© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Clinical considerations when adding a sodium-glucose
co-transporter-2 inhibitor to insulin therapy in patients with diabetes
mellitus
Kathryn Tan, MBBCH, MD1; WS Chow, FRCP
(Edin), FHKAM (Medicine)2; Jenny Leung, MB, BS, FRCP (Lond)3;
Andrew Ho, FHKCP4; Risa Ozaki, MB, ChB, FHKCP5;
Grace Kam, FHKCP, FRCP (Glasgow)6; June Li, MB, ChB, FHKCP7;
CH Choi, MB, ChB, FRCP (Lond)8; MW Tsang, MB, BS, FRCP9;
Norman Chan, MD, FRCP9; KK Lee, MB, BS, FHKAM (Medicine)1;
KW Chan, FRCP (Edin), FHKAM (Medicine)10
1 Department of Medicine, The University
of Hong Kong, Pokfulam, Hong Kong
2 Department of Medicine, Queen Mary
Hospital, Pokfulam, Hong Kong
3 Department of Integrated Medical
Service, Ruttonjee and Tang Shiu Kin Hospitals, Hong Kong
4 Department of Medicine and Geriatrics,
Tuen Mun Hospital, Tuen Mun, Hong Kong
5 Department of Medicine and
Therapeutics, The Chinese University of Hong Kong, Shatin, Hong Kong
6 Department of Medicine and Geriatrics,
United Christian Hospital, Kwun Tong, Hong Kong
7 Department of Medicine, Yan Chai
Hospital, Tsuen Wan, Hong Kong
8 Department of Medicine, Queen
Elizabeth Hospital, Jordan, Hong Kong
9 Specialist in Endocrinology, Private
Practice
10 Department of Medicine and
Geriatrics, Princess Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Prof Kathryn Tan (kcbtan@hku.hk)
Abstract
A consensus meeting was held to discuss add-on
therapy of sodium-glucose co-transporter-2 (SGLT2) inhibitors in
patients with diabetes mellitus treated with insulin. The objectives
were to affirm the efficacy and safety of SGLT2 inhibitors as an add-on
to insulin, empower clinicians to minimise the risk of adverse events,
and provide clinical guidance. Administration of SGLT2 inhibitors as an
add-on therapy to insulin is associated with significant reductions
compared with placebo in glycosylated haemoglobin A1c, fasting plasma
glucose, insulin dose, and body weight without an increased risk of
hypoglycaemia. Compared with traditional therapies, SGLT2 inhibitors
have shown cardiovascular and renal benefits. Adding an SGLT2 inhibitor
to insulin increases the risk of urinary tract and genital tract
infections. The use of SGLT2 inhibitor is also associated with a
slightly increased incidence of diabetic ketoacidosis. Patients who may
benefit most from add-on therapy with SGLT2 inhibitors include those
with established atherosclerotic cardiovascular disease, heart failure,
chronic kidney disease, high insulin doses, obesity, and metabolic
syndrome. Routine monitoring for diabetic ketoacidosis is controversial,
and patient and clinician education is essential to minimise risk. The
decision to adjust insulin dose when adding an SGLT2 inhibitor is
dependent on patient factors, but the insulin dose should not be reduced
beyond 20% prior to the first dose of SGLT2 inhibitor. Patients should
temporarily discontinue SGLT2 inhibitors during fasting, acute illness,
or low/reduced carbohydrate intake. If ketonuria is detected, SGLT2
inhibitors but not insulin should be immediately discontinued and
medical advice sought.
