Hong
Kong Med J 2019 Oct;25(5):382–91 | Epub 9 Oct 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Piloting a partially self-financed mode of human
immunodeficiency virus pre-exposure prophylaxis delivery for men who have
sex with men in Hong Kong
SS Lee, MD, FRCP1; TH Kwan, BSc2; NS Wong, PhD1;
Krystal CK Lee, MB, BS, FHKAM (Community Medicine)1,3; Denise PC Chan, PhD1; Teddy TN Lam, PharmD, PhD4;
Grace CY Lui, MB, ChB, FRCP1,5
1 Stanley Ho Centre for Emerging
Infectious Diseases, The Chinese University of Hong Kong, Shatin, Hong
Kong
2 Jockey Club School of Public Health
and Primary Care, The Chinese University of Hong Kong, Shatin, Hong Kong
3 Department of Psychiatry, Queen Mary
Hospital, Pokfulam, Hong Kong
4 School of Pharmacy, The Chinese
University of Hong Kong, Shatin, Hong Kong
5 Department of Medicine and
Therapeutics, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Prof Grace CY Lui (gracelui@cuhk.edu.hk)
Abstract
Introduction: Pre-exposure
prophylaxis (PrEP) with tenofovir disoproxil fumarate (TDF) 300
mg/emtricitabine (FTC) 200 mg is a proven strategy for preventing human
immunodeficiency virus (HIV) transmission in men who have sex with men
(MSM). This study aimed to test the feasibility and acceptability of
PrEP delivered at a pilot clinic for MSM in Hong Kong, where PrEP
service is currently unavailable.
Methods: Partially self-financed
PrEP was provided to HIV-negative adult MSM with high behavioural risk
of HIV transmission after excluding hepatitis B infection and renal
insufficiency. Participants received daily TDF/FTC for 30 weeks at 13.3%
of the drug cost. Adherence and behaviours were monitored through
questionnaires while creatinine and HIV/STI (sexually transmitted
infection) incidence were monitored with point-of-care and laboratory
tests. Preference for continuing with PrEP was evaluated at the end of
the prescription period.
Results: Seventy-one PrEP-naïve
MSM were included in the study, of whom 57 (80%) were retained at the
end of 28 weeks. Satisfactory adherence and self-limiting adverse events
were reported, while none of the participants contracted HIV. Risk
compensation was observed, with an STI incidence of 3.17 per 100
person-years. At the end of the prescription period, a majority (89%)
indicated interest in continuing with PrEP. Preference for PrEP was
associated with age ≥28 years and peer influence (P=0.04), while stigma
was a concern. Price was a deterrent to self-financed PrEP, and only
half (51%) considered a monthly cost of ≤HK$500 (US$1=HK$7.8) as
reasonable.
Conclusions: A partially
self-financed mode of PrEP delivery is feasible with good retention in
MSM in Hong Kong.
New knowledge added by this study
- A workable model for delivering affordable pre-exposure prophylaxis (PrEP) to men who have sex with men (MSM) at high risk of human immunodeficiency virus (HIV) infection in Hong Kong is important.
- Risk compensation as reflected by diagnosis of sexually transmitted infections (STIs) following PrEP is a concern in a proportion of MSM.
- Adverse events from the use of tenofovir disoproxil fumarate 300 mg and emtricitabine 200 mg for PrEP are not uncommon though normally self-limiting, but cessation may be required in a small proportion of PrEP users.
- Partially self-financed daily PrEP administered in conjunction with STI/HIV monitoring is operationally feasible and acceptable to MSM in Hong Kong, where PrEP is currently otherwise unavailable as a service.
Introduction
Pre-exposure prophylaxis (PrEP) with tenofovir
disoproxil fumarate (TDF) 300 mg/emtricitabine (FTC) 200 mg is a key
strategy for protecting people at high risk of human immunodeficiency
virus (HIV) transmission. The effectiveness of PrEP for HIV prevention has
been demonstrated in large-scale national studies1
2 3
and extensively reviewed in the literature.4
Mathematical modelling parameterised by data from the Netherlands
concluded that PrEP for men who have sex with men (MSM) is cost-effective
in the context of a stable HIV epidemic.5
Following approval of the Food and Drug Administration in the US, PrEP
guidelines have been promulgated by the World Health Organization,6 the Centers for Disease Control and Prevention,7 and the European AIDS Clinical Society.8 Despite promotions and advocacies at different levels,
uptake of PrEP has remained low internationally, with wide disparities
across countries. Of note, Asia has been reported to account for fewer
than 5% of all PrEP initiations recorded worldwide.9 Notably, cost remains an important deterrent to its
introduction in most cities/countries where generic TDF/FTC cannot be
prescribed legally, and Hong Kong is no exception. Elsewhere, different
service models for PrEP delivery have been developed,10 but there exists a “purview paradox” causing
obstructions in societal implementation.11
In Hong Kong, PrEP is currently unavailable as an
HIV prevention service, where MSM have continued to account for a high
proportion of newly reported HIV infections (67% in 2017; www.aids.gov.
hk). A study was conceptualised to test the feasibility and acceptability
of PrEP delivery by piloting a designated clinic to deliver lower-cost
TDF/FTC to MSM at high risk of HIV transmission. The results of this study
are expected to serve as a useful reference for the future development of
sustainable PrEP programmes in Hong Kong.
Methods
Pre-exposure prophylaxis clinic
A research clinic was set up at Prince of Wales
Hospital, the teaching hospital of The Chinese University of Hong Kong. In
collaboration with HIV services and community-based organisations,
eligible HIV-negative MSM were referred or self-referred to join the
study. A bilingual (Chinese and English) website was set up to provide
information about PrEP, with linkages to eligibility screening and
behavioural and adherence surveys through the online system.
