tomography scanning of patients with sepsis.
- Renal function improved within 48-72 hours after computed tomography scans, relative to initial measurements in all patients, suggesting that sepsis (not the administration of contrast media) was the primary determinant of clinical outcomes.
- The lack of a significant correlation between the administration of contrast agents and the risk of acute kidney injury in patients with sepsis conflicts with the tendency to withhold contrast-enhanced computed tomography for the diagnostic assessment and management of sepsis in the emergency setting.
- After weighing the benefits and risks of contrast administration, clinicians could utilise contrast-enhanced computed tomography scanning in a reasonable manner in critically ill patients with sepsis, in order to identify occult infection foci earlier and facilitate prompt medical management.
Background
Sepsis is a life-threatening condition that
contributes to nearly 850 000 emergency department (ED) visits annually in
the US.1 According to the practice
guidelines published by the Surviving Sepsis Campaign, a care bundle of
sepsis treatment—including fluid resuscitation, antimicrobial therapy, and
source control—is recommended as life-saving treatment for patients with
sepsis.2 Computed tomography (CT)
scanning is a popular method for identifying the focus of infection and
guiding the implementation of an appropriate antimicrobial strategy in
emergency medical care settings.3
The utilisation of CT scans in the ED has increased considerably, such
that more than 70 million CT scans are performed in the US annually.4 Approximately one in seven patients undergoes a CT scan
during evaluation in the ED.5
Although the use of iodinated contrast media is an
important method for improving the diagnostic accuracy of CT examination,6 there are concerns regarding the
potential for precipitating renal dysfunction, especially in patients who
already have impaired renal function.7
8 The third leading cause of acute
kidney injury (AKI) in hospitalised patients is reported to be
contrast-associated (CA)-AKI9;
CA-AKI is associated with increased risks of major adverse events,
including myocardial infarction, renal failure, and mortality.7 10
Nevertheless, it remains controversial whether an association exists
between intravenous administration of contrast media during CT scans and
the development of CA-AKI.11 12 13 This
controversy exists largely because the introduction of refined iso- or
low-osmolar contrast agents has reduced the risk of AKI14 and because the majority of previous studies on
CA-AKI were performed in patients who underwent coronary angiography,15 16 17 which utilises different dosages and routes of
contrast administration relative to those of conventional
contrast-enhanced CT scans.18
Previous studies on CA-AKI in an emergency setting have been inconclusive.6 7
19 20
21 22
23 24
Although those studies investigated the benefits and risks of contrast
administration in many clinical settings, including acute stroke,
pulmonary embolism, and trauma, very few of them evaluated the impact of
contrast administration on patients with sepsis. Notably, sepsis remains a
leading cause of mortality in critically ill patients2 and CT imaging studies play important roles in both
identifying the source of infection and facilitating infection control in
patients with sepsis. Therefore, the aim of the current study was to investigate
whether intravenous contrast administration in patients with sepsis is
associated with an increased risk of AKI and increased incidences of other
adverse clinical outcomes.
Methods
Study design
This retrospective cohort study was conducted at a
tertiary referral medical centre with approximately 50 000 ED visits per
year. The study population included all adult (age ≥18 years) patients who
visited the ED and underwent CT scans (including brain, chest, abdomen or
extremities) and serial serum creatinine measurements during their initial
ED visits and any follow-ups within 48 to 72 hours from 1 January 2012 to
31 December 2016. Patients with sepsis were identified by principal
diagnosis and serum lactate measurement, in accordance with Sepsis-3
guidelines.25 Patients who
received haemodialysis, underwent contrast-enhanced CT scan within 3
months, or experienced a cardiac arrest event before ED arrival were
excluded from the analysis. This study protocol followed the STROBE
(Strengthening the Reporting of Observational Studies in Epidemiology)
guidelines.
Data collection
Demographic characteristics of the enrolled
patients (ie, age and sex) and clinical information (eg, co-morbidities,
chronic medications, laboratory results, acute illness, types and dosage
of contrast agent, and initial and final diagnoses) were obtained from
written medical charts and electronic medical records. Co-morbidities were
coded based on International Classification of Diseases, Ninth Edition,
Clinical Modification diagnostic codes reported in medical records. In
accordance with World Health Organization criteria, anaemia was defined as
baseline haematocrit values below 39% and below 36% for men and women,
respectively.26 Chronic kidney
disease was defined as a baseline estimated glomerular filtration rate
(eGFR) <60 mL/min/1.73 m2, calculated using the Modification
of Diet in Renal Disease equation.27
Baseline renal function was calculated according to each patient’s serum
creatinine level at 24 hours before the CT scan. The presence of shock was
identified by the need for vasopressors to maintain haemodynamic stability
despite adequate fluid administration during ED stay.