Introduction
Sodium-glucose co-transporter-2 (SGLT2) inhibitors
are an important therapeutic option in the management of type 2 diabetes
mellitus (T2DM). These oral agents treat hyperglycaemia by blocking the
reabsorption of glucose in renal tubules, which results in increased
urinary glucose excretion.1 As
monotherapy, SGLT2 inhibitors have been shown to significantly lower
glycated haemoglobin A1c (HbA1c), fasting glucose, and postprandial
glucose compared with placebo in subjects with T2DM that was inadequately
controlled with diet and exercise.2
3 4
The significant and consistent reduction in HbA1c observed with SGLT2
inhibitors is similar to or better than that produced by metformin,
sulfonylureas, and dipeptidyl peptidase-4 (DPP-4) inhibitors, with a
minimal risk of hypoglycaemia. These SGLT2 inhibitors can also bring about
reductions in body weight and blood pressure. Sodium-glucose
co-transporter- 2 inhibitor therapy is associated with an elevated risk of
genital tract infections (GTIs) and, to a lesser degree, urinary tract
infections (UTIs).1 5 In addition, in rare cases, SGLT2 inhibitors have been
associated with diabetic ketoacidosis (DKA) and euglycaemic ketoacidosis.
Many patients with T2DM fail to achieve glycaemic
goals despite receiving two or more antidiabetic drug classes that target
different core defects of the disease.6
Whereas the majority of antidiabetic drugs have an insulin-dependent mode
of action, SGLT2 inhibitors have an insulin-independent mode of action,
suggesting that the use of these drugs could offer therapeutic synergy
when used in combination.6 7 Randomised controlled trials (RCTs),8 9 10 11 as well
as real-world studies12 have confirmed the efficacy and tolerability of
SGLT2 inhibitors when used as monotherapy and as an add-on therapy to
insulin.13 14 At the time of writing, SGLT2 inhibitors are not
approved for use in type 1 diabetes mellitus (T1DM); however, there are
RCTs providing evidence of a potential role for SGLT2 inhibitors in
patients with T1DM.15 16
A meeting of esteemed endocrinologists in Hong Kong
was held in August 2018 to develop a consensus on the role of add-on
therapy with SGLT2 inhibitors in insulin-treated patients with diabetes
mellitus. The expert panel considered all evidence relating to T2DM and
also considered T1DM where possible. All statements pertaining to T1DM
should be interpreted carefully given the paucity of data available for
this condition and not construed as recommendations for off-label use.
Herein we present the consensus findings of the expert panel, with the
major objectives of this review summarised as follows: (1) to summarise
the clinical approach and rationale for intensifying insulin therapy with
SGLT2 inhibitors (2) to affirm the efficacy and safety of SGLT2 inhibitors
in insulin-treated patients; (3) to empower clinicians to minimise the
risk of hypoglycaemia and DKA; (4) to provide practical clinical guidance
on adding SGLT2 inhibitors in insulin-treated patients; and (5) to guide
clinicians on patient selection.
Clinical approach and rationale
Statement 1.1: The decision to add an additional
therapy or intensify insulin therapy is dependent on individual patient
factors that contribute to inadequate control
Because T2DM is a progressive disease, many
patients will need intensification of therapy. If the patient has
experienced an episode of severe hypoglycaemia while on insulin therapy,
then the addition of another therapy with a low risk of hypoglycaemia may
be a better option compared with intensifying insulin therapy. Patient
preference is an important consideration. Intensifying insulin heightens
the potential risks for weight gain and hypoglycaemia, especially at high
insulin doses. Moreover, a large proportion of patients with T2DM have
co-morbid obesity and/or metabolic syndrome, with the latter characterised
by hypertension and dyslipidaemia in addition to poor glycaemic control.
