Hong Kong Med J 2019 Oct;25(5):372–81 | Epub 9 Oct 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Sexual function, self-esteem, and general well-being in
Chinese adult survivors of childhood cancers: a cross-sectional survey
CF Ng, FHKAM (Surgery)1; Cindy YL Hong, MSc1;
Becky SY Lau, MPH1; Jeremy YC Teoh, FHKAM (Surgery)1; Samuel CH Yee, FHKAM (Surgery)1;
Alex WK Leung, FHKAM (Paediatrics)2; John WM Yuen, PhD3
1 SH Ho Urology Centre, Department of
Surgery, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Paediatrics Oncology Team, Department
of Paediatrics, The Chinese University of Hong Kong, Shatin, Hong Kong
3 School of Nursing, The Hong Kong
Polytechnic University, Hung Hom, Hong Kong
Corresponding author: Prof CF Ng (ngcf@surgery.cuhk.edu.hk)
Abstract
Introduction: This study
was conducted to evaluate sexual function in adult survivors of
childhood cancers and investigate possible relationships between sexual
function and quality of life, as measured by general well-being,
self-esteem, body image, and depressive symptoms.
Methods: This cross-sectional
survey was performed in our centre from 14 August 2015 to 8 September
2017. Adult patients who had a history of childhood cancers, and who
were disease-free for >3 years, were approached for the study during
clinical follow-up. Clinical information was collected from medical
records. Self-administered questionnaires regarding quality of life and
sexual functioning were given to the patients and resulting data were
analysed.
Results: Two hundred patients
agreed to participate in the study. The overall response rate was 64.8%.
Ninety-one (45.5%) patients were women, and the mean age was 25.4 ± 5.57
years. The overall sexual functioning score was 28.3 ± 20.09.
Forty-eight (24.0%) patients reported at least one sexual problem. Among
patients who reported no sexual problems, more had haematological
cancers (P=0.009), fewer underwent surgery (P=0.004), fewer underwent
surgery with external effects (P=0.032), and fewer were regular social
drinkers (P=0.013); additionally, they had a higher mean Rosenberg
self-esteem scale score (P=0.010), lower mean body image scale score
(P=0.008), and lower mean Patient Health Questionnaire score (P=0.001).
Conclusion: Aspects of life
beyond disease condition and physical function should be considered in
adult survivors of childhood cancers. Appropriate referral and
intervention should be initiated for these patients when
necessary.
New knowledge added by this study
- Approximately one-quarter of young Chinese cancer survivors in Hong Kong had at least one sexual problem.
- Sexual problems were more common in men, in patients diagnosed with cancer at an older age, and in patients who were married or had a history of sexual experiences.
- The presence of sexual problems in adult survivors of childhood cancer was significantly associated with a history of surgery, as well as a history of surgery with external effects; patients with sexual problems generally had lower physical well-being scores, lower self-esteem scores, higher body image scale scores, and an increased number of depressive symptoms.
- Rather than entirely focusing on disease condition and physical function, physicians and medical staff should ensure that they consider other aspects of life in survivors of childhood cancer, to support holistic recovery of these patients.
- Multidisciplinary care, such as involvement of adult urologists and gynaecologists, would facilitate the transition of these young cancer survivors into adult life.
Introduction
Sexual health, defined by the World Health
Organization as a state of physical, emotional, mental and social
well-being related to sexuality, has been recognised as an integral part
of overall health and quality of life.1
Improvements in disease understanding and treatment options have changed
the circumstances involved in the management of sexual dysfunction and
reproductive medicine.
With improvements in cancer care, the long-term
outlook of paediatric cancer patients has significantly improved in recent
decades.2 However, there is
increasing awareness of the problems experienced by these cancer survivors
when they reach adulthood.3
Potential problems experienced by adult survivors of childhood cancers
include (1) physical and functional complications related to the cancer
and its therapies (eg, delay in pubertal development, hormonal production,
azo-/oligospermia, ovarian failure, and vaginal stenosis); and (2)
psychosocial problems related to the cancer and its therapies (eg,
concerns regarding cancer recurrence, self-esteem, and relationship
problems). These physical and psychological dysfunctions affect the sexual
health and overall health of the patients. The findings of many reports
have suggested significant associations between sexual function and health
status,4 and have revealed that
these problems are relatively common among cancer survivors.5 6
Unfortunately, discussions of sexual dysfunction
remain infrequent in traditional Chinese culture. The situation may be
more difficult among young adult cancer survivors. Therefore, information
regarding cancer-related sexual dysfunction, including its prevalence in
the Chinese population, remains limited and may lead to an underestimation
of the seriousness of the problem. To address this lack of knowledge and
facilitate future development of childhood cancer care, this
cross-sectional study was conducted to evaluate sexual function in adult
survivors of childhood cancers and the relationships of sexual function
with the general well-being, self-esteem, body image, and depressive
symptoms of these patients.
Methods
Patients
The study was conducted in accordance with the
Declaration of Helsinki and was performed at The Chinese University of
Hong Kong. The sample size was based on a convenience sample of all
patients that we could recruit during the 2-year study period. Consecutive
patients who were returning to the paediatric oncology clinic for
follow-up and who fulfilled the inclusion and exclusion criteria were
invited to participate in the study. After patients provided informed
consent, basic demographic data and disease-related information were
collected.
The inclusion criteria were as follows: (1)
diagnosed with cancer at age <18 years; (2) aged 18 to 40 years at the
time of inclusion in the study; (3) not undergoing treatment and
disease-free >3 years after completing treatment (excluding use of
chemopreventive agents). The exclusion criteria were as follows: (1)
original tumours that were hormone-dependent, such as breast cancer; (2)
ongoing sex hormone supplementation; and (3) sensory/cognitive impairment
that would interfere with the patient’s ability to independently complete
the questionnaires.
Questionnaire data collection
A series of self-administered questionnaires were
completed by the patients in a private room in the clinic. The following
questionnaires were used.