Outcome measures
We divided the eligible patients for this study
into two groups: contrast-enhanced CT and non-enhanced CT; primary and
secondary outcomes were recorded and compared between groups. The primary
outcome was the incidence of AKI, which was defined as an absolute
increase of 0.5 mg/dL or >50% increase in baseline serum creatinine
concentration within 48 to 72 hours after CT scan.28 The secondary outcomes included the incidences of
emergent dialysis (defined as initiation of dialysis during the hospital
stay) and short-term mortality (defined as death within 30 days after CT
scan), as well as the difference in length of hospital stay for survivors.
Sample size estimation
The estimation of sample size was performed with
PASS 11 software in accordance with the results of previous studies
regarding AKI incidence in patients with sepsis29
and odds ratio (OR) of CA-AKI.30
With a 30% incidence of AKI in patients with sepsis and an OR of 2.7 for
CA-AKI, we determined that 109 patients were needed to detect a
significant association with probability (power) of 0.8 and Type 1 error
of 0.05.
Statistical analysis
Data are presented as means±standard deviations or
medians with 25th to 75th percentiles (ie, interquartile range) for
continuous variables, and as numbers (%) for categorical variables.
Two-sample t tests and Chi squared tests were used to compare
continuous and categorical variables, respectively. A two-tailed P value
of <0.05 was considered statistically significant. Propensity score
matching was performed to reduce potential selection bias and other
confounding factors. We calculated the propensity score for each patient
by modelling the probability of receiving contrast medium. Variables in
the model were composed of factors that influence outcomes related to
renal function or influence the selection of contrast medium. We used
total 21 variables including age, sex, co-morbidities (ie, diabetes
mellitus, hypertension, liver cirrhosis, coronary artery disease, left
heart failure, chronic kidney disease, anaemia, chronic obstructive
pulmonary disease, dyslipidaemia, and malignancy), nephrotoxic medications
(ie, statins, non-steroidal anti-inflammatory drugs,
angiotensin-converting enzyme inhibitors or angiotensin II receptor
blockers, nephrotoxic antibiotics such as aminoglycosides and vancomycin),
laboratory data (ie, initial serum creatinine, eGFR, and serum lactate),
measures of illness severity (ie, initial presence of septic shock and
need for intensive care unit [ICU] admission) to calculate the propensity
scores for all patients. A multivariable logistic regression analysis
model using nearest-neighbour matching, calliper 0.1, was generated to
predict the probability of receiving contrast medium. We used the
resulting propensity scores to match the contrast-enhanced CT group
members with non-enhanced CT group members at a ratio of 1:1. Patients
without a corresponding match were excluded. All statistical analyses were
performed using SPSS (Windows version 22.0; IBM Corp, Armonk [NY], US).
Results
Study population and contrast agents
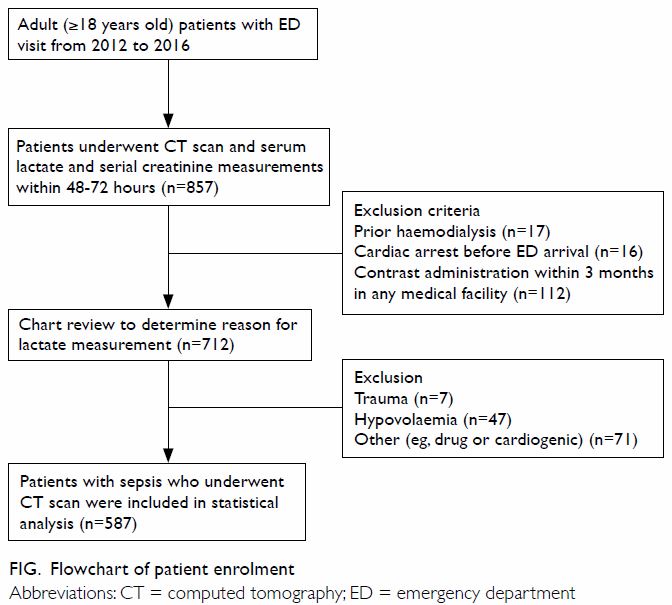
During the study period, 200 427 adult patients
visited the ED; of these, 712 met the criteria for inclusion in this
study. After further exclusion of patients with elevated serum lactate
levels from shock with non-septic aetiology, the remaining 587 patients
(enhanced CT group: 105; non-enhanced CT group: 482) were analysed (Fig).
