Hong Kong Med J 2019 Oct;25(5):363–71 | Epub 9 Oct 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Epidemiology of respiratory syncytial virus infection
and its effect on children with heart disease in Hong Kong: a multicentre
review
SH Lee, MB, BS, FRCPCH1; KL Hon, MB, BS, MD2; WK Chiu, MB, BS, MD3;
YW Ting, MB, ChB, FHKAM (Paediatrics)4; SY Lam, MB, BS, FRCPCH4
1 Department of Paediatrics and
Adolescent Medicine, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Department of Paediatrics, The Chinese
University of Hong Kong, Shatin, Hong Kong
3 Department of Paediatrics and
Adolescent Medicine, United Christian Hospital, Kwun Tong, Hong Kong
4 Department of Paediatrics and
Adolescent Medicine, Tuen Mun Hospital, Tuen Mun, Hong Kong
Corresponding author: Dr SH Lee (leeshm@ha.org.hk)
Abstract
Objectives: There is no
guideline in Hong Kong regarding respiratory syncytial virus (RSV)
immunoprophylaxis for children with heart disease because of a lack of
local data on RSV infection. Therefore, this study evaluated the
epidemiology and impact of RSV infection on children with heart disease
in Hong Kong, with the goal of providing recommendations regarding RSV
immunoprophylaxis.
Methods: This multicentre
retrospective case-control study on paediatric RSV infection was
conducted in four local regional hospitals from 2013 to 2015. The
patients’ demographic and clinical data were retrieved and analysed.
Results: There were 3538 RSV
hospitalisations during the study period, and the mortality rate was
0.14%. Some RSV seasonality was present in Hong Kong, primarily in
spring and summer. Respiratory syncytial virus infection was positively
correlated with relative humidity and negatively correlated with wind
speed and atmospheric pressure. Patients with heart disease had a more
severe outcome than those without, including longer median hospital stay
(4 vs 2 days, P<0.001), higher complication rate (28.6% vs 9.8%,
P<0.001), and higher rates of intensive care (11.6% vs 1.4%,
P<0.001) and mechanical ventilation (3.6% vs 0.4%, P=0.003).
Complications in non-cardiac patients included myocarditis and Kawasaki
disease. Predictors of severe RSV infection in patients with heart
disease were heart failure, pulmonary hypertension, and severe airway
abnormalities associated with congenital heart disease.
Conclusions: Respiratory
syncytial virus infection occurs mainly in spring and summer in Hong
Kong, and is related to meteorological conditions. Respiratory syncytial
virus infection poses a heavy disease burden on children with heart
disease. A local guideline on RSV immunoprophylaxis for these children
is therefore needed.
New knowledge added by this study
- This study reviewed the epidemiology and impact of respiratory syncytial virus (RSV) infection on children with heart disease (HD) in Hong Kong.
- RSV infections are common in Hong Kong, and the incidence peaks from March to August; prevalence is greatest in children aged <1 year, and there is a mild male preponderance. Infection is favoured by high relative humidity, low wind speed, and low atmospheric pressure.
- HD, both congenital and acquired, is a distinct risk factor for severe RSV infection in terms of hospital length of stay, reinfection, complication, respiratory failure, and the requirements for intensive care unit care and mechanical ventilation.
- A local guideline on RSV immunoprophylaxis is needed for children with HD.
- In Hong Kong, an RSV immunoprophylaxis scheme administered monthly for 5 months, beginning in the first year of life, should be considered in children with HD who exhibit any of the following severity predictors: heart failure, pulmonary hypertension, and severe airway abnormalities associated with congenital HD.
- The optimal timing for immunoprophylaxis may be during the local peak of infection, from March to August.
Introduction
Respiratory syncytial virus (RSV) infection poses a
heavy disease burden in children worldwide.1
2 Haemodynamically significant
congenital heart disease (hs-CHD) has been mainly studied and identified
as a risk factor for severe RSV infection.3
4National immunoprophylaxis
policies for RSV in children have been adopted worldwide.5 6 7 8 9 10 11 In Hong Kong, under the Paediatric Coordinating
Committee of the Hospital Authority, guidelines and government funding for
RSV immunoprophylaxis for children with bronchopulmonary dysplasia of
prematurity were established in 2012.12
However, no consensus has been reached regarding guidelines for RSV
immunoprophylaxis for children with congenital or acquired heart disease
(HD), because the epidemiology and impact of RSV infection on these
patients have not been delineated in Hong Kong.13
14 15
16 It has been suggested that the
high morbidity and mortality rates of RSV infection in children with
hs-CHD observed during the pre-palivizumab era3
are no longer applicable, owing to advances in healthcare. To widen the
scope of the study regarding HD there is a need to conduct an updated
local study regarding the epidemiology and impact of RSV infection on
children with all types of HD, with the aim of providing evidence-based
recommendations for RSV immunoprophylaxis.
Methods
Study design
Hong Kong has a population of 7.48 million,
including a paediatric population (aged ≤18 years) of approximately 1.1
million. Over 90% of in-patient service and nearly all tertiary service is
provided by the public health system. There are 12 public hospitals
providing approximately 1500 acute paediatric beds, of which 45 are
paediatric intensive care beds; the total annual discharges and deaths are
approximately 88 500. The present study was a multicentre retrospective
case-control study of RSV infection in children in four hospitals with
large paediatric departments, including 22 paediatric intensive care beds
and approximately 630 acute paediatric beds, from 1 January 2013 to 31
December 2015.
Recruitment criteria
Paediatric patients were recruited using the
Clinical Data Analysis and Reporting System electronic database of the
Hong Kong Hospital Authority. The inclusion criteria were: (1) any
discharge diagnosis of RSV infection, including bronchiolitis and
pneumonia [International Classification of Diseases, Ninth Revision,
codes: 079.6 (0), 466.0 (9), 466.11, 480.1]; and (2) laboratory
confirmation of RSV infection from patients’ nasopharyngeal or
endotracheal secretions, either by immunofluorescent antigen staining (D3
Ultra 8 DFA; Diagnostic Hybrids Inc, Athens [OH], US) or RNA detection by
reverse transcriptase polymerase chain reaction (Xpert Xpress Flu/RSV;
Cepheid, Sunnyvale [CA], US).
