© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Type A aortic dissection involving the superior
mesenteric artery with peripheral malperfusion managed with a hybrid
approach: a case report
Mina Cheng, FRCS, FHKAM (Surgery); KY Lee, FRCS,
FHKAM (Surgery)
Department of Surgery, Queen Elizabeth Hospital,
Jordan, Hong Kong
Corresponding author: Dr Mina Cheng (minacheng0505@gmail.com)
Case report
A 62-year-old man presented to Queen Elizabeth
Hospital in November 2015 with sudden-onset chest pain radiating to his
back and left lower limb numbness for 3 hours. He had previously suffered
a stroke from which he had made a good recovery and could walked unaided.
There was decreased sensation of the entire left lower limb that was
cooler than the right. All pulses over the left lower limb were absent but
motor power was intact. He did not complain of abdominal pain and his
abdomen was neither distended nor tender. He was haemodynamically stable,
and auscultation revealed normal heart sounds.
Contrast-enhanced computed tomography (CT) scan
revealed a type A dissection from the aortic root down to the left
external iliac artery. No haemopericardium or pericardial effusion was
noted. Celiac artery and superior mesenteric artery (SMA) originated from
both a true and false lumen. The origin and proximal segment of the SMA
and celiac artery were compressed by the thrombosed false lumen (Fig
1). Bowel wall was well enhanced. There was cranial extension of the
dissection into the right common and internal carotid arteries. The
cerebral arteries were still opacified. Computed tomography brain showed
early ischaemic change in the right cerebellar hemisphere.
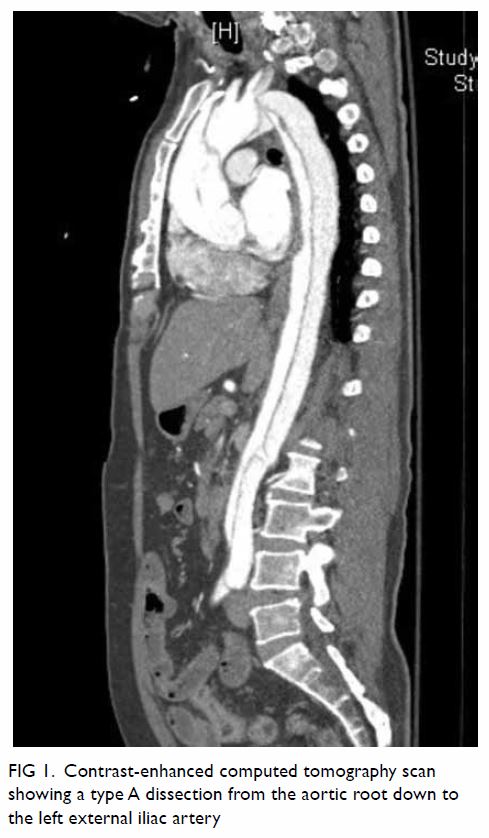
Figure 1. Contrast-enhanced computed tomography scan showing a type A dissection from the aortic root down to the left external iliac artery
Arterial blood gas analysis and serum lactate level
were normal. Transthoracic echocardiography detected an intimal flap at
the aortic root. Mild aortic regurgitation was present. Left ventricular
function was good.
The patient had a type A aortic dissection with
left lower limb acute ischaemia. Emergency interpositional ascending
aortic grafting was performed to close the entry site of the ascending
aorta dissection and to re-expand the true lumen. Postoperatively, the
patient was haemodynamically stable and all left lower limb pulses
reappeared.
A new CT scan of the abdomen revealed improved
perfusion to the celiac artery. Nonetheless, the SMA remained severely
stenotic with only a thin line of contrast enhancement evident in the true
lumen. Enhancement of the jejunal wall was decreased. The SMA had both
restricted inflow at the orifice and outflow due to compression by the
thrombosed false lumen.
Emergency endovascular stenting and open
fenestration of the SMA was performed in the hybrid operation theatre. A
5-French arterial sheath was inserted into the right common femoral
artery. The true lumen orifice of the SMA was cannulated with a 5-French
Yashiro Glidecath catheter (Terumo, Japan) and a 0.035-inch hydrophilic
guidewire (Terumo, Japan). The 0.035-inch hydrophilic guidewire (Terumo,
Japan) was exchanged for a Rosen guidewire (Cordis, US). The 5-French
arterial sheath was exchanged for an 8-French Arrow-Flex guiding sheath
(Arrow International, US). A 10-mm × 12-mm Genesis balloon expandable
stent (Cordis, US) was deployed at the SMA orifice. Arteriogram showed
improvement of flow at the SMA orifice only (Fig 2). Laparotomy was performed. The small and
large bowel were not infarcted but lacked peristalsis. The SMA distal to
the middle colic artery was bluish in appearance due to thrombosis.
Longitudinal arteriotomy was made. All the thrombus in the false lumen was
removed. Longitudinal fenestration was made at the dissection flap. The
true lumen circulation was re-established with thrombectomy using a
3-French Fogarty catheter. The arteriotomy was closed with a saphenous
vein patch. The small bowel appeared healthy after revascularisation.
On-table duplex ultrasonography revealed good flow along the SMA.