In the contrast-enhanced CT group, 23 patients received intravenous
iopromide (Ultravist 370; Bayer Parma AG, Berlin, Germany) and 82 patients
received intravenous iohexol (Omnipaque; Bayer Parma AG, Berlin, Germany).
Only one patient received a contrast volume >100 mL (120 mL).
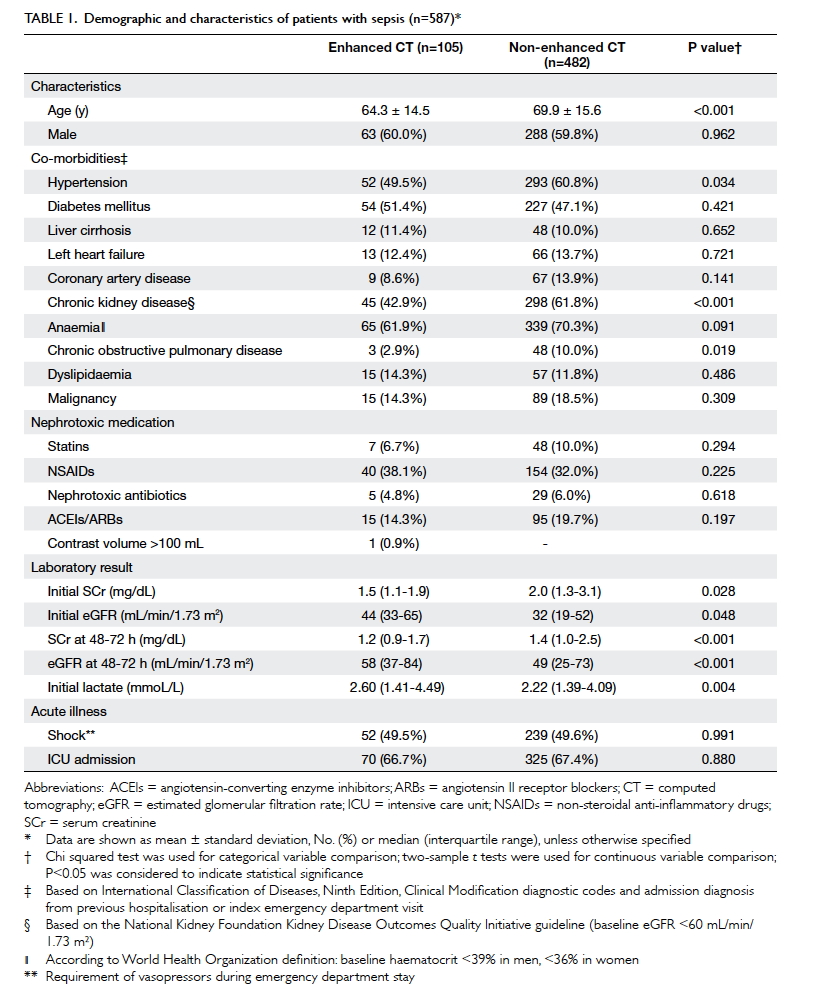
Prior to propensity score matching, patients in the
contrast-enhanced CT group were significantly younger; moreover, they had
lower prevalences of hypertension, chronic kidney disease, and chronic
obstructive pulmonary disease, compared to patients in the non-enhanced CT
group. Patients in the enhanced CT group also had significantly lower
initial and follow-up serum creatinine levels, and had higher initial
serum lactate levels than those in the non-enhanced CT group. There were
no significant differences in the incidences of shock and ICU admission
between the two groups (Table 1).