Data collection
Information was collected regarding epidemiologic
characteristics, demographics, and clinical information (eg, laboratory
and pharmacy data, pre-existing co-morbidities, complications,
reinfection, and intensive care unit [ICU] and ventilator requirements).
Complications were defined as new secondary diagnosis related to RSV
infection in a specific episode. Heart disease was defined as the presence
of any active HD, including structural defects and cardiomyopathies.
Patients who showed complete resolution of HD were considered normal.
Diagnosis was confirmed by echocardiography, with or without cardiac
catheterisation. Details of HD were retrieved, such as haemodynamic
status, need for medication, and need for operation. One designated
paediatrician from each hospital verified and retrieved information from
the patients’ paper records, if needed. The numbers of hospital admissions
for acute respiratory infections in the same period were also retrieved
from each hospital. Meteorological information was obtained from the
electronic database of the Hong Kong Observatory.
Main outcomes
First, the epidemiology of RSV infection and its
association with meteorological conditions in the entire cohort was
studied. Then patients were excluded if they had significant
co-morbidities other than HD, including chronic lung disease,
neuromuscular problems, and immunocompromised status, which are expected
to increase the severity of RSV infection.17
18 19
Patients with social problems awaiting placement in the hospital causing
undue prolonged hospital stay were also excluded. The remaining patients
were then divided into heart disease (HD) and control (without any
co-morbidities) groups to compare the severity of RSV infection. Cardiac
patients alone were analysed to identify cardiac predictors of severe
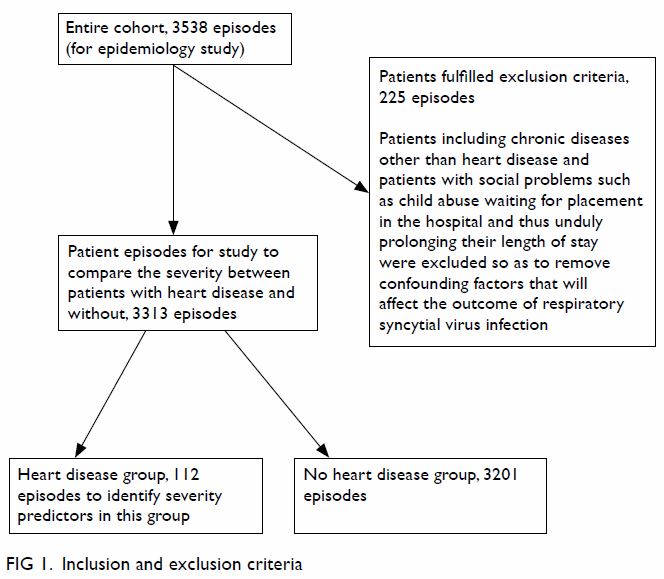
outcome from RSV infection (Fig 1).
Statistical analysis
Statistical analysis was conducted using SPSS
(Windows version 23.0; IBM Corp, Armonk [NY], US). Continuous data were
tested for normality. There was a large difference in sample size between
the HD and control groups; therefore, non-parametric tests were used to
compare these groups: the Mann-Whitney U test was used for
univariate analysis of continuous data, and the Chi squared test or
Fisher’s exact test were used for categorical variables, where
appropriate. The same statistical analyses were repeated using a smaller
control group (1/10 of the original size), which was obtained by
generating an age- and sex-matched random sample from the original control
group. This repeat analysis yielded similar results, which showed that the
statistical tests used were acceptable despite the large discrepancy in
sample size. Univariate and multivariate regression analyses were also
performed. A backward variable selection method was used for model
building. Results were considered statistically significant when P≤0.05.
Results
Epidemiology
There were 3538 RSV-related hospital admissions in
the four paediatric departments during the 3-year period, which
constituted 11.8% of acute respiratory infection admissions to the four
departments and 43% of all RSV admissions to public hospitals in Hong
Kong. The age distribution of the admitted patients is shown in Table
1. The RSV infections primarily affected children aged <5 years:
96.2% in the entire cohort and 97.3% in the HD group. Furthermore, RSV
infections were most common in children aged <1 year: 44.6% in the
entire cohort and 56.3% in the HD group. There was a mild male sex
preponderance in the entire cohort, as well as in the HD group:
male-to-female ratios of 1.32 and 1.29). The mortality rates were 0.14%
(n=5) in the entire cohort and 0% in the HD group. All patients who died
had an underlying chronic illness, such as congenital hereditary muscular
dystrophy, spinal muscular atrophy, acute leukaemia, or asthma.
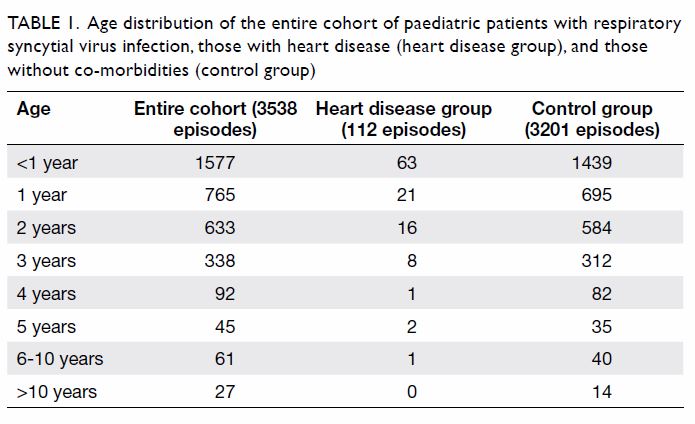
Table 1. Age distribution of the entire cohort of paediatric patients with respiratory syncytial virus infection, those with heart disease (heart disease group), and those without co-morbidities (control group)
The aggregated monthly trend of RSV infection is
shown in Figure 2. Most infections (87.7% of those in the
entire cohort and 91.1% of those in the HD group) occurred during the
period from January to September, peaking from March to August (the spring
and summer months). With respect to meteorological factors, bivariate
correlation analysis of the entire cohort showed that the aggregated
monthly RSV incidence had a statistically significant positive correlation
with the monthly mean relative humidity (r=0.71, P=0.010) and
statistically significant negative correlations with the monthly mean wind
speed (r=-0.80, P=0.002) and monthly mean atmospheric pressure (r=-0.62,
P=0.032) [Table 2]. Backward linear regression revealed that
only monthly mean wind speed was significantly and independently
associated with monthly RSV incidence (B=-31.716, 95% confidence interval
[CI]=-48.61 to -14.82; P=0.002).