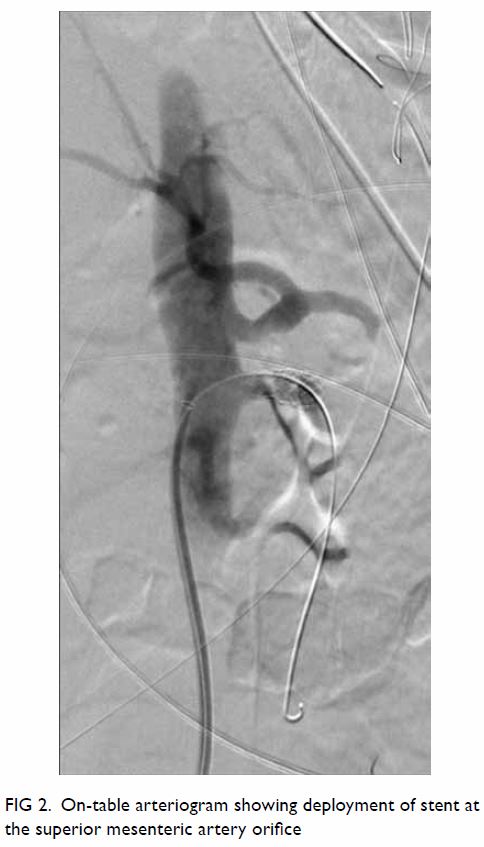
Figure 2. On-table arteriogram showing deployment of stent at the superior mesenteric artery orifice
The patient was transferred to the intensive care
unit and extubated 4 days after surgery. He had no neurological deficits
or abdominal symptoms. All distal pulses were normal. He recovered well
and was discharged from hospital on aspirin 42 days after admission.
Serial follow-up CT scans after the operation
showed a patent SMA and celiac artery. The patient remained asymptomatic
at a follow-up examination 2 years later.
Discussion
Acute dissection is one of the most lethal surgical
emergencies of the aorta. It results from a tear in the aortic wall intima
that extends into the media to create a false lumen and a dissection flap.
It is categorised according to the Stanford classification. Visceral
malperfusion occurs in 16% to 33% of cases1
due to anterograde propagation of the dissection from the ascending aorta
to the level of the aortic visceral branches. This leads to mesenteric
ischaemia, organ dysfunction, and systemic metabolic abnormalities.
Although the results of surgical treatment for acute type A aortic
dissection have improved due to technical advances, mortality remains as
high as 89% in the presence of visceral ischaemia.2 It has been suggested that unless pericardial tamponade
is present, restoration of visceral perfusion by endovascular techniques
should take precedence, especially in cases with a high degree of
mesenteric ischaemia and metabolic disturbance.3
This can improve metabolic status and reduce the intra-operative risk of
subsequent dissection repair. However, the extent of intestinal
malperfusion is difficult to assess since clinical signs typically present
late: 75% of patients have no clinical evidence at presentation, as in our
patient. This can delay diagnosis and management contributing to the high
mortality. The use of biomarkers such as serum lactate has been suggested
as potentially useful indicators of mesenteric ischaemia.4 5 If the
initial lactate level is high with no other cause and radiological or
clinical evidence of bowel ischaemia is present, revascularisation using
percutaneous endovascular techniques should be performed first to
alleviate intestinal ischaemia, followed by serial measurements of lactate
level to look for improvement in peripheral perfusion.3 If the lactate
level is persistently high, surgical revascularisation must be considered.
Our patient had no clinical or biochemical signs
suggestive of bowel ischaemia. Therefore, interpositional ascending aortic
grafting was performed first, since proximal extension of the dissection
would be dangerous and entry site closure may improve blood flow along the
SMA. The left lower limb pulses reappeared after surgery. However, CT did
not show similar improvement in the SMA; on the contrary, the jejunum
showed decreased enhancement. Superior mesenteric artery revascularisation
was indicated. An anterograde approach was adopted for endovascular
stenting of the SMA orifice, followed by open fenestration and closure
with vein patch distally. No bowel resection was required. The successful
outcome in this patient demonstrates good treatment prioritisation, prompt
decision making, and multidisciplinary cooperation in the management of
type A aortic dissection with peripheral malperfusion.
Author contributions
The authors have made substantial contributions to
the concept or design of this study, acquisition of data, analysis or
interpretation of data, drafting of the article, and critical revision for
important intellectual content. The authors had full access to the data,
contributed to the study, approved the final version for publication, and
takes responsibility for its accuracy and integrity.
Conflicts of interest
The authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the
Declaration of Helsinki. The patient provided informed consent for all
procedures.
References
1. Okita Y, Takamoto S, Ando M, Morota T,
Kawashima Y. Surgical strategies in managing organ malperfusion as a
complication of aortic dissection. Eur J Cardiothorac Surg 1995;9:242-6. Crossref
2. Girardi LN, Krieger KH, Lee LY, Mack CA,
Tortolani AJ, Isom OW. Management strategies for type A dissection
complicated by peripheral vascular malperfusion. Ann Thorac Surg
2004;77:1309-14. Crossref
3. Slonim SM, Nyman U, Semba CP, Miller DC,
Mitchell RS, Dake MD. Aortic dissection: percutaneous management of
ischemic complications with endovascular stents and balloon fenestration.
J Vasc Surg 1996;23:241-51. Crossref
4. Muraki S, Fukada J, Morishita K,
Kawaharada N, Abe T. Acute type A aortic dissection with intestinal
ischemia predicted by serum lactate elevation. Ann Thorac Cardiovasc Surg
2003;9:79-80.
5. Murray MJ, Gonze MD, Nowak LR, Cobb CF.
Serum D(-)-lactate levels as an aid to diagnosing acute intestinal
ischemia. Am J Surg 1994;167:575-8. Crossref