Medical outcomes study sexual functioning scale
This is a validated instrument that has been widely
used to identify sexual impairment and dysfunction associated with serious
health conditions or side-effects of treatments.7
It consists of four questions for both male and female patients, which
evaluate sexual problems including lack of interest in sexual activity,
difficulty in becoming aroused, difficulty in relaxing and enjoying sex,
and difficulty in achieving orgasm. Each outcome is measured with an
ordinal scale ranging from 0 (‘not a problem’) to 4 (‘very much a
problem’). The category of ‘not applicable’ was recoded as 0 during
calculation. Total scores were calculated and transformed to a 0-100
scale; a higher score indicates more sexual problems. The questionnaire
was translated and validated by Department of Nursing, The Polytechnic
University of Hong Kong.
General Health Questionnaire Short Form-12
This questionnaire is used for general measurement
of health status in terms of physical component score (PCS) and mental
component score (MCS).8 The summary
scores are calculated based on the standard scoring algorithm described in
the manual9; a higher score
represents better physical or mental health.
Rosenberg self-esteem scale
This tool is commonly used to evaluate self-esteem.10 11
It comprises 10 questions which assess both positive and negative feelings
about the self. Patients respond to questions using a 4-point scale,
ranging from ‘strongly agree’ to ‘strongly disagree’; a higher summary
score indicates higher self-esteem.
Body image scale
Body image scale (BIS) is an 11-item scale used to
assess body image changes in cancer survivors after cancer treatment.12 13 Body
image changes can be rated in an ordinal scale ranging from 0 (‘not at
all’) to 3 (‘very much’); a higher total score indicates greater body
image distress.
Patient Health Questionnaire
Patient Health Questionnaire (PHQ-9) is widely used
to measure depressive symptoms. It assesses the extent to which the
symptoms were experienced by the patient in past 2 weeks.14 15 Patients
respond to items using an ordinal scale ranging from 0 (‘not at all’) to 3
(‘nearly every day’). The degree of depression is graded based on the
total score of the nine items: mild (5 ≤ score ≤ 9), moderate (10 ≤ score
≤ 14), and severe (score ≥15).
Statistical analysis
Descriptive statistics are presented as counts and
percentages for categorical data, and as means and standard deviations for
continuous data. The Chi squared test, Fisher’s exact test, analysis of
variance, correlation, and simple linear regression methods were used for
simple analyses and subgroup comparisons. More sophisticated analyses were
performed using multiple linear regression and multivariable logistic
regression, to control for potential confounders. All statistical analyses
were performed using SPSS (Windows version 24.0; IBM Corp, Armonk [NY],
United States). All levels of significance were set at the 0.05 level and
all tests were two-sided. Missing data were excluded from analysis.
Results
Patient demographic characteristics and cancer
treatment histories
The study was performed from 14 August 2015 to 8
September 2017. Three hundred seventy-two consecutive patients were
approached, and 241 patients agreed to participate in the study. The
overall response rate was 64.8%. Forty-one patients were excluded from
analysis due to incomplete data collection or failure to appropriately
meet the inclusion/exclusion criteria. Therefore, a total of 200 patients
were included in the analysis.
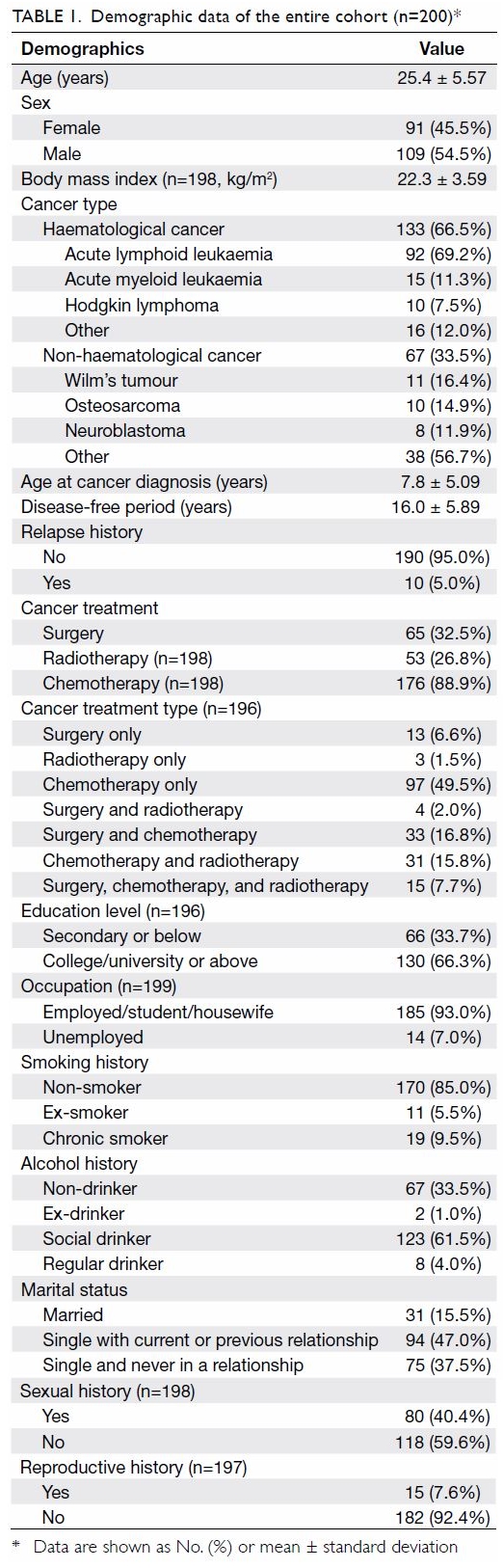
Among the 200 patients, 91 (45.5%) were women; the
mean age of all patients was 25.4 ± 5.57 years, and the mean age at
diagnosis was 7.8 ± 5.09 years. In total, 133 (66.5%) patients had
haematological cancer, among whom 92 (46.0%) had acute lymphoid leukaemia,
15 (7.5%) had acute myeloid leukaemia, and 10 (5.0%) had Hodgkin lymphoma.