Participant recruitment
Participants were MSM aged ≥18 years who were
normally resident in Hong Kong and could communicate in written and spoken
English or Chinese. Potential participants who had not previously used
PrEP were targeted. The main inclusion criteria were: firstly, history of
unprotected anal sex in the preceding 6 months; and secondly, negative HIV
antibody test result within the last 3 months; plus at least one of the
following in the past 6 months: (a) diagnosis of sexually transmitted
infection [STI], (b) sex partner(s) with positive or unknown HIV status,
(c) history of recreational drug use during sex, ie, “chem-sex”; and/or
(d) multiple sex partners. The exclusion criteria were: (a) having any
form of mental illnesses; (b) inability or refusal to give consent; (c)
incarceration; (d) known hepatitis B virus infection; and (e) known renal
insufficiency with creatinine clearance <60 mL/min/1.73 m2.
Pre-exposure prophylaxis regimen and monitoring
Participants completed a pre-assessment to confirm
their eligibility for inclusion in the study. A 2-week course of daily
TDF/FTC was given free at the first visit to evaluate tolerance before the
participant was asked to provide partial payment by instalment, covering a
total prescription period of 30 weeks. The prescription was partially
self-financed, as each person was required to pay HK$1316 (US$1=HK$7.8) 4
times for four consecutive prescription periods. As an incentive, the same
payment entitled the participant to receive an increasing duration of
medication. At week 28, a 2-week supply of medication was given free. The
total cost was equivalent to 13.3% of the market price of patented
TDF/FTC, or HK$702 per month, in Hong Kong.
Three forms of monitoring were implemented: (a)
questionnaires for periodic data collection on HIV risk behaviours,
adverse reactions to antiretrovirals, and adherence to daily
self-administered PrEP by tablet PC, at each consultation; (b)
point-of-care and laboratory tests: fourth-generation HIV antibody/antigen
tests; plasma creatinine and estimated glomerular filtration rate; STI:
syphilis serology, and urine tests for Neisseria gonorrhoeae and Chlamydia
trachomatis by nucleic acid amplification test (NAAT); and (c) online
diary for tracking daily intake of TDF/FTC and sexual activities. Finger
prick was used for monitoring HIV, creatinine, and syphilis serology at
selected time-points. Archived blood samples collected at baseline and
week 28 were tested for hepatitis C virus antibody. A weekly email
reminder was sent to participants requesting completion of the online
diary.
Analyses
Complete case analyses were performed addressing:
(a) acceptability/feasibility: characteristics of participants; proportion
of MSM interested in continuing with PrEP following the study; retention
in the programme; (b) outcome evaluation: drug adherence; coverage of
unprotected sex; adverse reactions; detection of STIs at baseline;
subsequent diagnoses while on PrEP; and preference for continuing PrEP use
and service delivery models including price, setting, and regimen.
Variables were assessed using univariate analysis. Categorical variables
were tested with the Chi squared test if the expected value in each cell
was at least 5; otherwise, Fisher’s exact test was used. Continuous
variables were examined using the Mann-Whitney U test. The STROBE
guideline was implemented in reporting the study.
Results
Between September 2017 and May 2018, 292 MSM were
assessed.12 A total of 71 (median
age, 32 years) MSM were included in the study. Their demographic and
behavioural profiles are shown in Table 1. Half of the participants were
self-referred; the rest were referred from community-based organisations
and collaborating HIV services. Thirty-three (46%) participants reported a
history of STIs, 15 of whom had a diagnosis in the preceding 6 months. All
participants were PrEP-naïve, but 10 (14%) had previously been put on
post-exposure prophylaxis after high-risk sexual exposure. Engagement in
chem-sex was reported by 25 (35%) of the participants. The self-perceived
risk of HIV infection was high in one-fifth of the participants. Over the
30-week prescription period, there was a total follow-up of 1639
person-weeks. At the end of the study period, 57 (80%) participants
remained in the programme. Fourteen withdrew from the study, nine of whom
(64%) did so within the initial 2 months. The following reasons for
withdrawal were given by 10 participants: low or no perceived risk (6);
adverse events (2); concern about adverse drug effects or drug
interactions (1); unaffordability (1); inconvenience or too busy to attend
the clinic (1); and on the advice of friends (1).