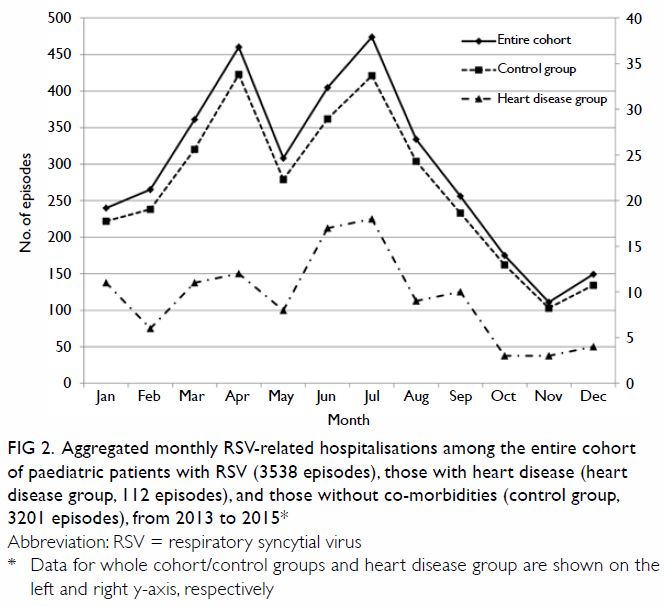
Figure 2. Aggregated monthly RSV-related hospitalisations among the entire cohort of paediatric patients with RSV (3538 episodes), those with heart disease (heart disease group, 112 episodes), and those without co-morbidities (control group, 3201 episodes), from 2013 to 2015
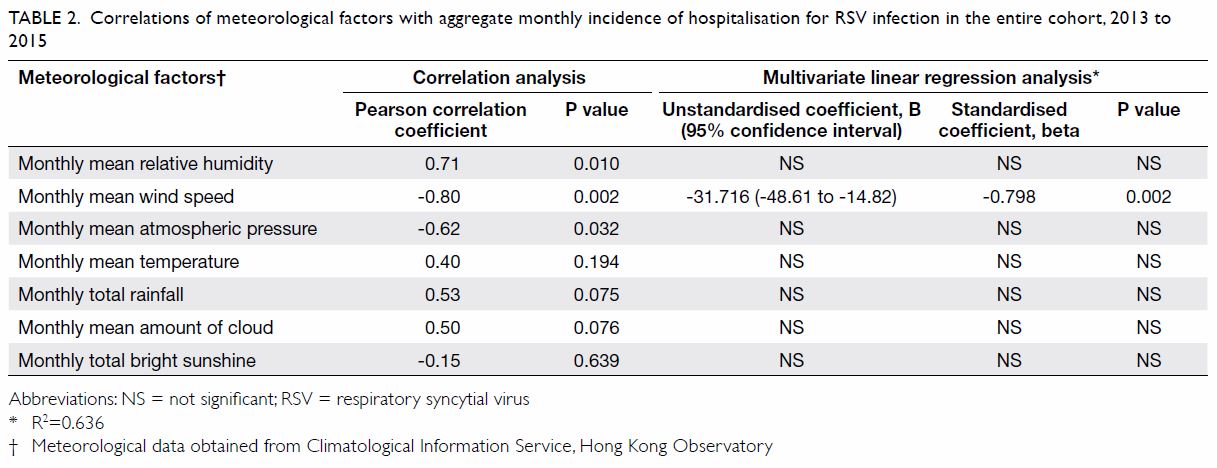
Table 2. Correlations of meteorological factors with aggregate monthly incidence of hospitalisation for RSV infection in the entire cohort, 2013 to 2015
Impact of respiratory syncytial virus infection on
children with heart disease
Overall, 225 episodes were excluded because they
involved patients who had co-morbidities other than HD. The HD group had
112 episodes involving 105 patients, while the control group had 3201
episodes involving 3155 patients. The demographic and clinical details of
both groups are depicted in Table 3. The HD and control groups had similar age
distribution (median [interquartile range]=0.9 years [0.46-1.97 years] vs
1.19 years [0.45-2.4 years]; P=0.068) and sex proportion (male
preponderance of 56.3% vs 56.9%, P=0.899). There was no statistically
significant difference in mortality rate (0% in HD group vs 0.03% in
control group; P=1). Patient-based analysis revealed that the HD group had
a higher RSV re-infection rate (>1 episode in the study period) than
the rate in the control group (7.6% vs 1.7%, P<0.001). Respiratory
syncytial virus infection was statistically significantly more severe in
the HD group than in the control group in terms of the hospital length of
stay (median=4 days [3-6 days] vs 2 days [2-4 days]; P<0.001),
respiratory failure requiring respiratory support (17.0% vs 5.1%;
P<0.001), requirement of ICU care (11.6% vs 1.4%, P<0.001),
requirement of invasive or non-invasive mechanical ventilation (3.6% vs
0.4%, P=0.003), and occurrence of complications (28.6% vs 9.8%,
P<0.001). Respiratory failure and dehydration were common complications
in both groups. However, heart failure exacerbation (n=13) and arrhythmia
(n=3) only occurred in the HD group, while febrile convulsion (n=96),
acute myocarditis (n=2), and Kawasaki disease (n=8) were only observed in
the control group. There was no statistically significant difference
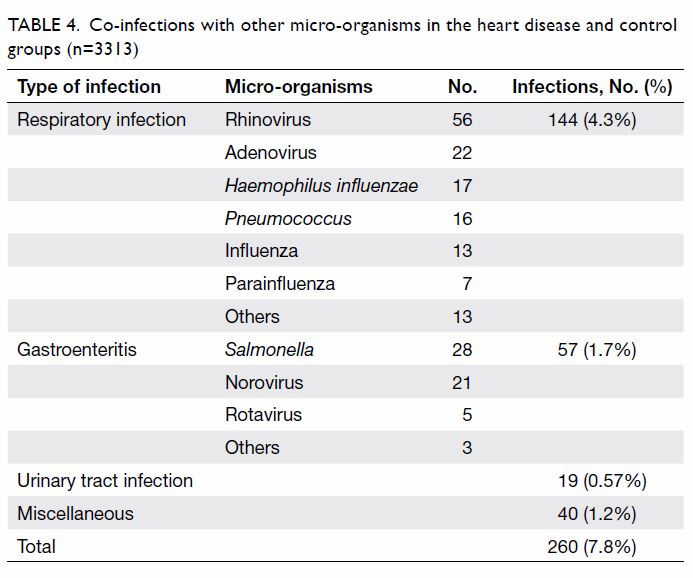
between the two groups in terms of co-infection rate (8.9% vs 7.8%;
P=0.665). More than half of co-infections (55.4%) were due to respiratory
organisms: rhinovirus was most common, followed by adenovirus, Haemophilus
influenzae, and Pneumococcus bacteria (Table
4).
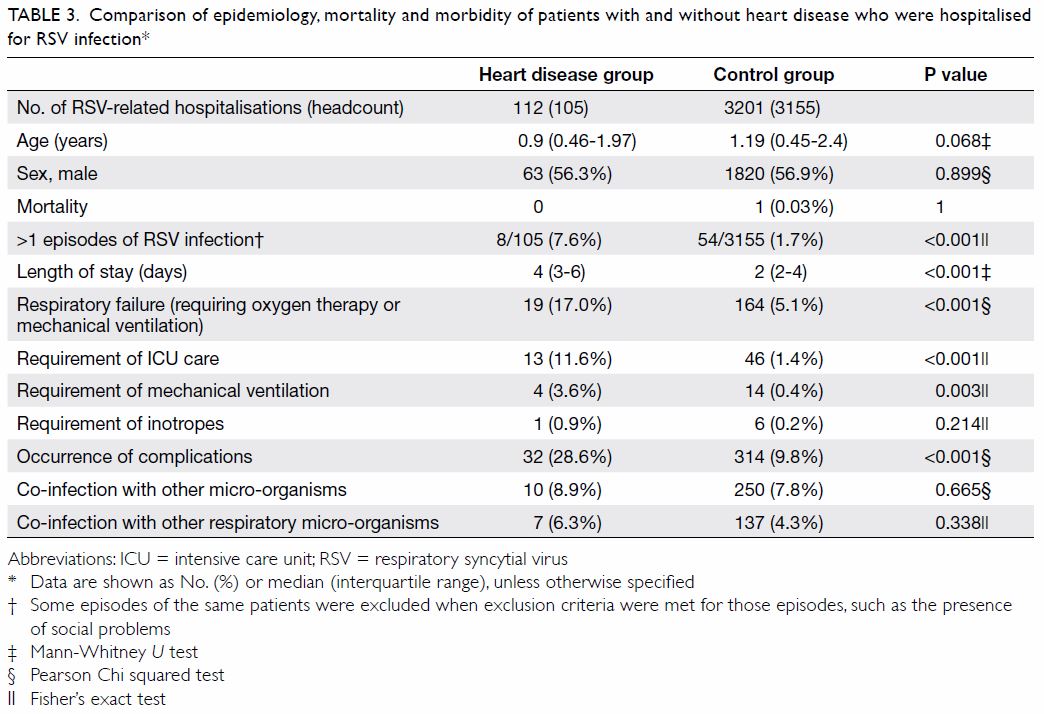
Table 3. Comparison of epidemiology, mortality and morbidity of patients with and without heart disease who were hospitalised for RSV infection
Predictors of severe outcome in cardiac patients
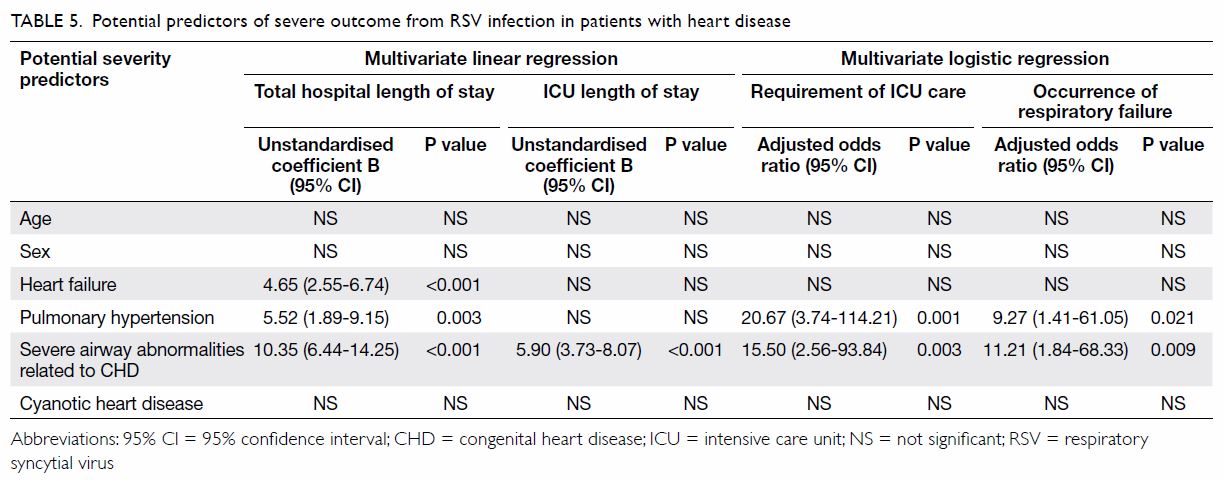
In the HD group, 26 patients had a history of