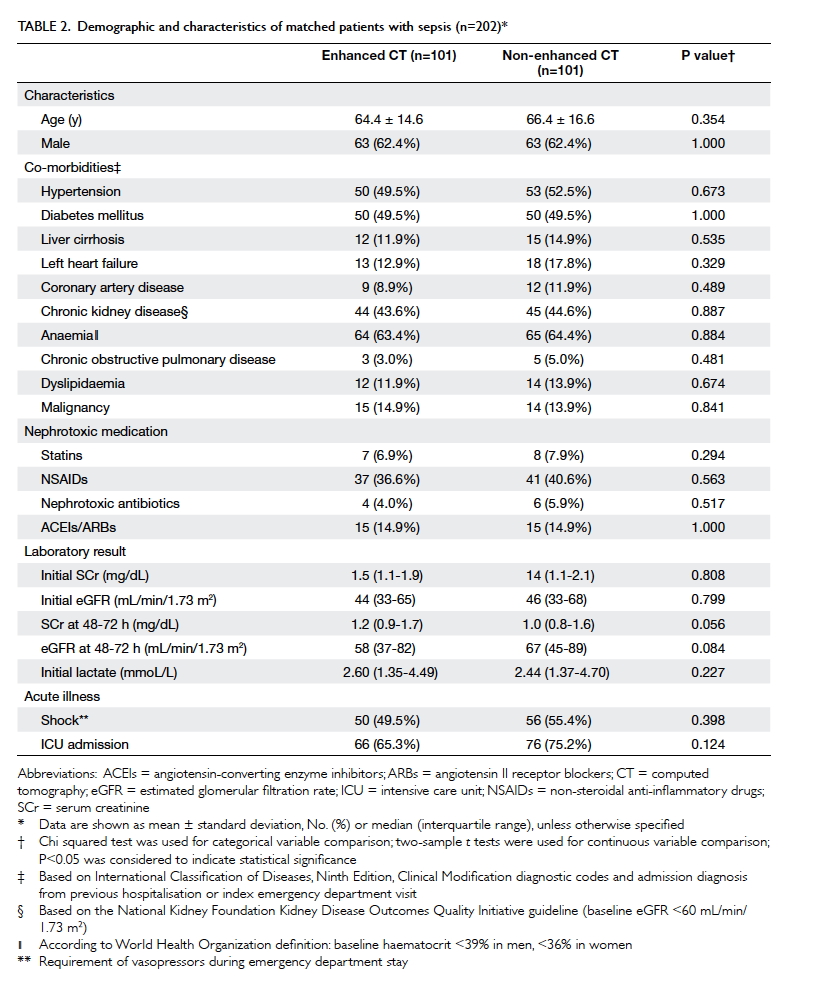
By using propensity score with 1:1 matching, 101
patients with sepsis in the contrast-enhanced CT group were successfully
paired with an equal number of patients in the non-enhanced CT group.
After matching, there were no statistically significant differences
between the two groups in any covariates (Table 2).
Treatment outcomes
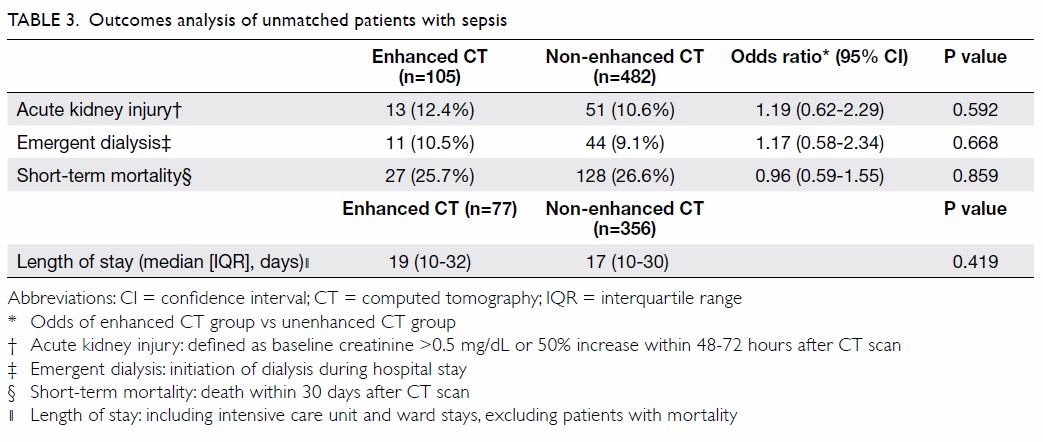
Before propensity score matching, the risks of AKI,
emergent dialysis, and short-term mortality were not significantly greater
in the contrast-enhanced CT group than in the non-enhanced CT group. Five
of 44 patients with sepsis in the non-enhanced CT group who received
emergency haemodialysis subsequently required chronic dialysis; however,
no patients required chronic dialysis in the contrast-enhanced CT group.