Sixty-seven (33.5%) patients had non-haematological cancer, among whom 11
(5.5%) had Wilm’s tumour, 10 (5.0%) had osteosarcoma, and eight (4.0%) had
neuroblastoma. Sixty-five (32.5%) patients underwent surgery, and 23
(11.5%) exhibited visible external effects, such as limb resection.
Fifty-three (26.8%) patients received radiotherapy and 176 (88.9%)
received chemotherapy. Ten (5.0%) patients had experienced cancer relapse.
Thirty-one (15.5%) patients were married, 94 (47.0%) were single with a
current or previous relationship, and 75 (37.5%) were single and had never
been in a relationship (Table 1). Eighty (40.4%) patients reported a history
of sexual experiences and 15 (7.6%) had impregnated their partners or ever
conceived a child.
Sexual impairment and dysfunction related to childhood
cancer and treatment
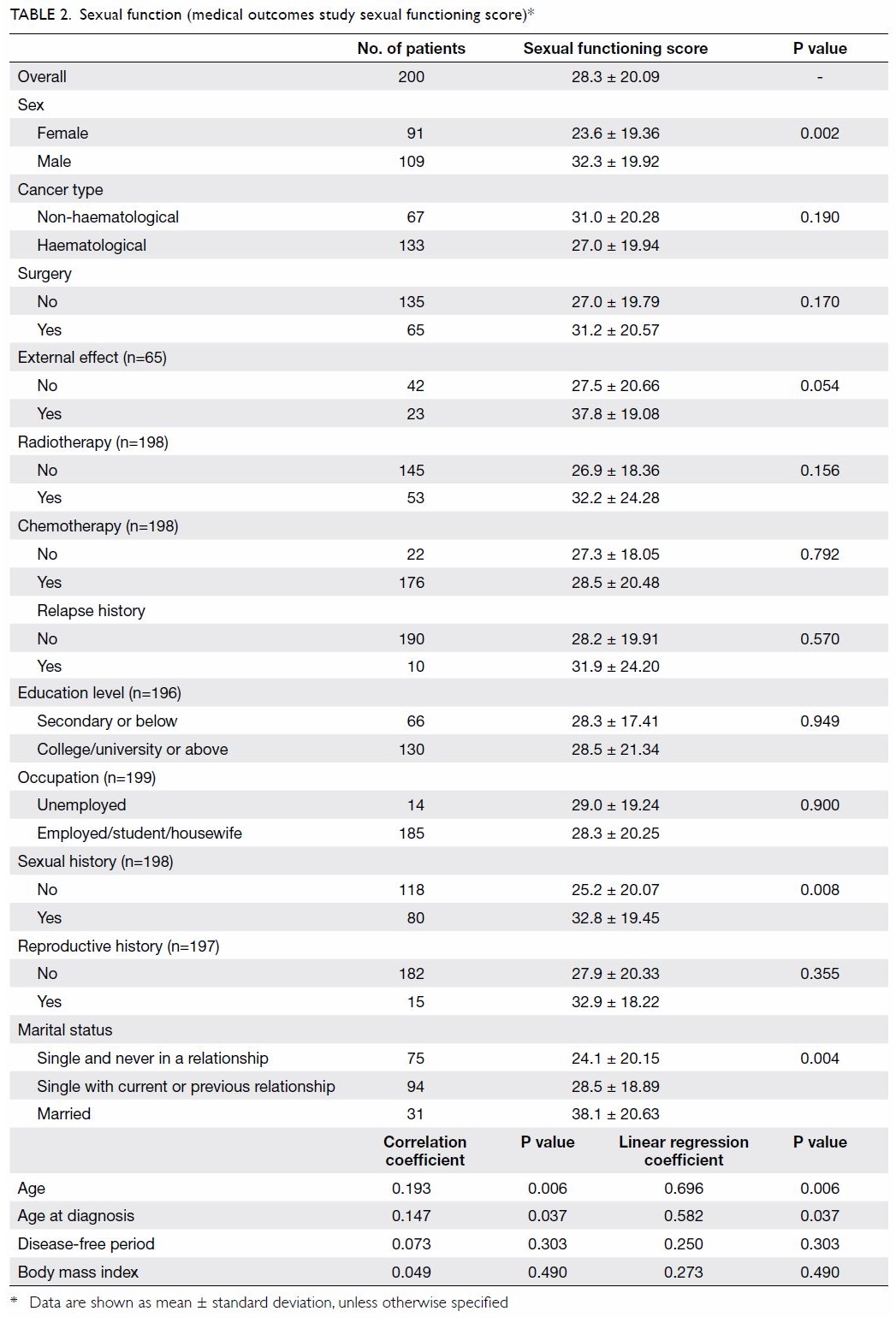
The overall medical outcomes study sexual
functioning score was 28.3 ± 20.09. Men (32.3 ± 19.92) had a significantly
higher mean sexual functioning score (ie, more sexual problems) than women
(23.6 ± 19.36, P=0.002) [Table 2]. Age at the time of this study (r=0.193,
P=0.006) and age at cancer diagnosis (r=0.147, P=0.037) were
significantly positively correlated with sexual functioning score (ie,
more sexual problems). Patients who had a history of sexual experiences
(32.8 ± 19.45, P=0.008) or who had been married (38.1 ± 20.63, P=0.004)
had a significantly higher mean sexual functioning score than patients who
had no history of sexual experiences and who had not been married (Table
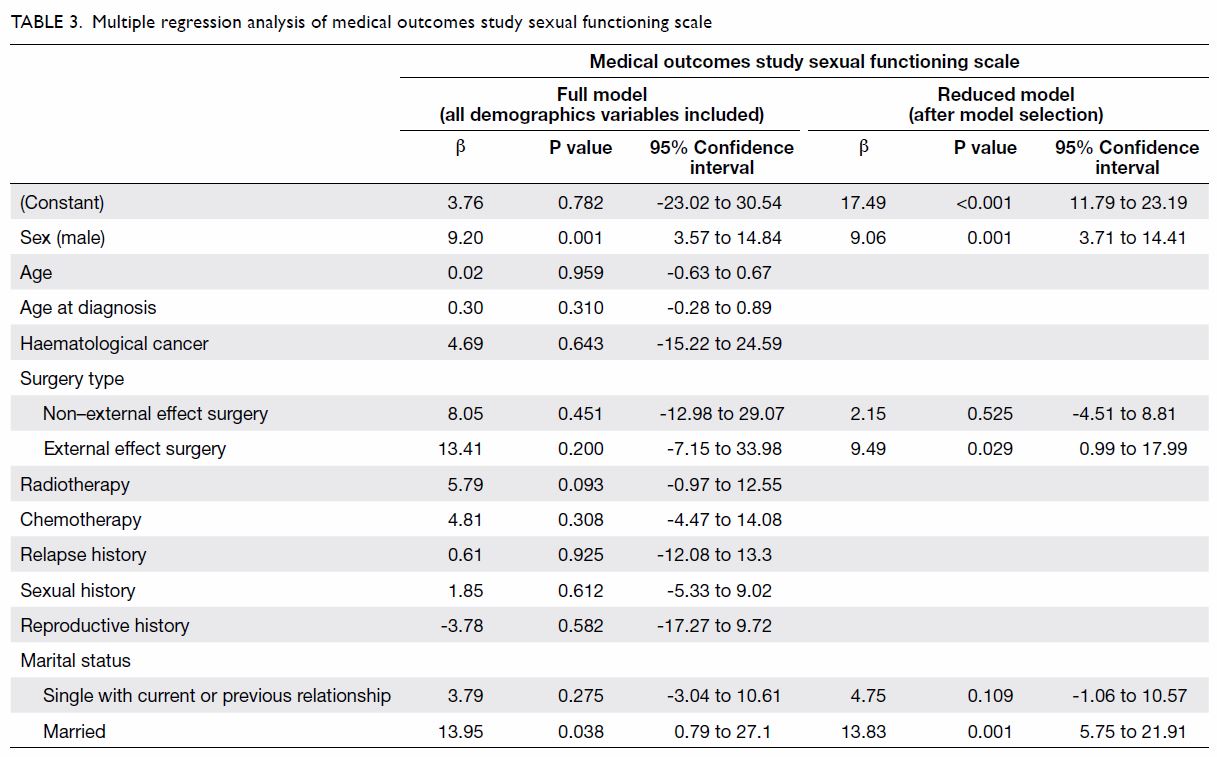
2). Multiple regression analysis controlling for all potential
confounders suggested that male sex (β=9.20, P=0.001) and marital status
of ‘married’ (β=13.95, P=0.038) were significantly associated with higher
sexual functioning score (ie, more sexual problems) [Table
3].
Assessments of self-esteem, body image, and depression
in all patients
The mean Rosenberg self-esteem scale score was 29.9
± 4.25. Multiple regression analysis showed that a history of relapse