partial surgical repair of heart lesions, while nine others required
urgent cardiac surgery after recovery from RSV infection. Four cardiac
risk factors were identified: (1) heart failure requiring medications
(n=26); (2) pulmonary hypertension (n=7); (3) severe airway abnormalities
associated with congenital heart disease (CHD) such as pulmonary artery
sling (n=6); and (4) cyanotic CHD (n=1). Each patient could have multiple
risk factors; 75 patients had no risk factors. Regression analyses
involving age, sex, and the four risk factors were conducted to identify
variables that could predict the severity of RSV infection. Backward
multivariate linear regression showed that total hospital length of stay
was positively and independently associated with heart failure (B=4.65,
95% CI=2.55-6.74, P<0.001), pulmonary hypertension (B=5.52, 95%
CI=1.89-9.15, P=0.003), and airway abnormalities (B=10.35, 95%
CI=6.44-14.25, P<0.001). The ICU length of stay was positively and
independently associated with airway abnormalities (B=5.90, 95%
CI=3.73-8.07, P<0.001). Backward binary logistic regression revealed
that the requirement of ICU care was positively associated with pulmonary
hypertension (adjusted odds ratio [aOR]=20.67, 95% CI=3.74-114.21,
P=0.001) and airway abnormalities (aOR=15.50, 95% CI=2.56-93.84, P=0.003).
Similarly, respiratory failure was positively associated with both
pulmonary hypertension (aOR=9.27, 95% CI=1.41-61.05, P=0.021) and airway
abnormalities (aOR=11.21, 95% CI =1.84-68.33, P=0.009) [Table
5].
Discussion
To the best of our knowledge, this is the first
study reviewing the epidemiology and impact of RSV infection on children
with HD in Hong Kong. The representative sample in this study included 43%
of all RSV-related hospitalisations in the public sector. Respiratory
infection due to RSV was common in children in Hong Kong, causing 11.8% of
hospital admissions for acute respiratory infection; it was most common in
children aged <5 years, similar to the findings of another study in
Hong Kong (96.2% vs 98.4%).13
However, the incidence of respiratory infection due to RSV in patients
aged <1 year in the present study was lower than that in Western
studies (44.6% vs 75%-90%).2 This is
potentially because babies in Hong Kong are typically cared for at home
with the help of grandparents or domestic helpers, rather than attending
nursery facilities. Coupled with the small number of children in most Hong
Kong families, this may have reduced the rate of cross-infection. 20 Male sex was identified as a risk factor for
RSV-related hospitalisation, as in prior studies, but the male
preponderance in this study was slightly lower than that of other studies
(1.32:1 vs 1.44-1.65:1).2 13 20 21 22 23 The mortality rate of RSV-related hospitalisation was
extremely low (0.14%), which was similar to the rates in local and