Intensifying insulin in these patients could contribute to a vicious cycle
of increasing appetite, further weight gain, and increasing insulin
resistance.17
Statement 1.2: Sodium-glucose co-transporter-2
inhibitors offer weight loss and cardiovascular-renal benefits with a low
risk of hypoglycaemia compared with other oral agents
Sodium-glucose co-transporter-2 inhibitors offer
the benefit of improving glycaemic control without increasing the risks of
hypoglycaemia or weight gain in insulin-treated patients. Compared with
most other therapies, SGLT2 inhibitors have shown cardiovascular benefits
in patients with established atherosclerotic cardiovascular disease, which
appears to be a class effect.18
Because of their insulin-independent mechanism, SGLT2 inhibitors are often
effective in patients in whom other therapies have failed. Add-on therapy
with an SGLT2 inhibitor may have particular benefits in obese patients, as
insulin intensification or add-on therapy with sulfonylurea or
thiazolidinedione may exacerbate weight gain. Treatment with SGLT2
inhibitors can improve metabolic syndrome through reductions in weight and
blood pressure while counteracting insulin resistance and improving
insulin sensitivity. Continuous glucose monitoring studies have shown
improvement in glucose excursions after initiating SGLT2 inhibitor
therapy. In patients with T1DM, the patients who mainly benefit from SGLT2
inhibitor therapy are those on high insulin doses and those in whom
features of T2DM—including excessive weight, high blood pressure, and
other indices of metabolic syndrome—have accrued.
Efficacy and safety
Statement 2.1: Administration of sodium-glucose
co-transporter-2 inhibitors as add-on therapy to insulin in type 2
diabetes mellitus is associated with significant reductions in haemoglobin
A1c, fasting plasma glucose, insulin dose and body weight
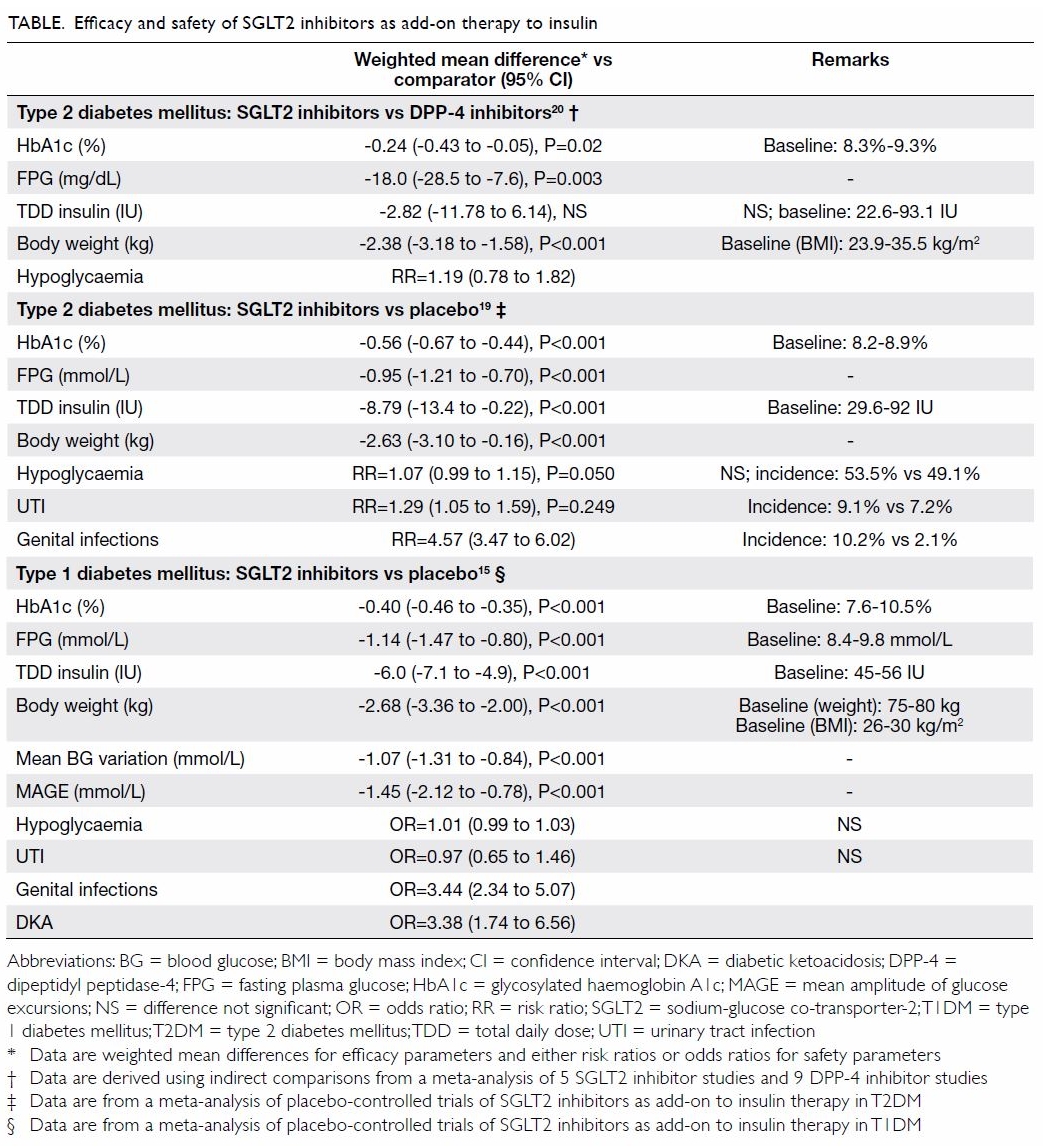
The efficacy of SGLT2 inhibitors as add-on therapy
to insulin in T2DM has been demonstrated in randomised, placebo-controlled
trials, with significant reductions in HbA1c, fasting plasma glucose,
total daily insulin dose, and body weight compared with placebo achieved
over periods of up to 24 weeks (Table).19 So
far, no head-to-head trials have compared SGLT2 inhibitors and DPP-4
inhibitors as an add-on therapy to insulin. Indirect comparison via
network meta-analysis has suggested that SGLT2 inhibitors showed better
glycaemic control and greater weight reduction than DPP-4 inhibitors in
patients with T2DM that was inadequately controlled with insulin (Table).20 In addition, SGLT2 inhibitors
have proven cardiovascular-renal benefits, while most DPP-4 inhibitors