(β=-2.89, P=0.044) was significantly associated with Rosenberg self-esteem
scale score following adjustment for other variables (ie, patients who had
a history of relapse had lower self-esteem) [online Supplementary Appendices 1 and 2].
The mean BIS score was 5.6 ± 4.45. Age at diagnosis
was statistically significantly positively correlated with BIS score (r=0.260,
P<0.001). Patients who had not undergone surgery (4.9 ± 4.09) had
significantly lower BIS score than patients who had undergone surgery (7.1
± 4.81, P=0.002). Patients who had a history of haematological cancer (4.9
± 4.11) also had significantly lower BIS score than patients who had a
history of non-haematological cancer (7.1 ± 4.77, P=0.002) [online
Supplementary Appendix 1]. Multiple regression analysis suggested
that age at diagnosis (β=0.22, P<0.001) was associated with BIS score (online Supplementary Appendix 2).
The mean PHQ score was 4.80 ± 4.27. The numbers of
patients who reported minimal depressive symptoms and major depression
were 68 (34%) and 24 (12%), respectively (online Supplementary Appendix 3). No statistical
significance was found across demographics variables (online
Supplementary Appendices 1 and 2).
The General Health Questionnaire Short Form-12
analysis revealed that the overall PCS was 51.2 ± 6.44. Age at the time of
this study (r=-0.159, P=0.025) and age at diagnosis (r=-0.170,
P=0.017) were significantly negatively correlated with PCS. Patients who
had undergone surgery without external effects (51.9 ± 5.20) had
significantly higher mean PCS (ie, better physical health) than patients
who had undergone surgery with external effects (47.5 ± 7.39, P=0.018) [online Supplementary Appendix 1]. Multiple
regression analysis showed that male sex (β=2.04, P=0.024) was
significantly associated with PCS, following adjustment for other
variables (online Supplementary Appendix 2). In contrast, the
overall MCS was 49.0 ± 9.00. There were no statistically significant
relationships between MCS and any demographic variables (online
Supplementary Appendices 1 and 2).