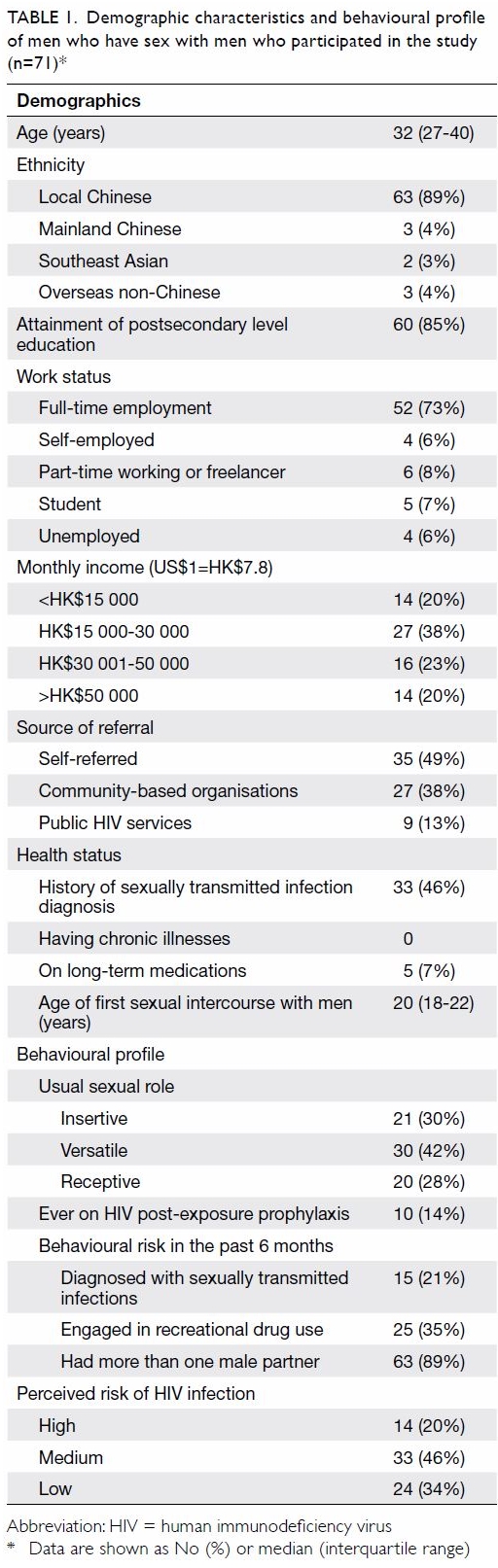
Table 1. Demographic characteristics and behavioural profile of men who have sex with men who participated in the study (n=71)
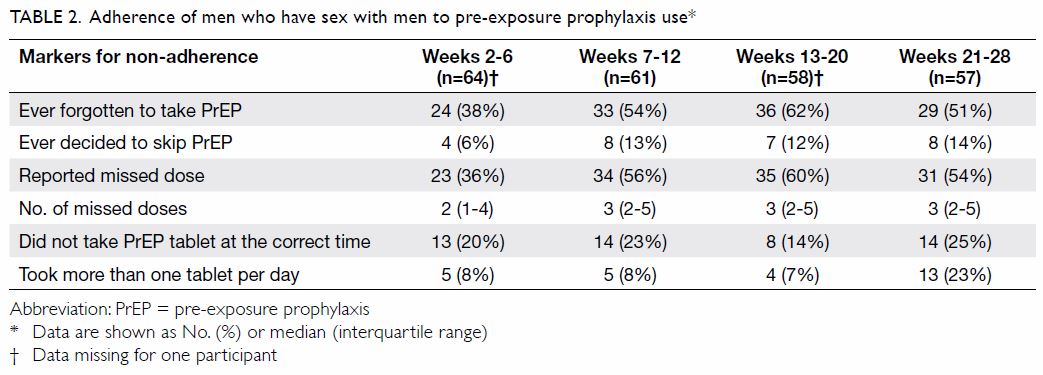
Full adherence to attending all visits was achieved
by all participants who completed the study, though 16% (74/460) of the
pre-arranged appointments required rescheduling. The rates of adherence to
HIV testing (six time-points), plasma creatinine testing (three
time-points), and STI screening (three time-points) were 100%, 99.5%, and
100%, respectively. Adherence to daily use of TDF/FTC, as derived from the
questionnaires administered at each visit, is shown in Table
2. Overall, 60 out of 69 (87%) participants with diary data reported
having ever omitted at least one dose. A median of two to three doses was
missed between each pair of consultations, which took place at intervals
of 4 to 8 weeks. The total number of doses omitted ranged from 1 to 71
(median, 6; interquartile range=3-14). Occasions of condomless sex without
concurrent use of TDF/FTC were noted, which occurred in 20 out of 953 (2%)
person-days. While participants were advised to take the TDF/ FTC tablet
at about the same time each day, some 14% to 25% reported not being able
to stick to the strict 24-hour dosing interval.
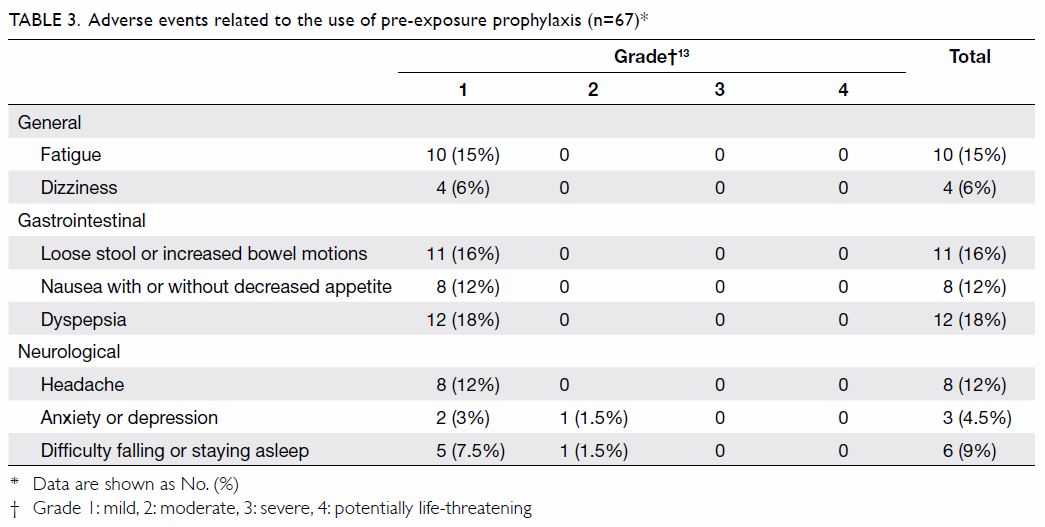
Adverse events relating to the use of TDF/FTC were
reported by 35 (52%) out of the 67 participants who attended the clinic at
least twice (Table 313).
The main adverse events were: dyspepsia (18%), loose stool or increased
bowel motions (16%), fatigue (15%), headache (12%), and nausea with or
without decreased appetite (12%). Other adverse events included difficulty
falling or staying asleep, dizziness, and anxiety or depression. These
adverse events were generally mild (grade 1), self-limiting, and did not
bother the participants, and the majority resolved within the first week.