have only achieved cardiovascular safety.
Statement 2.2: Adding sodium-glucose co-transporter- 2
inhibitors to insulin in type 2 diabetes mellitus patients with a mean
baseline haemoglobin A1c of 8.4% (range, 8.2%-8.9%) does not increase the
risk of hypoglycaemia but increases the risks of urinary and genital tract
infections
In patients with T2DM, the use of SGLT2 inhibitors
as add-on therapy to insulin is associated with a similar incidence of
hypoglycaemia to placebo (Table).19
However, SGLT2 inhibitors are associated with higher risks of UTIs and
GTIs compared with placebo.19
Statement 2.3: Administration of sodium-glucose
co-transporter-2 inhibitors as add-on therapy to insulin in type 1
diabetes mellitus is associated with significant reductions in haemoglobin
A1c, fasting plasma glucose, daily total insulin dose, body weight, and
glycaemic excursions
The use of SGLT2 inhibitors in T1DM is currently
off-label. The addition of an SGLT2 inhibitor in patients with T1DM offers
therapeutic value in patients who are obese or with problems with large
glucose excursions. Careful patient selection and meticulous patient
education of precautions are important to minimise the risk of DKA.21 Evidence from several clinical trials showed that in
patients with T1DM, dapagliflozin, or empagliflozin as adjunctive therapy
to insulin improved glycaemic control and weight.16
Statement 2.4: Adding sodium-glucose co-transporter-2
inhibitors to insulin in type 1 diabetes mellitus does not appear to
increase the risks of hypoglycaemia or urinary tract infections but
increases the risk of genital tract infections
In patients with T1DM, the use of SGLT2 inhibitor
therapy is not associated with an increased risk of hypoglycaemia or UTIs
compared with placebo but is associated with an increased risk of GTIs (Table).15
Evidence from the EASE trials showed that in patients with T1DM,
empagliflozin as adjunctive therapy to insulin did not increase the risk
of hypoglycaemia.16
Adverse events of special interest
Statement 3.1: The incidence of genital tract
infections is higher in patients with sodium-glucose co-transporter-2
inhibitors added to insulin therapy versus other agents or placebo;
however, most events are classified as mild or moderate in intensity and
readily respond to therapy
In patients with T1DM or T2DM, the addition of an
SGLT2 inhibitor to insulin therapy is associated with a significantly
increased risk of GTIs (Table).15 19 The estimated risk ratio ranges
from 3 to 5 compared with placebo. However, treatment cessation is not
necessary, and most events are mild or moderate in intensity and readily
respond to therapy.