Furthermore, there was no significant difference in the length of hospital
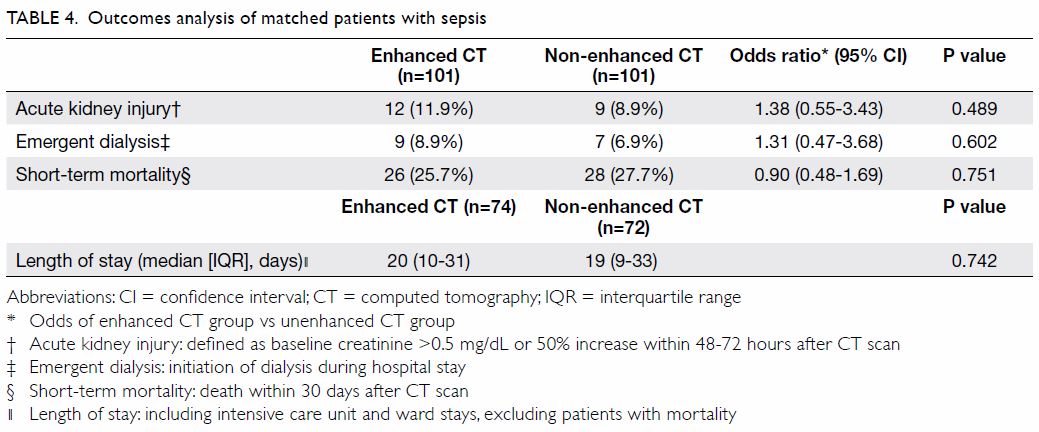
stay between the two groups (Table 3). The same results were observed after
propensity score matching: there were no notable differences in the risks
of AKI, emergent dialysis, or short-term mortality; the median length of
hospital stay was also similar between the matched contrast-enhanced and
non-enhanced CT groups (Table 4).
Discussion
In this ED-based single-centre retrospective study,
we performed a subgroup analysis to investigate the possible adverse
clinical impacts of contrast agent administration in patients with sepsis.
By using propensity score matching, we demonstrated that intravenous
administration of contrast media in patients with sepsis was not
associated with increased risks of AKI or other adverse outcomes,
following contrast-enhanced CT scans to identify foci of infection. During
revision of this manuscript, Hinson et al31
reported a retrospective cohort study; they concluded that contrast medium
administration was not associated with increased incidence of AKI in
patients with sepsis, consistent with our findings. Compared with the
study by Hinson et al, the patients in our study had more severe sepsis
(ie, higher incidences of shock and ICU admission); moreover, our findings
revealed that administration of reasonable volumes of contrast medium did
not increase the risks of emergency dialysis or short-term mortality.
Thus, clinicians can use contrast-enhanced CT scans in a reasonable manner
in septic patients, in order to identify occult infection foci earlier and
facilitate prompt medical management.
Our patients had surprisingly high prevalences of
hypertension, diabetes mellitus, and chronic kidney disease, which could
have been related to their older age, as reported in a prior study.32 Before propensity score matching, patients in the
contrast-enhanced CT group were significantly younger and had fewer
co-morbidities, including hypertension, chronic kidney disease, and
chronic obstructive pulmonary disease. Moreover, patients in the
contrast-enhanced CT group had lower initial serum creatinine levels and
higher eGFRs, as observed in other studies.6
32 This could be related to the
common clinical practice of using contrast-enhanced CT for younger
patients with few co-morbidities and relatively good renal function, based
on considerations of the potential nephrotoxicities of the contrast agents7; a few patients with poor renal
function (24 of 482 patients with sepsis in the non-enhanced CT group) may
also have avoided contrast agents following an explanation of the
potential for nephrotoxicity. Clinicians may have hesitated to administer
contrast media to patients with respiratory disease because of the risk of
immediate hypersensitivity reaction; however, asthma and chronic
obstructive pulmonary disease have not been established as consistent risk
factors for contrast media-related adverse drug reactions.33 The lack of significant differences in risks of AKI,
emergent dialysis, and short-term mortality between the non-enhanced and
enhanced CT groups before propensity score matching in our study may have
been influenced by the above-mentioned tendency for clinicians to perform
contrast-enhanced CT in presumably healthier patients. However, it is
difficult to evaluate the causal relationship between administration of
contrast agents and risk of AKI in patients with sepsis by comparing two
patient groups with many different demographic and characteristics;
therefore, we used propensity score matching to minimise the impacts of
potential confounders.
Although the mean initial serum lactate level in
the contrast-enhanced CT group was slightly but significantly higher than
that in the non-enhanced CT group, this difference was not correlated with
the incidences of acute illness (eg, shock), ICU admission, and short-term
mortality between the two groups. In addition, the levels of renal
function, reflected by serum creatinine levels and eGFRs within 48 to 72
hours after CT scans, improved relative to initial measurements in both
groups. These seemingly paradoxical findings suggested that sepsis, rather
than the administration of contrast media, was the determinant of clinical
outcomes in the present study.