Two participants withdrew from the study because of grade 2 adverse events
that lasted over 2 weeks. One complained of nausea, diarrhoea, stomach
upset, and anxiety, which resolved 2 days after stopping TDF/FTC at week
8. The other had headache shortly after initiation on PrEP and depressed
mood and fleeting suicidal ideation in the ensuing 2 weeks, which resolved
upon stopping at week 4. Separately, plasma creatinine was measured at
baseline, week 12, and week 28; there was a >20% increase of plasma
creatinine in 7/63 (11%) and 6/57 (11%) of the participants compared with
baseline, respectively. Three (5%) participants who completed the study
had a 20% increase in plasma creatinine readings at both weeks 12 and 28.
None had an estimated glomerular filtration rate level <60 mL/min/1.73
m2 at any time in the course of receiving PrEP.
None of the participants contracted HIV in the
course of the study. Condomless sex was reported by 70 out of 71 (99%) of
the participants. Their behavioural profiles and STI diagnoses at
different time intervals are shown in Table 4. Out of the 59 MSM followed up through week
20 or beyond, compared with baseline, condom use decreased in 22 (49%),
increased in 9 (20%), and was unchanged in 14 (31%) for sex with known
partners (n=45), and the corresponding rates for newly met partners (n=47)
were 19 (40%), 7 (15%), and 21 (45%), respectively. Reduction of condom
use with newly met partners was associated with the attainment of
postsecondary education (odds ratio [OR]=2.00, 95% confidence interval
[CI]=1.46-2.75, P=0.07 by Fisher’s exact test). There was no association
between reduction of condom use and demographic characteristics, reasons
for taking PrEP, or history of risk behaviours. The proportion of PrEP
users engaging in chem-sex was similar before (41%) and after PrEP
(32%-40%).
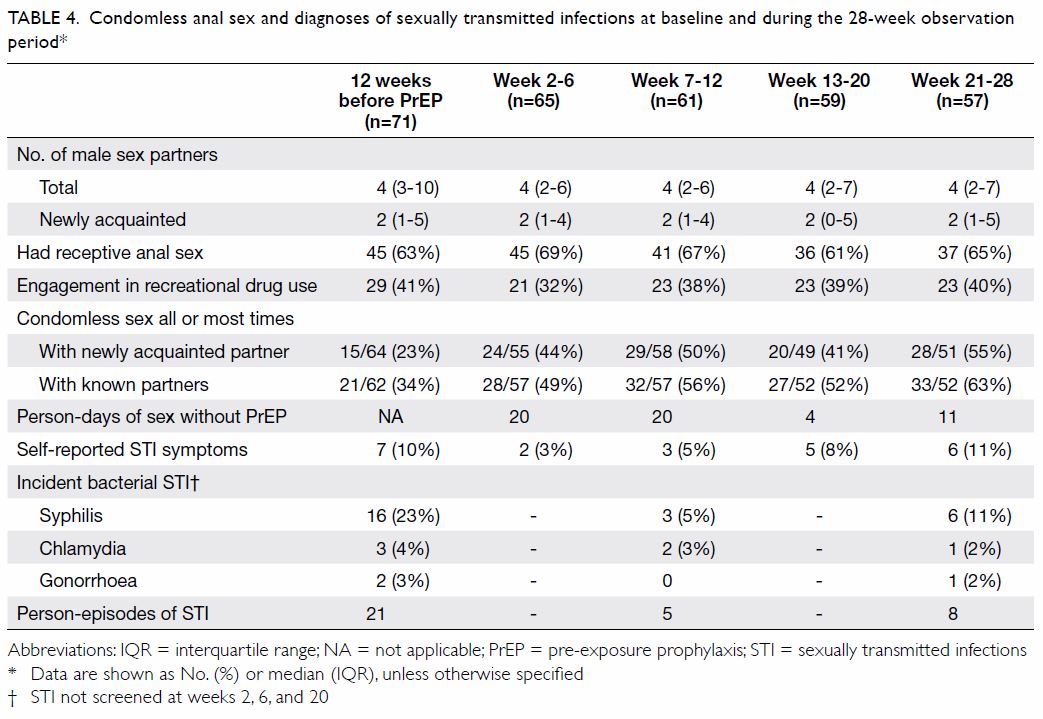
Table 4. Condomless anal sex and diagnoses of sexually transmitted infections at baseline and during the 28-week observation period
With regard to STIs, 2 (3%), 3 (4%), and 16 (23%)
were positive for N gonorrhoeae (NAAT), C trachomatis
(NAAT), and syphilis serology (nine treponemal only, seven treponemal and
non-treponemal) at baseline, respectively. One of the 16 (6%) syphilis
serology-positive MSM was a newly diagnosed infection. Over a follow-up
period of 1639 person-weeks among those retained in the study for at least
12 weeks, 13 incident STIs (one N gonorrhoeae [incidence rate=3.17
per 100 personyears], three C trachomatis [incidence rate=9.52 per
100 person-years], and nine syphilis [incidence rate=28.55 per 100
person-years]) had occurred. The participants with incident STI were more
likely to be poppers users (46% and 18% of participants with and without
incident STIs used poppers, respectively; OR=3.81, 95% CI=1.03-14.10,
P=0.06). None of the participants had positive results for hepatitis C
virus antibody at baseline or follow-up.