Statement 3.2: Administration of sodium-glucose
co-transporter-2 inhibitors is associated with an increased, albeit low,
incidence of euglycaemic diabetic ketoacidosis, a risk that is strongly
associated with use of insulin
In RCTs, the incidence of DKA among patients with
T1DM or T2DM receiving SGLT2 inhibitor therapy is estimated to be <1
case per 1000, whereas in cohort studies, the incidence has been reported
to be 1.6 cases per 1000 person-years.22
In patients with T1DM, the DEPICT-1,18
DEPICT-2,23 inTandem1,24 and inTandem325
trials showed a DKA incidence ranging from 1.5% to 4.0% in patients
treated with selective SGLT2 inhibitors or dual SGLT1 and SGLT2 inhibitors
for up to 1 year.
Statement 3.3: The risk of diabetic ketoacidosis is
heightened in patients with high haemoglobin A1c levels, frail and elderly
patients, those with inadequate food intake, and patients with poor
disease awareness/adherence or frequent complications
In patients with T2DM, those with HbA1c levels of
≥10% may have a higher risk of developing DKA than patients with lower
HbA1c levels. Risk may also be elevated in latent autoimmune diabetes in
adults, frail and elderly patients with poor disease awareness, those who
are repeatedly admitted to hospital for complications, and patients with
long-term diabetes with depleted ß-cell reserves.
In patients with T1DM, poor glycaemic control is
also indicative of heightened risk of DKA, along with insulin pump use and
suboptimal adherence.26 27 28 Other
risk groups include those taking weight loss medications, those without
steady dietary control, postoperative patients, and frail or elderly
patients, particularly those with cognitive impairment.
Patient selection
Statement 4.1: Sodium-glucose co-transporter-2
inhibitors have the greatest overall benefit/risk profile in patients with
obesity, cardiovascular or renal diseases, high insulin requirement, or
large glycaemic excursions
Randomised controlled trials support the use of
SGLT2 inhibitors as an add-on therapy to insulin therapy in T2DM patients
with obesity, cardiovascular or renal diseases, high insulin requirement,
or large glycaemic excursions in whom insulin intensification would
otherwise be the next step in achieving glycaemic control.9 29 30 31 32 33 34 35
In patients with T1DM, SGLT2 inhibitors should only
be prescribed by an endocrinologist, as the use of these therapies in T1DM
is currently off-label. The use of SGLT2 inhibitors may be a useful
adjunct to insulin therapy in patients with T1DM and obesity or large
glycaemic variability. Hypoglycaemia unawareness is not an absolute
contra-indication provided that the patient is compliant and knowledgeable
about the disease. Patients should be capable of detecting insulin pump
failure, and the ability to monitor urine or serum ketone levels is
mandatory. In RCTs of patients with T1DM, patient selection included those
aged 18 to 75 years with baseline HbA1c levels of 7.0% to 11.0% and a body
mass index ≥18.5 kg/m2.21
23 24
25 In the DEPICT trials, patients
using insulin for ≥12 months with a total daily insulin dose ≥0.3 IU/kg
for ≥3 months were selected, and patients were additionally required to
have a creatinine clearance of >60 mL/min and C-peptide level <0.7
ng/mL.21 23 In the inTandem trials, patients were required to
have treatment with insulin at a stable dose via continuous subcutaneous
insulin infusion or multi-dose insulin treatment, with no change in
insulin delivery within 3 months.24
25 Patients were additionally
required to perform self-monitoring of blood glucose (SMBG) and have an
estimated glomerular filtration rate of >45 mL/min/1.73 m2.