Previous studies in emergency medical settings have
shown wide variation in the incidence of post-contrast AKI (3.2%-12%);
this may be partially explained by the variety of diseases encountered in
the ED, as well as differences in the definitions of AKI adopted in each
study.6 7
19 20
21 22
23 24
31 Nearly half of the patients
(49%) in the present study experienced septic shock; thus, the increased
incidence of post-contrast AKI in our patients (12.4%), compared with that
observed in prior studies, may be attributed to the impaired physical
status of our patients. This may also explain the considerably higher
rates of emergent dialysis and short-term mortality, as well as the
increased median length of hospital stay for survivors among our patients,
compared to those parameters measured in other studies that did not focus
on patients with sepsis.21 34
Thus far, the pathophysiology of CA-AKI remains
poorly characterised. Based on the results of some animal studies,
proposed mechanisms include acute tubular necrosis caused by medullary
hypoxia from vasoconstriction, as well as direct cytotoxic effects of the
contrast agent on renal tubular cells.35
36 Compared with AKI caused by
other aetiologies, CA-AKI involves relatively rapid recovery of renal
function; this is potentially because of the reduced extent of tubular
necrosis, which leads to minor and transient functional impairment of
tubular epithelial cells.37
Nevertheless, sepsis is the leading cause of AKI in critically ill
patients and is associated with a higher mortality rate among patients in
the ICU, compared with patients who have AKI caused by other aetiologies.38 Therefore, hesitation to perform
contrast-enhanced CT scans for patients with sepsis, in order to identify
occult infection foci, could result in delayed diagnoses of
life-threatening conditions that carry considerable risks of morbidity and
mortality, even in patients with serum creatinine up to 4.0 mg/dL.6
A number of studies performed in the past several
years have been designed to maintain a balance between the benefits and
adverse effects of contrast-enhanced CT scans in many clinical settings.6 7
12 20
21 22
The vast majority of those studies showed no significant association
between the use of contrast agents and an increased risk of AKI.
Consistent with the prior findings, contrast-enhanced CT scans of our
patients with sepsis were not associated with increased risks of AKI and
other adverse clinical outcomes. Among all aetiologies of AKI in patients
requiring emergent medical attention, such as sepsis, dehydration, and
nephrotoxic medication use,18 the
contribution of CA-AKI is regarded as considerably less important37; notably, our findings support this view.
Furthermore, it has been consistently shown that the performance of a
contrast-enhanced CT scan is justified in patients for whom the
examination is indicated, provided that other risk factors of AKI are well
controlled.39
There are several limitations in our study,
largely in relation to its single-centre and retrospective design. First,
the non-enhanced CT group consisted of older patients with a higher
prevalence of hypertension and worse renal function; this suggested a
selection bias. Although we routinely checked serum lactate for patients
with suspected sepsis in the ED, there were a few patients diagnosed with
sepsis who did not have lactate measurement data; this may also have
resulted in selection bias. Second, although propensity score matching was
used to minimise the impacts of potential confounders, unmeasured
confounding variables remained, leading to potentially biased results.
Therefore, further large-scale cohort or well-controlled prospective
randomised studies are warranted. Finally, the definition of AKI used in
this study (elevation of serum creatinine concentration by 0.5 mg/dL or by
50% increase relative to baseline within 48 to 72 hours after contrast
administration) may not accurately reflect the clinical condition because
the relationship between increases in serum creatinine level and
deterioration of renal function is reportedly non-linear.40
Conclusion
Our study demonstrated that the intravenous
administration of contrast media during CT scans was not associated with
increased risks of AKI, emergent dialysis, or short-term mortality for
patients with sepsis in the ED; moreover, the use of contrast-enhanced CT
was not associated with prolonged length of hospital stay in these
patients. The lack of a significant correlation between the administration
of contrast agents and the risk of AKI in patients with sepsis conflicts
with the tendency to withhold contrast-enhanced CT for the diagnostic
assessment and management of sepsis in the emergency setting. Further
studies are necessary to confirm these findings and provide further
guidance for clinical practice.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: YC Hsu.
Acquisition of data: HY Su, CW Hsu, CY Liang.
Analysis or interpretation of data: TB Chen.
Drafting of the article: YC Hsu.