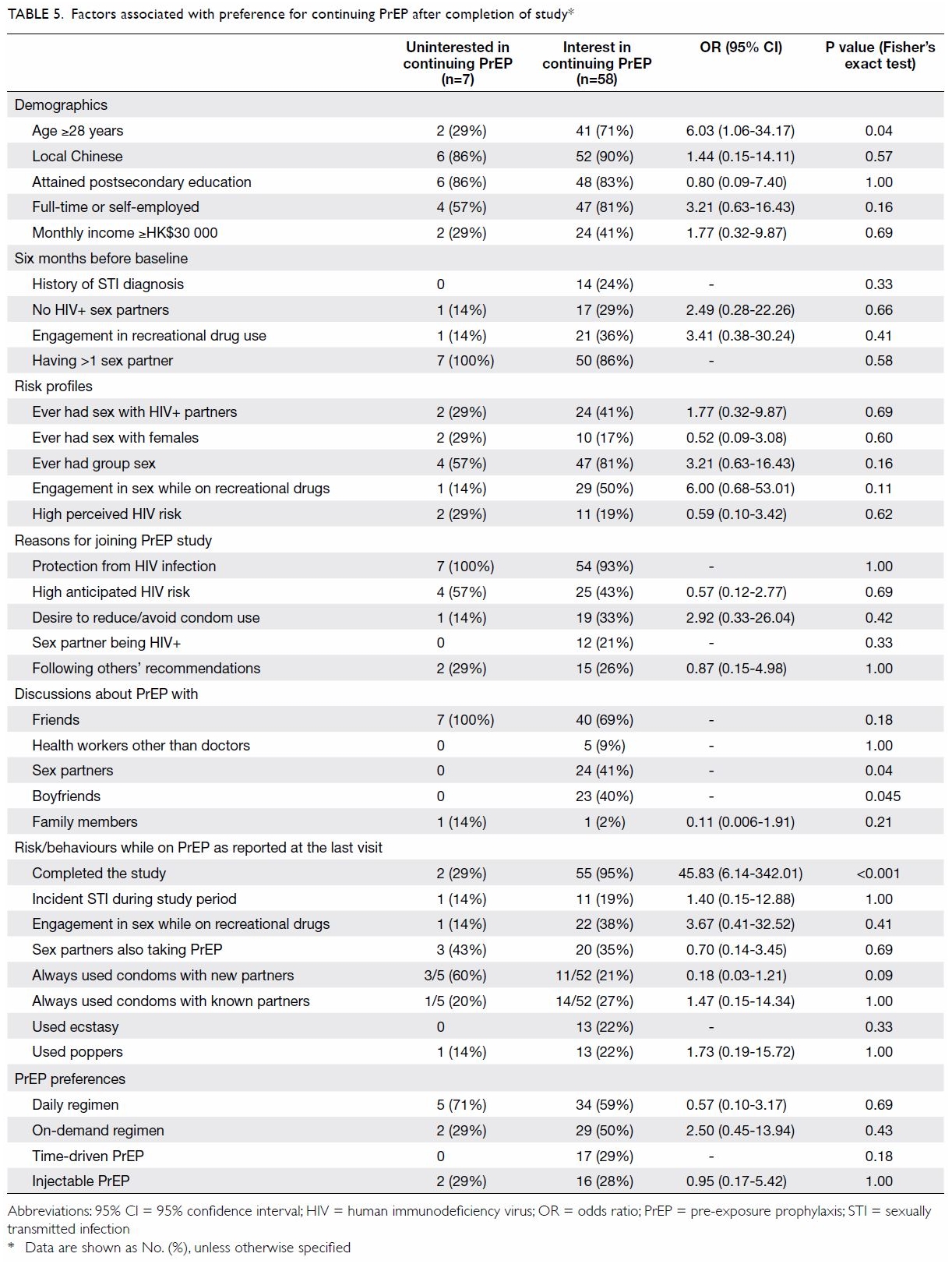
At the last visit during the prescription period,
participants (n=65) were asked about their future intentions regarding
PrEP. Fifty-eight (89%) responded that they would like to continue with
PrEP after the study. The MSM who preferred to continue PrEP after the
study were more likely to be aged ≥28 years (OR=6.03, 95% CI=1.06-34.17,
P=0.04) [Table 5]. Peer influence was important, as none of
those uninterested in continuing on had discussed PrEP use with their
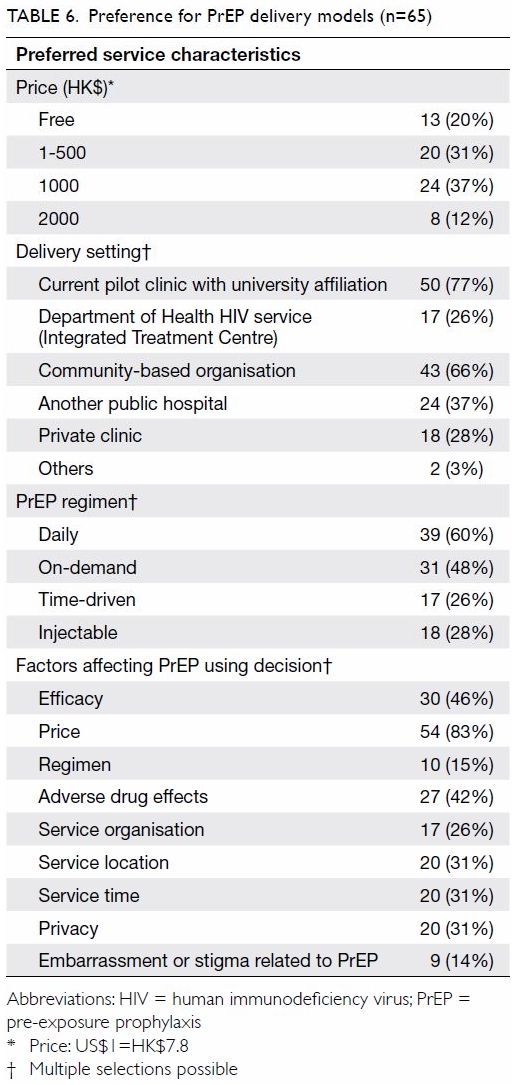
boyfriends (P=0.04) or sex partners (P=0.04). Price (83%) was the most
concerning factor affecting their PrEP-using decision, followed by
efficacy (46%) and potential adverse drug effects (42%) [Table
6]. Some 14% were worried about embarrassment or stigma related to
PrEP. The majority, 63 of 68 (93%), had disclosed their PrEP status to
their partners, and 9 (14%) of them reported ever experiencing stigma.