Switching versus adding
Statement 5.1: In patients with type 2 diabetes
mellitus, the decision to switch a sodium-glucose co-transporter-2
inhibitor to another oral agent or add a sodium-glucose co-transporter-2
inhibitor to an existing treatment regimen is based on factors such as
efficacy, tolerability of the existing treatment, and cost
In T2DM patients on combination therapy of insulin
and oral antidiabetic agents, replacing one of the oral agents with an
SGLT2 inhibitor can be considered. When the effectiveness of the DPP-4
inhibitor is no longer sustained owing to its limited durability or when
the patient develops fluid retention with a glitazone, patients would be
expected to benefit from a switch to an SGLT2 inhibitor. Switching to an
SGLT2 inhibitor might be more cost-effective compared with adding an SGLT2
inhibitor to the existing drug regimen.
Implementation
Statement 6.1: Adjustment of the insulin dose when
adding a sodium-glucose co-transporter-2 inhibitor may be appropriate in
some but not all patients
In patients with T2DM, the decision to adjust
insulin dose upon initiation of an SGLT2 inhibitor is dependent on patient
factors. If the patient is obese and insulin resistant with high HbA1c,
then maintaining the insulin dose is a reasonable approach. Conversely, if
a patient’s HbA1c is close to target, a reduction in insulin dose will be
appropriate. For patients with frequent large glycaemic excursions, the
expert panel recommends reduction of the insulin dose by up to 10% before
initiating an SGLT2 inhibitor. Initiation of the SGLT2 inhibitor at the
lowest available dose is also recommended.
In the DEPICT trials with T1DM, after the first
dose of study drug, basal and bolus insulin were reduced symmetrically by
up to 20%.21 23 In the inTandem trials, in which dual SGLT1 and SGLT2
inhibitor therapy was employed, bolus insulin was reduced by 30%, with
insulin dosing subsequently adjusted according to SMBG data to meet
targets.24 25 The consensus of the expert panel was that the
insulin dose should not be reduced beyond 20% upon initiation of an SGLT2
inhibitor. When the patient is receiving both basal and bolus doses of
insulin, the bolus dose may be reduced, with addition of the SGLT2
inhibitor as appropriate.
Monitoring
Statement 7.1: Early and regular monitoring for
diabetic ketoacidosis is recommended following initiation of
sodium-glucose co-transporter-2 inhibitor therapy
Patients should be monitored and closely followed
after initiating an SGLT2 inhibitor. Self-monitoring of blood glucose is
important, and titration of insulin may be necessary. Early follow-up in
the form of telecommunication or nurse clinic instead of clinician visits
may be more feasible, and monitoring of renal function within 4 weeks is
recommended. Daily ketone monitoring is not practical because of the high
cost and short shelf life of ketone strips. However, monitoring of ketones
is recommended during acute illness. Education of patients and clinicians
is essential for improved awareness, and the importance of sick day
management needs to be emphasised.
The risk of DKA is higher in T1DM. Urine or blood
ketone monitoring should be considered during initiation of SGLT2
inhibitors and mandatory during acute stress. Education regarding optimal
nutrition, situations of nausea/vomiting, and temporary cessation of SGLT2
inhibitor therapy is appropriate. Instruction on measures to reverse
ketosis and prevent progression to DKA (including carbohydrates and fluid
intake as well as additional correction of insulin doses) should be given.
In T1DM, the STICH protocol is an appropriate strategy for mitigating DKA
risk in patients receiving SGLT2 inhibitors. When DKA is suspected, the
patients should stop SGLT2 inhibitor therapy, inject bolus insulin,
consume 30 g of carbohydrates, and hydrate with water.36
Statement 7.2: Sodium-glucose co-transporter-2
inhibitor therapy should be stopped in the event of high ketone levels
In the event that a patient detects high ketone
levels, they should be instructed to stop their SGLT2 inhibitor, continue
insulin, ensure carbohydrate intake, and seek medical advice.