Critical revision for important intellectual content: CK Sun, CW Hsu.
Acquisition of data: HY Su, CW Hsu, CY Liang.
Analysis or interpretation of data: TB Chen.
Drafting of the article: YC Hsu.
Critical revision for important intellectual content: CK Sun, CW Hsu.
Acknowledgement
Dr Chi-feng Hsieh is acknowledged for providing
technical support in sample size calculation.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Institutional Review
Board of E-Da hospital (EMRP-106-037) and the requirement for informed
patient consent was waived because of the retrospective observational
nature of the study.
References
1. Wang HE, Jones AR, Donnelly JP. Revised
national estimates of emergency department visits for sepsis in the United
States. Crit Care Med 2017;45:1443-9. Crossref
2. Rhodes A, Evans LE, Alhazzani W, et al.
Surviving Sepsis Campaign: international guidelines for management of
sepsis and septic shock: 2016. Intensive Care Med 2017;43:304-77. Crossref
3. Jimenez MF, Marshall JC; International
Sepsis Forum. Source control in the management of sepsis. Intensive Care
Med 2001;27 Suppl 1:S49-62. Crossref
4. Berrington de González A, Mahesh M, Kim
KP, et al. Projected cancer risks from computed tomographic scans
performed in the United States in 2007. Arch Intern Med 2009;169:2071-7. Crossref
5. Kocher KE, Meurer WJ, Fazel R, Scott PA,
Krumholz HM, Nallamothu BK. National trends in use of computed tomography
in the emergency department. Ann Emerg Med 2011;58:452-62.e3. Crossref
6. Hinson JS, Ehmann MR, Fine DM, et al.
Risk of acute kidney injury after intravenous contrast media
administration. Ann Emerg Med 2017;69:577-86.e4. Crossref
7. Mitchell AM, Kline JA, Jones AE, Tumlin
JA. Major adverse events one year after acute kidney injury after
contrast-enhanced computed tomography. Ann Emerg Med 2015;66:267-74.e4. Crossref
8. McCullough PA, Adam A, Becker CR, et al.
Epidemiology and prognostic implications of contrast-induced nephropathy.
Am J Cardiol 2006;98:5K-13K. Crossref
9. Nash K, Hafeez A, Hou S.
Hospital-acquired renal insufficiency. Am J Kidney Dis 2002;39:930-6. Crossref
10. Coca SG, Peixoto AJ, Garg AX, Krumholz
HM, Parikh CR. The prognostic importance of a small acute decrement in
kidney function in hospitalized patients: a systematic review and
meta-analysis. Am J Kidney Dis 2007;50:712-20. Crossref
11. Katzberg RW, Newhouse JH. Intravenous
contrast medium–induced nephrotoxicity: is the medical risk really as
great as we have come to believe? Radiology 2010;256:21-8. Crossref
12. McDonald JS, McDonald RJ, Comin J, et
al. Frequency of acute kidney injury following intravenous contrast medium
administration: a systematic review and meta-analysis. Radiology
2013;267:119-28. Crossref
13. Kashani K, Levin A, Schetz M.
Contrast-associated acute kidney injury is a myth: We are not sure.