worldwide studies (0.15%-1%).2 13 15 Most
deaths occurred in patients who had underlying chronic illnesses.
Although the seasonality of RSV infection in Hong
Kong, a subtropical area, is not well-defined in the manner observed in
temperate regions, there is a degree of seasonal variation. As in other
studies from Hong Kong and Singapore,13
14 15
16 24
this study showed that RSV infection peaked in the humid spring and summer
months, but was less common from October to December during the dry and
windy season. Indeed, analysis of meteorological data showed that RSV
infection was positively correlated with relative humidity and negatively
correlated with both wind speed and atmospheric pressure. Relative
humidity has been consistently associated with RSV infection rate in other
studies.13 16 25 Notably,
RSV in large particle aerosol form is more stable at higher humidity and
may thus favour transmission.25
While temperature, rainfall, and sunlight were associated with RSV
infection in previous studies,13 16 25
this study showed that wind speed was negatively associated with RSV
infection. We presume that strong wind disturbs the stability of RSV in
aerosol. We recommend that individuals who care for young children
maintain a dry and well-ventilated environment to decrease the likelihood
of cross-infection. In addition, this study highlighted the differences in
RSV seasonality in Hong Kong, relative to other parts of Asia. Taiwan has
two biennial peaks (spring and autumn), while Malaysia has a single peak
infection period (September to December).22
23 Thus, local epidemiological and
climatic data are pivotal in determining the appropriate timing for RSV
immunoprophylaxis.
In this study, we included all types of HD, rather
than CHD alone; notably, acquired HD, such as cardiomyopathy, can increase
vulnerability to RSV infection.26
The characteristics of RSV infection in cardiac patients have changed.
First, the percentage of cardiac patients in this study was lower than
that in prior studies (3.2% vs 8%-16.4%).3
22 27
28 29
Second, the outcome of RSV infection in this study was less severe than
that of prior studies with respect to the requirement of ICU care (11.6%
vs 30.4%-63%), requirement of mechanical ventilation (3.6% vs 19%-24%),
and rate of mortality (0% vs 2.5%-37%).3
22 27
28 29
These differences may be related to the ongoing tendencies for economic
and healthcare improvement; they may also be related to good antenatal
ultrasound service, termination of pregnancies involving severe CHD, and
easy access to medical service in Hong Kong. However, as in other studies,
children with HD in Hong Kong exhibit significantly more severe outcomes
from RSV infection than do children without co-morbidity.15 22 26 29 30 31
In the present study, myocarditis and Kawasaki
disease were complications of RSV infection in the non-cardiac group.