Subgroup analysis based on sexual functioning scores
Patients were divided into three groups based on
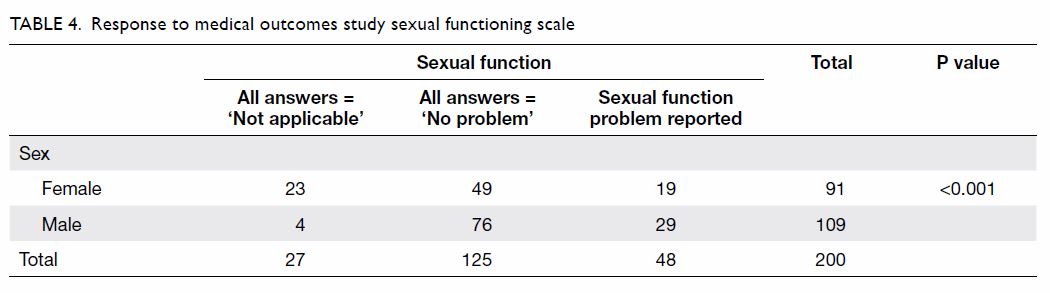
their sexual functioning scores. Forty-eight (24.0%) patients had
experienced at least one sexual problem, 125 (62.5%) patients reported
that they never had any sexual problem and/or stated ‘not applicable’, and
27 (13.5%) patients reported ‘not applicable’ for all items. Among women
in this study, 19 (20.9%) reported at least a small problem in at least
one aspect of sexual function, 49 (53.8%) reported ‘not a problem’ and/or
‘not applicable’ for all items, and 23 (25.3%) reported ‘not applicable’
for all items; among men in this study, these numbers were 29 (26.6%), 76
(69.7%), and four (3.7%), respectively (Table 4).
In the group with no sexual problems, more patients
had haematological cancers (n=89, 71.2% vs n=24, 50.0%; P=0.009), fewer
patients underwent surgery (n=34, 27.2% vs n=24, 50.0%; P=0.004), fewer
patients underwent surgery with external effects (n=9, 26.5% vs n=13,
54.2%; P=0.032), and fewer patients were regular social drinkers (n=2,
1.6% vs n=6, 12.5%; P=0.013). The group with no sexual problems also had
statistically significantly higher PCS (52.5 ± 5.62 vs 48.1 ± 7.96,
P=0.003), higher Rosenberg self-esteem scale score (30.6 ± 4.24 vs 28.4 ±
4.06, P=0.010), lower mean BIS score (4.9 ± 3.89 vs 7.5 ± 5.38, P=0.008),
and lower mean PHQ score (4.1 ± 4.44 vs. 6.8 ± 3.89, P=0.001) [Table
5]. However, in multivariable logistic regression analysis
controlling for all potential confounders, no variables were statistically
significant when comparing the group with no problems to the group with
problems. After model selection, a history of surgery with external
effects (odds ratio [OR]=6.09, P=0.001), PCS (OR=0.93, P=0.010), and
Rosenberg self-esteem scale score (OR=0.89, P=0.013) were significantly
related to the presence of sexual function problems (Table
6).
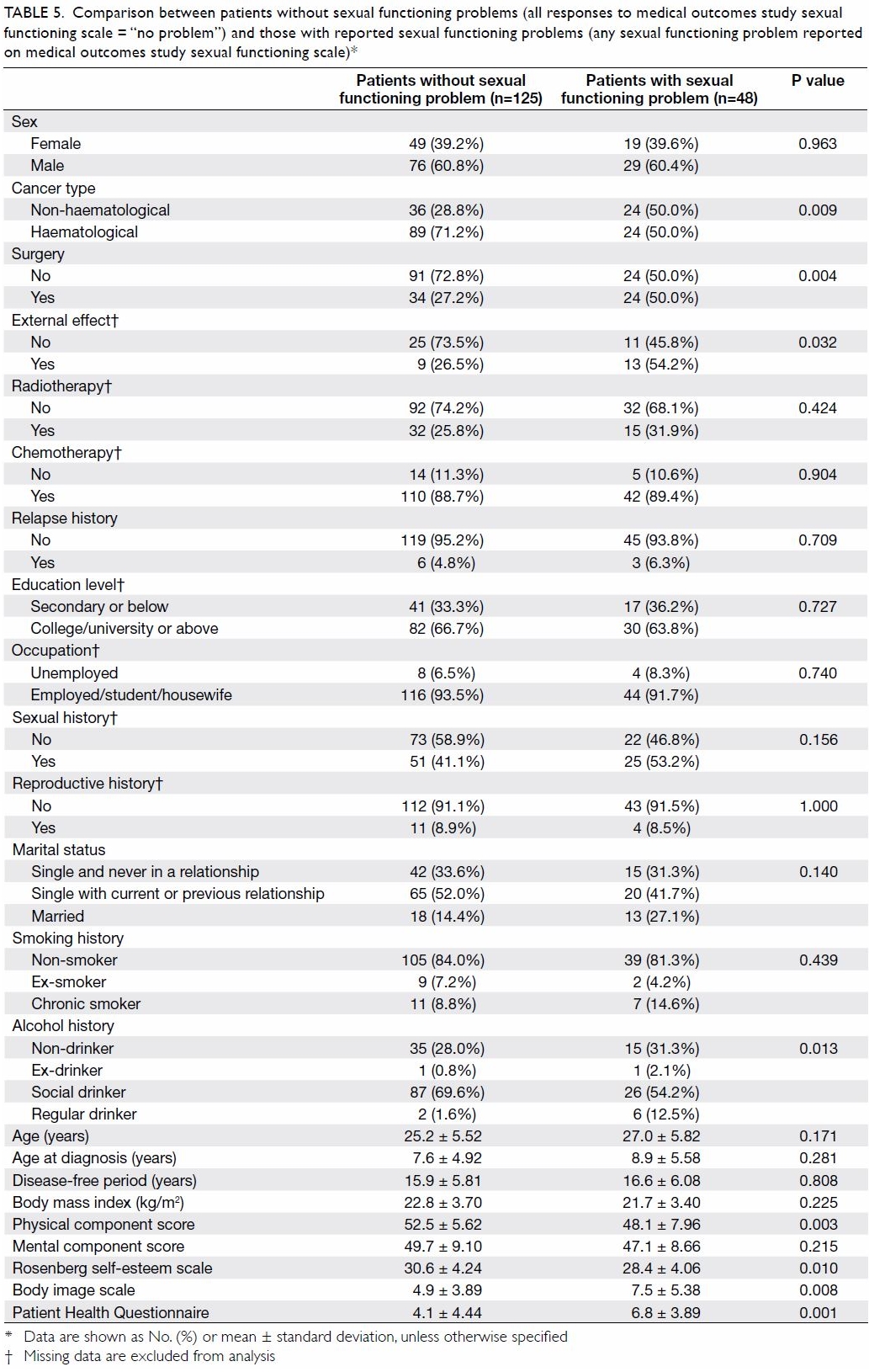