Intensive Care Med 2018;44:110-4. Crossref
14. Lameire N, Kellum JA; KDIGO AKI
Guideline Work Group. Contrast-induced acute kidney injury and renal
support for acute kidney injury: a KDIGO summary (Part 2). Crit Care
2013;17:205. Crossref
15. Mehran R, Aymong ED, Nikolsky E, et
al. A simple risk score for prediction of contrast-induced nephropathy
after percutaneous coronary intervention: development and initial
validation. J Am Coll Cardiol 2004;44:1393-9. Crossref
16. Mehran R, Nikolsky E. Contrast-induced
nephropathy: definition, epidemiology, and patients at risk. Kidney Int
Suppl 2006;(100):S11-5. Crossref
17. Rihal CS, Textor SC, Grill DE, et al.
Incidence and prognostic importance of acute renal failure after
percutaneous coronary intervention. Circulation 2002;105:2259-64. Crossref
18. Aycock RD, Westafer LM, Boxen JL,
Majlesi N, Schoenfeld EM, Bannuru RR. Acute kidney injury after computed
tomography: a meta-analysis. Ann Emerg Med 2018;71:44- 53.e4. Crossref
19. Mitchell AM, Kline JA. Contrast
nephropathy following computed tomography angiography of the chest for
pulmonary embolism in the emergency department. J Thromb Haemost
2007;5:50-4. Crossref
20. Sonhaye L, Kolou B, Tchaou M, et al.
Intravenous contrast medium administration for computed tomography scan in
emergency: a possible cause of contrast-induced nephropathy. Radiol Res
Pract 2015;2015:805786. Crossref
21. Heller M, Krieger P, Finefrock D,
Nguyen T, Akhtar S. Contrast CT scans in the emergency department do not
increase risk of adverse renal outcomes. West J Emerg Med 2016;17:404-8. Crossref
22. Ehrlich ME, Turner HL, Currie LJ,
Wintermark M, Worrall BB, Southerland AM. Safety of computed tomographic
angiography in the evaluation of patients with acute stroke: a
single-center experience. Stroke 2016;47:2045-50. Crossref
23. Lima FO, Lev MH, Levy RA, et al.
Functional contrast-enhanced CT for evaluation of acute ischemic stroke
does not increase the risk of contrast-induced nephropathy. AJNR Am J
Neuroradiol 2010;31:817-21. Crossref
24. Tremblay LN, Tien H, Hamilton P, et
al. Risk and benefit of intravenous contrast in trauma patients with an
elevated serum creatinine. J Trauma 2005;59:1162-6. Crossref
25. Singer M, Deutschman CS, Seymour CW,
et al. The third international consensus definitions for sepsis and septic
shock (Sepsis-3). JAMA 2016;315:801-10. Crossref
26. World Health Organ Tech Rep Ser.
Nutritional anaemias: report of a WHO scientific group. World Health Organ
Tech Rep Ser 1968;405:5-37.
27. National Kidney Foundation. K/DOQI
clinical practice guidelines for chronic kidney disease: evaluation,
classification, and stratification. Am J Kidney Dis 2002;39:S1-266.
28. Mehta RL, Kellum JA, Shah SV, et al.
Acute Kidney Injury Network: report of an initiative to improve outcomes
in acute kidney injury. Crit Care 2007;11:R31. Crossref
29. Bagshaw SM, George C, Bellomo R;
ANZICS Database Management Committee. Early acute kidney injury and
sepsis: a multicentre evaluation. Crit Care 2008;12:R47. Crossref
30. Song W, Zhang T, Pu J, Shen L, He B.
Incidence and risk of developing contrast-induced acute kidney injury
following intravascular contrast administration in elderly patients. Clin
Interv Aging 2014;9:85-93. Crossref
31. Hinson JS, Al Jalbout N, Ehmann MR,
Klein EY. Acute kidney injury following contrast media administration in
the septic patient: A retrospective propensity-matched analysis. J Crit
Care 2019;51:111-6. Crossref
32. McDonald, JS, McDonald RJ, Williamson
EE, Kallmes DF, Kashani K. Post-contrast acute kidney injury in intensive
care unit patients: a propensity score-adjusted study. Intensive Care Med
2017;43:774-84. Crossref
33. Bettmann MA, Heeren T, Greenfield A,
Goudey C. Adverse events with radiographic contrast agents: results of the
SCVIR Contrast Agent Registry. Radiology 1997;203:611-20. Crossref
34. McDonald RJ, McDonald JS, Carter RE,
et al. Intravenous contrast material exposure is not an independent risk
factor for dialysis or mortality. Radiology 2014;273:714-25. Crossref
35. Persson PB, Hansell P, Liss P.
Pathophysiology of contrast medium-induced nephropathy. Kidney Int
2005;68:14-22. Crossref
36. Heyman SN, Rosenberger C, Rosen S.
Regional alterations in renal haemodynamics and oxygenation: a role in
contrast medium-induced nephropathy. Nephrol Dial Transplant 2005;20 Suppl
1:i6-11. Crossref
37. Molitoris BA, Dahl R, Geerdes A.
Cytoskeleton disruption and apical redistribution of proximal tubule
Na(+)-K(+)-ATPase during ischemia. Am J Physiol 1992;263:F488-95. Crossref
38. Bellomo R, Kellum JA, Ronco C, et al.
Acute kidney injury in sepsis. Intensive Care Med 2017;43:816-28. Crossref
39. Petek BJ, Bravo PE, Kim F, et al.
Incidence and risk factors for postcontrast acute kidney injury in
survivors of sudden cardiac arrest. Ann Emerg Med 2016;67:469-76.e1. Crossref
40. Ostermann M, Joannidis M. Acute kidney
injury 2016: diagnosis and diagnostic workup. Crit Care 2016;20:299. Crossref