Half (51%) considered a monthly cost for PrEP of ≤HK$500 reasonable.
Two-thirds (66%) accepted community-based organisations as the portal for
receiving PrEP and monitoring. Over half (60%) and about half (48%)
favoured daily mode and on-demand PrEP, respectively. Fewer than one-third
favoured injection (28%) and time-driven PrEP (26%).
Discussion
Men who have sex with men have continued to be the
hardest-hit population by the global HIV epidemic,14 and Hong Kong is no exception. While PrEP has not yet
been implemented, its acceptance is generally high, at 78.6% among MSM in
late 2016,15 but only 1% have been
reported to have accessed PrEP.16
Our study was the first that has piloted PrEP delivery to MSM in Hong
Kong. Our results showed that the operation of a PrEP clinic in Hong Kong
is feasible and acceptable to the MSM community, as evidenced by our high
retention rate of 80% among users of a daily regimen over the 7-month
observation period. Severe adverse reactions were uncommon in our study,
echoing the conclusion on the safety profile of PrEP, as illustrated in
clinical studies and confirmed in reviews.4
17
None of the MSM in the study contracted HIV, but
the small sample size did not allow the efficacy of PrEP to be evaluated.
Elsewhere, a meta-analysis of multiple studies with different regimens
concluded that PrEP reduced the HIV infection risk by 70% in the presence
of high adherence.4 The failure of
PrEP is very uncommon, as shown by a large-scale PROUD study2 and by evaluating real world data.18 In our pilot study, adherence to daily TDF/FTC,
creatinine testing, and HIV/STI monitoring was high. Non-adherence to
TDF/FTC could potentially lead to resistance if HIV infection occurs in
the course of PrEP, though its incidence has remained low.19 Condomless sex in conjunction with the omission of
TDF/FTC, which can be referred as PrEP-unprotected condomless sex, was
relatively uncommon. Risk compensation, defined as the increased practice
of condomless sex in PrEP users, is an emerging concern.20 21 Our
results did not confirm any consistent increase of risk compensation
behaviours, an observation shared by other recent studies.4 22 23 Increased incidence of STI could be more prevalent in
the initial period of PrEP introduction19
24 or restricted to a
subpopulation of MSM regardless of PrEP use.20
It is uncommon to see MSM starting to engage in condomless anal sex after
PrEP initiation.23 One modelling
study showed that STI incidence would decline with increased PrEP
coverage.25 With increasing PrEP
coverage, non-PrEP users may become a neglected community when planning
STI/HIV interventions.26
The present results highlight that the major
obstacle to PrEP implementation is its cost, as patented TDF/FTC is too
expensive for out-of-pocket acquisition.16
In our study, requiring participants to pay an amount closer to the cost
of generic products (HK$750 or <US$100 per month) appealed to only a
fraction of the MSM approached. About half of the eligible MSM did not
join this project because of the high cost incurred.12 Making PrEP free or affordable should be an effective
strategy for preventing HIV transmission through high-risk behaviours.
Taking reference to the situation in the US, different models for PrEP
could be considered, including services at STI clinics, community health
centres, community-based organisations, pharmacies, and private primary
care providers.10 This study
highlighted issues for consideration in the establishment of a local PrEP
service. The need for dispensing prescription medicine alongside HIV/STI
testing and toxicity monitoring has made access to PrEP complex. Vertical
programmes that have conventionally offered HIV testing to high-risk
populations have been more prepared to implement PrEP than overburdened
primary care services tend to be.27
Other innovative models of PrEP delivery have been reported in other
countries, such as pharmacy-based clinics in Seattle in the US,28 community health centres in Bangkok, Thailand,29 and integrated sexual reproductive health services in
Wales.30 While those models
provide lessons, they may not be relevant to the situation in Hong Kong.
Finally, stigma could be a major deterrent to accessing PrEP, as expressed
by some of the MSM who refused to participate.31
In rolling out PrEP, a marketing strategy that focuses on health
protection rather than risk reduction may be more appropriate.32 A non-targeting approach regarding behavioural risk
could be less stigmatising and may still be cost-effective at achieving
HIV prevention in low-HIV incidence settings.33
The current study has some limitations. First, the
sample size was small, such that the results may not be generalisable to
the situation of the entire MSM community in Hong Kong. Second, sampling
bias could not be eliminated, as the study included self-referred MSM and
those referred from collaborating organisations. Individuals reluctant to
participate in a clinical trial and those unwilling or unable to pay for
TDF/FTC were excluded. Nevertheless, the study did manage to recruit MSM
with high-risk behaviours, including those who engaged in chem-sex.
Finally, we relied on self-reporting for tracking of HIV/STI risk, a
strategy that might have underestimated high-risk behaviours. By
subjecting the participants to STI screening at multiple time-points, we
detected otherwise-hidden STIs both at baseline and during the course of
PrEP. For maximum effectiveness, PrEP should go hand in hand with
community-based STI/HIV monitoring, so that prompt treatment can be
offered to those diagnosed with infections, while those testing negative
can continue to be prescribed TDF/FTC for HIV prevention.