Table 5. Comparison between patients without sexual functioning problems (all responses to medical outcomes study sexual functioning scale = “no problem”) and those with reported sexual functioning problems (any sexual functioning problem reported on medical outcomes study sexual functioning scale)
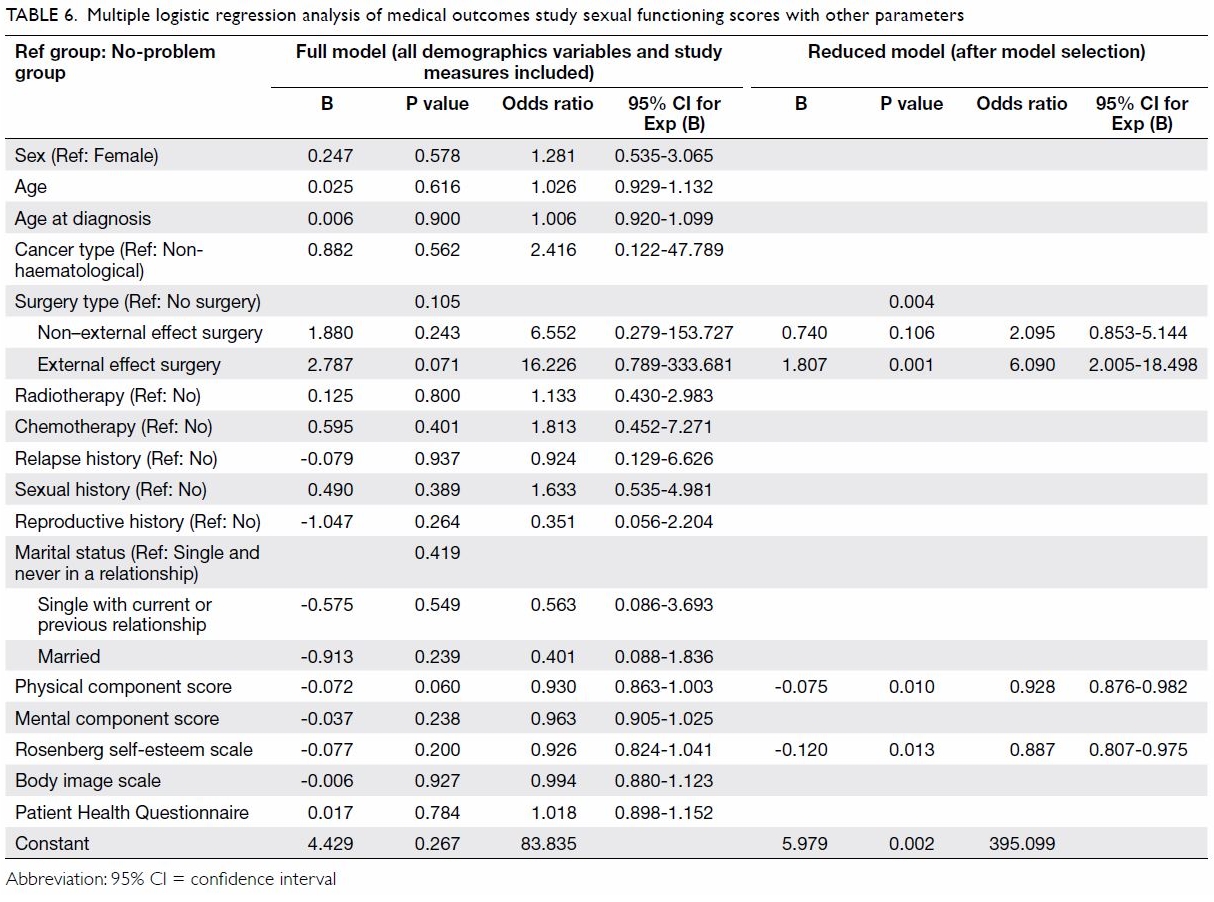
Table 6. Multiple logistic regression analysis of medical outcomes study sexual functioning scores with other parameters
Discussion
Summary
In this study, approximately one-quarter of young
Chinese cancer survivors in Hong Kong reported at least one sexual
problem. Sexual problems were more common in men, in patients diagnosed
with cancer at an older age, and in patients who were married or had a
history of sexual experiences. Moreover, the presence of sexual problems
was significantly associated with a history of surgery, as well as a
history of surgery with external effects; patients with sexual problems
generally had lower physical well-being scores, lower self-esteem scores,
higher BIS scores, and an increased number of depressive symptoms. This
new information can aid in understanding our patients’ needs and in
guiding the provision of necessary care.
Sex-related differences in sexual function outcomes
In a similar study in the United States,4 involving 599 cancer survivors aged 18 to 39 years, 52%
of female and 32% of male respondents reported at least ‘a small problem’
in one or more areas of sexual functioning. Overall, 42.7% of the patients
in that study reported at least one problematic symptom; the overall
sexual functioning score (indicative of more problems) was higher in women
(21.6) than in men (10.6). Interestingly, the findings in our study
contrasted with those of the prior study. Approximately one-quarter of
survivors (24% overall, 26.6% of men, 20.9% of women) had at least one
sexual problem. Furthermore, the overall sexual functioning scores for
male and female survivors were 32.3 and 23.6, respectively. Therefore,
fewer cancer survivors may experience sexual problems in Hong Kong.
However, the problems experienced by these survivors may be more severe,
as reflected by the higher sexual functioning score.