These associations have not been extensively reported in prior studies,
and further investigation is needed. The lack of these complications in
the HD group may be explained by the much smaller sample size in that
group (the HD group had 112 episodes involving 105 patients, while the
control group had 3201 episodes involving 3155 patients). The rate of
co-infection with other respiratory viruses in this study was low compared
with that reported in a meta-analysis (4.3% vs ≤50%)2; however, the findings were similar in that dual
infection led to a more severe disease course and that rhinovirus was the
most common co-infecting agent. Notably, adenovirus was the second most
common agent in this study, whereas it was human bocavirus in the
meta-analysis; the presence of this virus is not routinely assessed in
Hong Kong.
Within the HD group, we identified three severity
predictors for RSV infection, namely heart failure requiring medications,
pulmonary hypertension, and severe airway abnormalities associated with
CHD. The first two have been well described3
22 26
27 and were included in the
American Academy of Pediatrics guideline for RSV prophylaxis11; severe airway abnormality associated with CHD has
not been extensively investigated in previous studies of RSV infection.
Pulmonary artery sling with tracheal stenosis is not easily identified
during prenatal examinations and some affected patients remain undiagnosed
for a few months after birth. The disease may first be identified during
respiratory infection, such as RSV, and most patients exhibit severe
courses of disease. In contrast to the findings of the Taiwanese study,32 we did not find cyanotic CHD to
be a severity predictor. This was potentially because the number of such
patients was very small in our series (n=1). Indeed, the number of live
births of patients with severe cyanotic CHD is decreasing in Hong Kong due
to the high rate of termination of pregnancies involving the disease. The
number of surviving patients with severe untreated cyanotic CHD is also
small, as they either receive some form of surgical treatment or do not
survive early infancy.
Early studies stressed the importance of hygiene
and infection control for the prevention of RSV infection.20 The IMpact-RSV study in the late 1990s led to the
approval of palivizumab (a monoclonal antibody) for passive immunisation
in the US in 1998 and in Europe in 1999 for children with chronic lung
disease or prematurity.5 6 33 In 2003,
the use of palivizumab in the US was extended to children with hs-CHD
(heart failure, pulmonary hypertension, and cyanotic CHD) who were aged ≤2
years.7 34
Comparable national guidelines have been gradually adopted globally,
including in Asian countries (Japan in 2006 and Korea in 2009).8 9 In temperate
regions with a distinct RSV season length of approximately 6 months, the
typical palivizumab regimen is monthly intramuscular injection at 15
mg/kg/dose during the RSV season, with a maximum of five doses. However,
in subtropical regions without a clear RSV season, monthly injection may
be necessary for the entire year, which is costly. In 2011, a study in
Taiwan showed that a protocol of six consecutive monthly doses in at-risk
children, beginning when the risk was initially detected in the first year
of life, was also effective.10
Currently, the American Academy of Pediatrics limits routine RSV
immunoprophylaxis to children who have acyanotic CHD with heart failure or
pulmonary hypertension in the first year of life.11
In the United Kingdom, children who have cyanotic or acyanotic CHD with
significant co-morbidities are recommended to receive RSV
immunoprophylaxis until age 2 years.35
In 2017, however, an international steering committee broadened the
indications to children with unoperated hs-CHD or symptomatic airway
abnormalities who were aged ≤2 years, as well as children with
cardiomyopathy requiring treatment who were aged ≤1 year.36 A Canadian-Italian group questioned whether children
with hs-CHD need higher doses of immunoprophylaxis because of their
increased inherent risks.37
Numerous studies have shown the safety and efficacy of RSV
immunoprophylaxis and its long-term benefit in children with HD.6 30 38 39 40 It is therefore imperative to establish similar
guidelines in Hong Kong. In accordance with local experience regarding
bronchopulmonary dysplasia in premature babies,12
an RSV immunoprophylaxis scheme administered monthly for five doses,
beginning in the first year of life, should be considered in children with
HD who exhibit severity predictors such as heart failure, pulmonary
hypertension, and/or severe airway abnormality related to CHD. The optimal
timing for immunoprophylaxis may be during the local peak of infection,
from March to August.
Limitations of this study
Because this was a retrospective hospital-based
study, the incidence of RSV respiratory infection may have been
underestimated; however, we presume that only patients with relatively
mild disease were potentially omitted. As in all retrospective reviews,
this study did not use a pre-defined protocol for management and clinical
documentation. This limitation was partially addressed by analysing
objective clinical details which are relatively standard and easily
retrievable through electronic means. We also collected out-patient
follow-up records when necessary to provide additional information
regarding co-morbidities. However, instances of re-infection before and
after the study period were not assessed due to technical limitations,
which is not ideal.
Conclusions
Respiratory syncytial virus infections are common
in Hong Kong, and the incidence peaks from March to August; prevalence is
greatest in children aged <1 year, and there is a mild male
preponderance. Infection is favoured by high relative humidity, low wind
speed, and low atmospheric pressure. Heart disease, both congenital and
acquired, is a distinct risk factor for severe RSV infection in terms of
hospital length of stay, reinfection, complication, respiratory failure,
and the requirements for ICU care and mechanical ventilation. In Hong
Kong, an RSV immunoprophylaxis scheme administered monthly for five doses,
beginning during spring and summer in the first year of life, should be
considered in children with HD who exhibit any of the following severity
predictors: heart failure, pulmonary hypertension, and severe airway
abnormalities associated with CHD.
Author contributions
All authors contributed to the concept of study,
acquisition and analysis of data. SH Lee and KL Hon wrote the article and
had critical revision for important intellectual content. All authors had
full access to the data, contributed to the study, approved the final
version for publication, and take responsibility for its accuracy and
integrity.