Conclusion
While cost is a major obstacle to scaling up
implementation of PrEP, making it available free may pose an added
challenge to countries or cities where a policy decision to introduce PrEP
as a public health service has yet to be made. Our results suggested that
a partially self-financed mode of delivery is feasible and could appeal to
a proportion of risk-taking MSM. Fee-based PrEP provision has been
available in other Asian Pacific countries like Thailand.34 A partially self-financed model could be an interim
measure, and this is less demanding of resources in locations where
generic TDF/FTC is not available (as in Hong Kong). Operationally, PrEP
cannot be implemented in isolation but must be provided in conjunction
with periodic HIV/STI testing, as reflected in our study. Provision of
PrEP serves the dual purpose of HIV prevention and opportunistic STI
screening, which enables prompt treatment to be given so as to reduce
reinfections and the infection burden in the MSM community. Rectal C
trachomatis or N gonorrhoeae infection has been shown to be
associated with an increased risk of HIV transmission35 and should therefore be considered as part and parcel
of the HIV prevention package. Finally, as PrEP is a biomedical form of
HIV prevention, our results confirm that severe adverse events are
uncommon, but moderate but intolerable reactions may occur in a small
proportion of people on TDF/FTC, who would require clinical advice on
cessation.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: SS Lee, TH Kwan, TTN Lam.
Acquisition of data: TH Kwan, NS Wong, SS Lee, GCY Lui, DPC Chan, KCK Lee.
Analysis or interpretation of data: TH Kwan, NS Wong, SS Lee.
Drafting of the article: SS Lee.
Critical revision for important intellectual content: SS Lee, GCY Lui.
Acquisition of data: TH Kwan, NS Wong, SS Lee, GCY Lui, DPC Chan, KCK Lee.
Analysis or interpretation of data: TH Kwan, NS Wong, SS Lee.
Drafting of the article: SS Lee.
Critical revision for important intellectual content: SS Lee, GCY Lui.
Acknowledgement
Ms Mandy Li, Mr Chengqian Ye, Mr Choi-yin Lam, and
Dr See-long Lee are thanked for their assistance in participant enrolment
and management of the research clinic. We thank AIDS Concern, CHOICE
(Community Health Organisation for Intervention, Care and Empowerment),
Boys’ & Girls’ Clubs Association of Hong Kong, Integrated Treatment
Centre, HIV services of Queen Elizabeth Hospital, Princess Margaret
Hospital, and Prince of Wales Hospital for technical support and the
referral of potentially eligible participants to the study. The Li Ka
Shing Institute of Health Sciences and Stanley Ho Centre for Emerging
Infectious Diseases of The Chinese University of Hong Kong are
acknowledged for providing technical support in developing the analyses.
Gilead Sciences, Inc. is acknowledged for drug donation in support of part
of the study.
Declaration
The research data have been presented in part as
posters at the Lancet–CAMS Health Summit 2018 (27-28 October 2018,
Beijing, PR China), the 3rd Asia Pacific AIDS & Co-infections
Conference 2018 (28-30 June 2018, Hong Kong), HIV Glasgow 2018 (28-31
October 2018, Glasgow, United Kingdom), and the 10th IAS Conference on HIV
Science 2019 (21-24 July 2019, Mexico City, Mexico).
Conflicts of interest
GCY Lui has served as advisory committee member for
Gilead Sciences, Merck, Sanofi Pasteur, and ViiV; and as a speaker for
Gilead Sciences and Merck; and has received research grants/donations from
Gilead Sciences, Merck and GSK. SS Lee has served as advisory committee
member for Gilead Sciences, GSK and Merck; and as a speaker for Gilead
Sciences sponsored events; and has received funding from Gilead Grants for
community education activities.
Funding/support
This research was supported by the Council for the
AIDS Trust Fund (Ref MSS264R), Hong Kong SAR Government.
Ethics approval
The study was approved by The Joint Chinese
University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (Ref CREC 2016.470) and registered at the Centre for
Clinical Research and Biostatistics Clinical Trials Registry of The
Chinese University of Hong Kong (Ref CUHK_CCRB00533). A Clinical Trials
Certificate was obtained following application to Department of Health of
the Hong Kong SAR Government (Ref 100860).
References
1. Grant RM, Lama JR, Anderson PL, et al.
Preexposure chemoprophylaxis for HIV prevention in men who have sex with
men. N Engl J Med 2010;363:2587-99. Crossref
2. McCormack S, Dunn DT, Desai M, et al.
Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection
(PROUD): effectiveness results from the pilot phase of a pragmatic
open-label randomised trial. Lancet 2016;387:53-60. Crossref
3. Molina JM, Charreau I, Spire B, et al.
Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure
prophylaxis for HIV in men who have sex with men: an observational cohort
study. Lancet HIV 2017;4:e402-10.
4. Fonner VA, Dalglish SL, Kennedy CE, et
al. Effectiveness and safety of oral HIV preexposure prophylaxis for all
populations. AIDS 2016;30:1973-83. Crossref
5. Nichols BE, Boucher CA, van der Valk M,
Rijnders BJ, van de Vijver DA. Cost-effectiveness analysis of pre-exposure
prophylaxis for HIV-1 prevention in the Netherlands: a mathematical
modelling study. Lancet Infect Dis 2016;16:1423-29. Crossref
6. Department of HIV/AIDS, World Health
Organization. Consolidated guidelines on HIV prevention, diagnosis,
treatment and care for key populations. Switzerland: WHO Press; 2016.
Available from: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 28
Jan 2019.
7. Centers for Disease Control and
Prevention, US Government. Preexposure prophylaxis for the prevention of
HIV infection in the US—2017: a clinical practice guideline. Available
from:
https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf.
Accessed 3 Sep 2019. Crossref
8. European AIDS Clinical Society. EACS
Guidelines 8.0. Available from:
http://www.eacsociety.org/files/guidelines_8.0-english-revised_20160610.pdf.
Accessed 28 Jan 2019.