In our study, men had higher sexual functioning
scores (ie, more sexual problems) than women. However, compared with men
in the study (3.7%), many more women (25.3%) reported ‘not applicable’
(P<0.001). An overall lower score among women may not necessarily mean
that they experienced fewer sexual problems; it might indicate that they
were less sexually active. By excluding responses of ‘not applicable’ from
the overall sexual functioning scale assessment, we found no significant
sex-related difference in sexual functioning score (P=0.499). The mean
scores for women and men were 31.62 ± 15.72 and 33.51 ± 19.25,
respectively. Regarding patients with responses of ‘not applicable’ in the
overall sexual functioning scale, 85.2% did not have sexual experience.
Furthermore, women in the present study may have been less sexually active
than men. A larger proportion of female survivors might only have sexual
intercourse after marriage and thus be unaware of sexual problems prior to
that point. Therefore, long-term assessment of sexual function is
important for identifying sexual problems in cancer survivors, especially
women.
Sex-related differences in specific sexual problems
Overall, the most common sexual problems reported
were difficulties in relaxing and enjoying sex (19.5%) and difficulties in
achieving an erection or orgasm (18.5%). Comparatively fewer survivors
reported lack of sexual interest (13.0%) and problems in becoming sexually
aroused (13.5%). Frederick et al16 performed a semi-structured interview
study in a paediatric oncology and survivorship clinic, involving 22
childhood cancer survivors aged 18 to 39 who reported two or more sexual
problems. The most commonly reported sexual problems were also
difficulties in relaxing and enjoying sex (n=19, 86%) and difficulties in
achieving an erection or orgasm (n=18, 82%), as in the present study.
Frederick et al16 also reported
that for each of the sexual function items, the proportion of women who
reported problems (34.1%-39.5%) was greater than the proportion of men who
reported problems (15.3%-20.4%). However, our study showed similar
proportions of women and men experiencing problems in becoming sexually
aroused (women: 13.2%, men: 13.8%) and in achieving an erection or orgasm
(women: 18.7%, men: 18.3%). A greater proportion of men reported a lack of
sexual interest (women: 8.8%, men: 16.5%) and an inability to relax or
enjoy sex (women: 17.6%, men: 21.1%). The sexual problems experienced by
cancer survivors seemed to differ between sexes. In Chinese culture, men
play a more dominant role in a relationship, and typically initiate sexual
activity.17 This might be why more
men reported problems regarding sexual desire, including sexual interest,
relaxation, and enjoyment. Because Asian women are more passive in terms
of sexual activity, they might not view reduced sexual interest as a
problem.18 Instead, they might be
more concerned with an inability to achieve orgasm during sex.
The authors of previous studies proposed that
greater numbers of female survivors reported sexual problems because they
were more likely to experience cancer-related physical changes and
psychological distress.4 19 However, our study showed no significant sex-related
differences in physical health (P=0.072), mental health (P=0.354),
self-esteem (P=0.184), body image (P=0.057), or depressive symptoms
(P=0.349). This implies that the cancer survivors in our study did not
experience sex-specific effects of their childhood cancer experience on
their quality of life.
Implications for patient treatment
It is well-known that treatments for cancer may
cause adverse effects on sexual function. Both Kenney et al20 and Van Dorp et al21
reviewed the literature regarding reproductive health of male and female
survivors. They noted that alkylating agent chemotherapy and gonadal
irradiation carried dose-related risks of primary gonadal dysfunction,
which affected both sexual function and fertility. Chow et al22 also stated that surgery might involve long-term
consequences, disfiguration with psychosocial impact, and delayed
complications. Our study found that a larger proportion of survivors who
had undergone surgery, especially surgery with external effects, reported
problems involving sexual function, whereas survivors who had undergone
radiation or chemotherapy showed no significant difference between the
proportions of survivors who reported the presence or absence of problems
involving sexual function.
Adolescence and young adulthood are the points in
life when people focus intensely on their own bodies and can experience
dissatisfaction with their bodies and physical appearances.23 Any alterations in physical appearance may affect
their self-perceptions. Indeed, in a study involving focus groups and
questionnaire surveys among survivors aged 15 to 29 years and matched
controls to investigate body image and sexual health among adolescents and
young adult cancer survivors, Olsson et al24
found that survivors perceived themselves to be less sexually attractive
due to scars on their bodies and were less satisfied with their sexual
function, compared with their matched controls.
With the progression of surgical techniques, such
as the introduction of minimally invasive surgery, we presume that the
impacts of scarring and physical disfiguration may be minimised. Until
this change occurs, healthcare professionals should provide information
regarding the potential adverse effects of treatments on the reproductive
system and sexual function, as well as counselling to the survivors;
importantly, survivors interviewed in previous studies indicated they had
unmet needs for information, support, and counselling.20
Limitations
There were some limitations in our study. Because
we did not include a control arm, we could not assess whether there were
any differences between our patients and similar age-matched young adults
in terms of the measured parameters. Therefore, we plan to perform a
follow-up study that involves the application of the assessments in these
questionnaires to similarly aged individuals in the general population to
confirm our findings. Another limitation of this study was that it was
performed in a single centre and the findings may be biased due to the
specific patient population involved. However, this is one of the main
children’s cancer centres in Hong Kong, and is therefore a major referral
centre that receives patients from various regions of Hong Kong; combined
with the moderate sample size, we consider this to provide a good
representation of adult survivors of childhood cancer in Hong Kong.
Conclusion
In this cross-sectional study of 200 young Chinese
cancer survivors, approximately one-quarter of the patients reported at
least one sexual problem. A history of sexual problems was significantly
associated with a history of surgery, as well as a history of surgery with
external effects. Compared with patients without sexual problems, those
with sexual problems generally had lower physical well-being scores, lower
self-esteem scores, higher body image distress scores, and an increased
number of depressive symptoms. Given the findings in this study, aspects
of life beyond disease condition and physical function should be
considered in adult survivors of childhood cancers. Moreover, appropriate
referral and intervention should be initiated for these patients when
necessary.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: CF Ng, AWK Leung.