Acknowledgement
We thank Ms Kathy YC Tsang of Prince of Wales
Hospital who provided expert and continuous statistical support for this
study.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Declaration
The results from this research have been presented,
in part, at the following conferences:
1. The first phase of this research involving two hospitals on the impact of respiratory syncytial virus (RSV) infection in children with heart disease was presented in the 2nd Asian RSV Expert Forum, Singapore, 24 October 2016.
2. The preliminary data of this research involving four hospitals on the epidemiology and impact of RSV infection in children with heart disease were presented in: (a) "RSV infection in Hong Kong Children" forum organised by the Hong Kong Society of Paediatric Cardiology, 17 January 2017 and (b) the Working Group on Use of Palivizumab for RSV Infection Prophylaxis in Children under Hospital Authority Paediatric COC, 30 August 2017.
3. A modified abstract was selected as poster presentation in the Joint Annual Scientific Meeting of Hong Kong College of Paediatricians, 28 September 2019 (with prior permission by Editor-in-Chief of the Hong Kong Medical Journal).
1. The first phase of this research involving two hospitals on the impact of respiratory syncytial virus (RSV) infection in children with heart disease was presented in the 2nd Asian RSV Expert Forum, Singapore, 24 October 2016.
2. The preliminary data of this research involving four hospitals on the epidemiology and impact of RSV infection in children with heart disease were presented in: (a) "RSV infection in Hong Kong Children" forum organised by the Hong Kong Society of Paediatric Cardiology, 17 January 2017 and (b) the Working Group on Use of Palivizumab for RSV Infection Prophylaxis in Children under Hospital Authority Paediatric COC, 30 August 2017.
3. A modified abstract was selected as poster presentation in the Joint Annual Scientific Meeting of Hong Kong College of Paediatricians, 28 September 2019 (with prior permission by Editor-in-Chief of the Hong Kong Medical Journal).
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Research Ethics
Committees of the Hospital Authority of Hong Kong (Ref
KC/KE-16-0122/ER-2). There is no ethical concern and waiver for obtaining
consent was approved by the Cluster Research Ethics Committees of the
Hospital Authority of Hong Kong. All procedures performed in this study
involving human participants were in accordance with the ethical standards
of the institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards.
References
1. Nair H, Nokes DJ, Gessner BD, et al.
Global burden of acute lower respiratory infections due to respiratory
syncytial virus in young children: a systematic review and meta-analysis.
Lancet 2010;375:1545-55. Crossref
2. Bont L, Checchia PA, Fauroux B, et al.
Defining the epidemiology and burden of severe respiratory syncytial virus
infection among infants and children in Western countries. Infect Dis Ther
2016;5:271-98. Crossref
3. MacDonald NE, Hall CB, Suffin SC,
Alexson C, Harris PJ, Manning JA. Respiratory syncytial viral infection in
infants with congenital heart disease. N Engl J Med 1982;307:397-400. Crossref
4. Welliver RC. Review of epidemiology and
clinical risk factors for severe respiratory syncytial virus (RSV)
infection. J Pediatr 2003;143(5 Suppl):S112-7. Crossref
5. American Academy of Pediatrics:
Committee on Infectious Diseases and Committee on Fetus and Newborn.
Prevention of respiratory syncytial virus infections: indications for the
use of palivizumab and update on the use of RSV-IGIV. Pediatrics
1998;102:1211-6. Crossref
6. Simoes EA. Immunoprophylaxis of
respiratory syncytial virus: global experience. Respir Res 2002;3 Suppl
1:S26-33. Crossref
7. American Academy of Pediatrics:
Committee on Infectious Diseases and Committee on Fetus and Newborn.
Policy Statement: Revised indications for the use of palivizumab and
respiratory syncytial virus immune globulin intravenous for the prevention
of respiratory syncytial virus infections. Pediatrics 2003;112(6 Pt
1):1442-6. Crossref
8. Nakazawa M, Saji T, Ichida F, Oyama K,
Harada K, Kusuda S. Guidelines for the use of palivizumab in infants and
young children with congenital heart disease. Pediatr Int 2006;48:190-3. Crossref
9. Kim NK, Choi JY. Respiratory syncytial
virus prevention in children with congenital heart disease: who and how?
Korean J Pediatr 2011;54:197-200. Crossref
10. Chi H, Hsu CH, Chang JH, et al. A
novel six consecutive monthly doses of palivizumab prophylaxis protocol
for the prevention of respiratory syncytial virus infection in high-risk
preterm infants in Taiwan. PLoS One 2014;9:e100981. Crossref
11. American Academy of Pediatrics:
Committee on Infectious Diseases; American Academy of Pediatrics
Bronchiolitis Guidelines Committee. Updated guidance for palivizumab
prophylaxis among infants and young children at increased risk of
hospitalization for respiratory syncytial virus infection. Pediatrics
2014;134:415-20. Crossref
12. Lee SR, Kwok KL, Ng DK, Hon KL.
Palivizumab for infants <29 weeks in Hong Kong without a clear-cut
season for respiratory syncytial virus infection—a cost-effectiveness
analysis. J Trop Pediatr 2018;64:418-25. Crossref
13. Chan PK, Sung RY, Fung KS, et al.
Epidemiology of respiratory syncytial virus infection among paediatric
patients in Hong Kong: seasonality and disease impact. Epidemiol Infect
1999;123:257-62. Crossref
14. Hon KL, Leung TF, Cheng WY, et al.
Respiratory syncytial virus morbidity, premorbid factors, seasonality, and
implications for prophylaxis. J Crit Care 2012;27:464-8. Crossref
15. Leung TF, Lam DS, Miu TY, et al.
Epidemiology and risk factors for severe respiratory syncytial virus
infections requiring pediatric intensive care admission in Hong Kong
children. Infection 2014;42:343-50. Crossref
16. Chan PK, Tam WW, Lee TC, et al.
Hospitalization incidence, mortality, and seasonality of common
respiratory viruses over a period of 15 years in a developed subtropical
city. Medicine (Baltimore) 2015;94:e2024. Crossref
17. Hall CB, Powell KR, MacDonald NE, et
al. Respiratory syncytial viral infection in children with compromised
immune function. N Engl J Med 1986;315:77-81. Crossref
18. Groothuis JR, Gutierrez KM, Lauer BA.
Respiratory syncytial virus infection in children with bronchopulmonary
dysplasia. Pediatrics 1988;82:199-203.