9. To KW, Lee SS. HIV pre-exposure
prophylaxis in South East Asia: a focused review on present situation. Int
J Infect Dis 2018;77:113-7. Crossref
10. Mayer KH, Chan PA, Patel R, Flash CA,
Krakower DS. Evolving models and ongoing challenges for HIV preexposure
prophylaxis implementation in the United States. J Acquir Immune Defic
Syndr 2018;77:119-27. Crossref
11. Lee SS, Petersen EP. Overcoming
‘purview paradox’ to make way for the effective implementation of PrEP in
preventing HIV transmission. Int J Infect Dis 2018;77:105-6. Crossref
12. Kwan TH, Wong NS, Lui GC, Lee KC, Lee
SS. Acceptability of an incentivised PrEP programme for men who have sex
with men at high risk of HIV infection in Hong Kong: an implementation
study. Lancet 2018;392 Suppl 1:S77. Crossref
13. US Department of Health and Human
Services. Division of AIDS (DAIDS) Table for grading the severity of adult
and paediatric adverse events. Version 2.0, November 2014. Available from:
https://rsc.niaid.nih.gov/sites/default/files/daids-ae-grading-table-v2-nov2014.pdf.
Accessed 28 Jan 2019.
14. Beyrer C, Sullivan P, Sanchez J, et
al. The increase in global HIV epidemics in MSM. AIDS 2013;27:2665-78. Crossref
15. Kwan TH, Lee SS. Predictors of HIV
testing and their influence on PrEP acceptance in men who have sex with
men: a cross-sectional study. AIDS Behav 2018;22:1150-7. Crossref
16. Wang Z, Lau JT, Fang Y, Ip M, Gross
DL. Prevalence of actual uptake and willingness to use pre-exposure
prophylaxis to prevent HIV acquisition among men who have sex with men in
Hong Kong, China. PLoS One 2018;13:e0191671. Crossref
17. Trang TP, Dong BJ, Kojima N, Klausner
JD. Drug safety evaluation of oral tenofovir disoproxil
fumarate-emtricitabine for pre-exposure prophylaxis for human
immunodeficiency virus infection. Expert Opin Drug Saf 2016;15:1287-94. Crossref
18. Marcus JL, Hurley LB, Nguyen DP,
Silverberg MJ, Volk JE. Redefining human immunodeficiency virus (HIV)
preexposure prophylaxis failures. Clin Infect Dis 2017;65:1768-9. Crossref
19. Gibas KM, van den Berg P, Powell VE,
Krakower DS. Drug resistance during HIV pre-exposure prophylaxis. Drugs
2019;79:609-19. Crossref
20. Nguyen VK, Greenwald ZR, Trottier H,
et al. Incidence of sexually transmitted infections before and after
preexposure prophylaxis for HIV. AIDS 2018;32:523-30.
21. Beymer MR, DeVost MA, Weiss RE, et al.
Does HIV pre-exposure prophylaxis use lead to a higher incidence of
sexually transmitted infections? A case-crossover study of men who have
sex with men in Los Angeles, California. Sex Transm Infect 2018;94:457-62.
Crossref
22. Milam J, Jain S, Dubé MP, et al.
Sexual risk compensation in a pre-exposure prophylaxis demonstration study
among individuals at risk of HIV. J Acquir Immune Defic Syndr
2019;80:e9-e13. Crossref
23. Grov C, Whitfield TH, Rendina HJ,
Ventuneac A, Parsons JT. Willingness to take PrEP and potential for risk
compensation among highly sexually active gay and bisexual men. AIDS Behav
2015;19:2234-44. Crossref
24. Hoornenborg E, Coyer L, van Laarhoven
A, et al. Change in sexual risk behaviour after six months of pre-exposure
prophylaxis use: results from the Amsterdam pre-exposure prophylaxis
demonstration project. AIDS 2018;32:1527-32. Crossref
25. Jenness SM, Weiss KM, Goodreau SM, et
al. Incidence of gonorrhea and chlamydia following human immunodeficiency
virus preexposure prophylaxis among men who have sex with men: a modeling
study. Clin Infect Dis 2017;65:712-8. Crossref
26. Phanuphak N, Phanuphak P. Time to
focus more on condomless anal sex in non-PrEP users. Lancet HIV
2018;5:e410-1. Crossref
27. Venter WD. Pre-exposure prophylaxis:
the delivery challenge. Front Public Health 2018;6:188. Crossref
28. Tung EL, Thomas A, Eichner A, Shalit
P. Implementation of a community pharmacy-based pre-exposure prophylaxis
service: a novel model for pre-exposure prophylaxis care. Sex Health
2018;15:556-61. Crossref
29. Phanuphak N, Sungsing T, Jantarapakde
J, et al. Princess PrEP program: the first key population-led model to
deliver pre-exposure prophylaxis to key populations by key populations in
Thailand. Sex Health 2018;15:542-55. Crossref
30. Knapper C, Birley H, Couzens Z, Jones
AT, Parker I. How to do it: setting up a PrEP service in an integrated
sexual reproductive health service setting. Sex Transm Infect
2018;94:327-30.Crossref
31. Calabrese SK, Tekeste M, Mayer KH, et
al. Considering stigma in the provision of HIV pre-exposure prophylaxis:
reflections from current prescribers. AIDS Patient Care STDS
2019;33:79-88. Crossref
32. Rivet Amico K, Bekker LG. Global PrEP
roll-out: recommendations for programmatic success. Lancet HIV
2019;6:e137-40. Crossref
33. Wong NS, Kwan TH, Tsang OT, et al.
Pre-exposure prophylaxis (PrEP) for MSM in low HIV incidence places:
should high risk individuals be targeted? Sci Rep 2018;8:11641. Crossref
34. Zablotska I, Grulich AE, Phanuphak N,
et al. PrEP implementation in the Asia-Pacific region: opportunities,
implementation and barriers. J Int AIDS Soc 2016;19(Suppl 6):21119. Crossref
35. Bernstein KT, Marcus JL, Nieri G,
Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is
associated with increased risk of HIV seroconversion. J Acquir Immune
Defic Syndr 2010;53:537-43. Crossref