Acquisition of data: BSY Lau, CF Ng, AWK Leung.
Analysis or interpretation of data: CYL Hong, BSY Lau, CF Ng.
Drafting of the article: CYL Hong, BSY Lau, CF Ng.
Critical revision for important intellectual content: All authors.
Acquisition of data: BSY Lau, CF Ng, AWK Leung.
Analysis or interpretation of data: CYL Hong, BSY Lau, CF Ng.
Drafting of the article: CYL Hong, BSY Lau, CF Ng.
Critical revision for important intellectual content: All authors.
Conflicts of interest
As editors of the journal, CF Ng and JYC Teoh were
not involved in the peer review process. Other authors have no conflicts
of interest to disclose.
Funding/support
The project was supported by Hong Kong Children
Cancer Fund.
Ethics approval
Approvals (CRE-2014.674) from The Joint Chinese
University of Hong Kong—New Territories East Cluster Clinical Research
Ethics Committee were obtained.
References
1. World Health Organization. Sexual
health—a new focus for WHO. Prog Reprod Health Res 2004;67:1-8.
2. Gan HW, Spoudeas HA. Long-term follow-up
of survivors of childhood cancer (SIGN Clinical Guideline 132). Arch Dis
Child Educ Pract Ed 2014;99:138-43. Crossref
3. Jacobs LA, Pucci DA. Adult survivors of
childhood cancer: the medical and psychosocial late effects of cancer
treatment and the impact on sexual and reproductive health. J Sex Med
2013;10 Suppl 1:120-6. Crossref
4. Zebrack BJ, Foley S, Wittmann D, Leonard
M. Sexual functioning in young adult survivors of childhood cancer.
Psychooncology 2010;19:814-22. Crossref
5. Sundberg KK, Lampic C, Arvidson J,
Helström L, Wettergren L. Sexual function and experience among long-term
survivors of childhood cancer. Eur J Cancer 2011;47:397-403. Crossref
6. Bober SL, Zhou ES, Chen B, Manley PE,
Kenney LB, Recklitis CJ. Sexual function in childhood cancer survivors: a
report from project REACH. J Sex Med 2013;10:2084-93. Crossref
7. Sherbourne C. Social functioning: sexual
problems measures. In: Stewart AL, Ware JE, editors. Measuring Functioning
and Well-being: the Medical Outcomes Study Approach. Durham (NC), United
States: Duke University Press; 1992: 194-204.
8. Ware J Jr, Kosinski M, Keller SD. A
12-item short-form health survey: construction of scales and preliminary
tests of reliability and validity. Med Care 1996;34:220-33. Crossref
9. Lam CL, Tse EY, Gandek B. Is the
standard SF-12 health survey valid and equivalent for a Chinese
population? Qual Life Res 2005;14:539-47. Crossref
10. Rosenberg M. Society and the
Adolescent Self-image. Princeton [NJ], United States: Princeton University
Press; 1965.
11. Li HC, Chung OK, Ho KY, Chiu SY, Lopez
V. A descriptive study of the psychosocial well-being and quality of life
of childhood cancer survivors in Hong Kong. Cancer Nurs 2012;35:447-55. Crossref
12. Hopwood P, Fletcher I, Lee A, Al
Ghazal S. A body image scale for use with cancer patients. Eur J Cancer
2001;37:189-97. Crossref
13. Lin MS. An investigation on the body
image, medication adherence and quality of life in patients with systemic
lupus erythematosus after prednisolone treatment [thesis]. China Medical
University, Taichung City, Taiwan; 2010.
14. Kroenke K, Spitzer RL, Williams JB.
The PHQ-9: validity of a brief depression severity measure. J Gen Intern
Med 2001;16:606-13. Crossref
15. Yu X, Tam WW, Wong PT, Lam TH, Stewart
SM. The Patient Health Questionnaire-9 for measuring depressive symptoms
among the general population in Hong Kong. Compr Psychiatry
2012;53:95-102. Crossref
16. Frederick NN, Recklitis CJ, Blackmon
JE, Bober S. Sexual dysfunction in young adult survivors of childhood
cancer. Pediatr Blood Cancer 2016;63:1622-8. Crossref
17. Lo SS, Kok WM. Sexual behavior and
symptoms among reproductive age Chinese women in Hong Kong. J Sex Med
2014;11:1749-56. Crossref
18. Atallah S, Johnson-Agbakwu C,
Rosenbaum T, et al. Ethical and sociocultural aspects of sexual function
and dysfunction in both sexes. J Sex Med 2016;13:591-606. Crossref
19. The Family Planning Association of
Hong Kong. Report on youth sexuality study 2016. 2017. Available from:
https://www.famplan.org.hk/en/media-centre/press-releases/detail/fpahk-report-on-youth-sexuality-study.
Accessed 9 Jul 2018.
20. Kenney LB, Antal Z, Ginsberg JP, et
al. Improving male reproductive health after childhood, adolescent, and
young adult cancer: progress and future directions for survivorship
research. J Clin Oncol 2018;36:2160-8. Crossref
21. van Dorp W, Haupt R, Anderson RA, et
al. Reproductive function and outcomes in female survivors of childhood,
adolescent, and young adult cancer: a review. J Clin Oncol
2018;36:2169-80. Crossref
22. Chow EJ, Antal Z, Constine LS, et al.
New agents, emerging late effects, and the development of precision
survivorship. J Clin Oncol 2018;36:2231-40. Crossref
23. Cash T. Encyclopedia of Body Image and
Human Appearance. Oxford, United Kingdom: Elsevier Science; 2012.
24. Olsson M. Adolescent and Young Adult
Cancer Survivors— Body Image and Sexual Health. Gothenburg, Sweden:
University of Gothenburg; 2018.