19. Wilkesmann A, Ammann RA, Schildgen O,
et al. Hospitalized children with respiratory syncytial virus infection
and neuromuscular impairment face an increased risk of a complicated
course. Pediatr Infect Dis J 2007;26:485-91. Crossref
20. Simoes EA. Environmental and
demographic risk factors for respiratory syncytial virus lower respiratory
tract disease. J Pediatr 2003;143(5 Suppl):S118-26. Crossref
21. Purcell K, Fergie J. Driscoll
Children’s Hospital respiratory syncytial virus database: risk factors,
treatment and hospital course in 3308 infants and young children, 1991 to
2002. Pediatr Infect Dis J 2004;23:418-23. Crossref
22. Lee JT, Chang LY, Wang LC, et al.
Epidemiology of respiratory syncytial virus infection in northern Taiwan,
2001-2005—seasonality, clinical characteristics, and disease burden. J
Microbiol Immunol Infect 2007;40:293-301.
23. Khor CS, Sam IC, Hooi PS, Quek KF,
Chan YF. Epidemiology and seasonality of respiratory viral infections in
hospitalized children in Kuala Lumpur, Malaysia: a retrospective study of
27 years. BMC Pediatr 2012;12:32. Crossref
24. Chew FT, Doraisingham S, Ling AE,
Kumarasinghe G, Lee BW. Seasonal trends of viral respiratory tract
infections in the tropics. Epidemiol Infect 1998;121:121-8. Crossref
25. Welliver R. The relationship of
meteorological conditions to the epidemic activity of respiratory
syncytial virus. Paediatr Respir Rev 2009;10 Suppl 1:6-8. Crossref
26. Kristensen K, Stensballe LG, Bjerre J,
et al. Risk factors for respiratory syncytial virus hospitalisation in
children with heart disease. Arch Dis Child 2009;94:785-9. Crossref
27. Moler FW, Khan AS, Meliones JN, Custer
JR, Palmisano J, Shope TC. Respiratory syncytial virus morbidity and
mortality estimates in congenital heart disease patients: a recent
experience. Crit Care Med 1992;20:1406-13. Crossref
28. Wang EE, Law BJ, Stephens D. Pediatric
Investigators Collaborative Network on Infections in Canada (PICNIC):
prospective study of risk factors and outcomes in patients hospitalized
with respiratory syncytial viral lower respiratory tract infection. J
Pediatr 1995;126:212-9. Crossref
29. Jung JW. Respiratory syncytial virus
infection in children with congenital heart disease: global data and
interim results of Korean RSV-CHD survey. Korean J Pediatr 2011;54:192-6.
Crossref
30. Chang RK, Chen AY. Impact of
palivizumab on RSV hospitalizations for children with hemodynamically
significant congenital heart disease. Pediatr Cardiol 2010;31:90-5. Crossref
31. Chu PY, Hornik CP, Li JS, Campbell MJ,
Hill KD. Respiratory syncytial virus hospitalisation trends in children
with haemodynamically significant heart disease, 1997-2012. Cardiol Young
2017;27:16-25. Crossref
32. Chiu SN, Shao PL, Chen HC, et al. Risk
of respiratory syncytial virus infection in cyanotic congenital heart
disease in a subtropical area. J Pediatr 2016;171:25-30.e1. Crossref
33. The IMpact-RSV Study Group.
Palivizumab, a humanized respiratory syncytial virus monoclonal antibody,
reduces hospitalization from respiratory syncytial virus infection in
high-risk infants. Pediatrics 1998;102(3 Pt 1):531-7. Crossref
34. Feltes TF, Cabalka AK, Meissner HC, et
al. Palivizumab prophylaxis reduces hospitalization due to respiratory
syncytial virus in young children with hemodynamically significant
congenital heart disease. J Pediatr 2003;143:532-40. Crossref
35. UK Government. The Green Book.
Immunisation against infectious disease. Available from
https://webarchive.
nationalarchives.gov.uk/20130104181824/https://www.wp.dh.gov.uk/immunisation/files/2012/09/Green-Book-updated-040113.pdf.
Accessed 17 Jul 2019.
36. Tulloh RM, Medrano-Lopez C, Checchia
PA, et al. CHD and respiratory syncytial virus: global expert exchange
recommendations. Cardiol Young 2017;27:1504-21. Crossref
37. Manzoni P, Paes B, Lanctôt KL, et al.
Outcomes of infants receiving palivizumab prophylaxis for respiratory
syncytial virus in Canada and Italy: An international, prospective cohort
study. Pediatr Infect Dis J 2017;36:2-8. Crossref
38. Cohen SA, Zanni R, Cohen A, et al.
Palivizumab use in subjects with congenital heart disease: results from
the 2000-2004 Palivizumab Outcomes Registry. Pediatr Cardiol
2008;29:382-7. Crossref
39. Resch B, Michel-Behnke I. Respiratory
syncytial virus infections in infants and children with congenital heart
disease: update on the evidence of prevention with palivizumab. Curr Opin
Cardiol 2013;28:85-91. Crossref
40. Wegzyn C, Toh LK, Notario G, et al.
Safety and effectiveness of palivizumab in children at high risk of
serious disease due to respiratory syncytial virus infection: a systematic
review. Infect Dis Ther 2014;3:133-58. Crossref