Other practical advice
Statement 8.1: Sodium-glucose co-transporter-2
inhibitor therapy should be temporarily stopped to avoid diabetic
ketoacidosis during fasting or reduced intake of food, acute illness, and
hospitalisation
Although there are currently no guidelines on when
to discontinue SGLT2 inhibitor therapy, SGLT2 inhibitor therapy should be
withheld temporarily in the following situations37:
Sodium-glucose co-transporter-2 inhibitors may be
re-introduced when the patient is able to eat normally, with recovery of
renal function following acute illness.
Further evidence to explore
Statement 9.1: Additional real-world and renal and
cardiovascular outcome data in patients with type 1 diabetes mellitus are
needed to further support the use of sodium-glucose co-transporter-2
inhibitors in diabetes management
Real-world data are needed to better understand
long-term medication adherence and persistence, cost-effectiveness of
ketone monitoring, and the role of carbohydrate intake and SMBG in the
prevention of DKA. Renal and CVD outcome data are needed regarding the use
of SGLT2 inhibitors in T1DM.
Concluding remarks
Sodium-glucose co-transporter-2 inhibitors are an
effective and well tolerated therapeutic option as add-on therapy to
insulin in patients with T2DM. Addition of SGLT2 inhibitors in this
setting is associated with significant reductions in HbA1c, fasting plasma
glucose, total daily insulin dose, and body weight without increasing the
risk of hypoglycaemia. Sodium-glucose co-transporter-2 inhibitor use is
accompanied by a slightly increased risk of UTIs and GTIs. There is a low
risk of DKA (about 1 per 1000 person-years). Appropriate patient
selection, education, and monitoring are helpful in mitigating this risk.
The use of SGLT2 inhibitors in patients with T1DM is currently off-label
and should only be attempted under the supervision of an endocrinologist
in appropriately selected patients. Further research will help to clarify
the role of this important oral antidiabetic drug class in both T1DM and
T2DM.
Post-meeting note
The results of the EASE-2 and EASE-3 trials were
published after the meeting. Data from these two trials showed that adding
empagliflozin as an adjunct to insulin therapy in T1DM improved glycaemic
control and weight without increasing hypoglycaemia. The rate of
ketoacidosis was lower when a smaller dose of empagliflozin was used.
Author contributions
All authors have made substantial contributions to
the concept or design of this study; acquisition of data; analysis or
interpretation of data; drafting of the manuscript; and critical revision
for important intellectual content. All authors had full access to the
data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have declared no conflicts of interest.
Acknowledgement
Language editing and writing support, funded by an
unrestricted educational grant from AstraZeneca Hong Kong Limited, were
provided by Ben Searle and Howard Christian of MIMS (Hong Kong) Limited.
Funding/support
Editing and writing support was funded by an
unrestricted educational grant from AstraZeneca Hong Kong Limited.
References
1. Scheen AJ. SGLT2 inhibition: efficacy
and safety in type 2 diabetes treatment. Expert Opin Drug Saf
2015;14:1879- 904. Crossref
2. Stenlöf K, Cefalu WT, Kim KA, et al.
Efficacy and safety of canagliflozin monotherapy in subjects with type 2
diabetes mellitus inadequately controlled with diet and exercise. Diabetes
Obes Metab 2013;15:372-82. Crossref
3. Ji L, Ma J, Li H, et al. Dapagliflozin
as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus:
a randomized, blinded, prospective phase III study. Clin Ther
2014;36:84-100.e9. Crossref
4. Ferrannini E, Seman L, Seewaldt-Becker
E, Hantel S, Pinnetti S, Woerle HJ. A Phase IIb, randomized,
placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients
with type 2 diabetes. Diabetes Obes Metab 2013;15:721-8. Crossref
5. Levine MJ. Empagliflozin for type 2
diabetes mellitus: an overview of phase 3 clinical trials. Curr Diabetes
Rev 2017;13:405-23. Crossref
6. Fioretto P, Giaccari A, Sesti G.
Efficacy and safety of dapagliflozin, a sodium glucose cotransporter 2
(SGLT2) inhibitor, in diabetes mellitus. Cardiovasc Diabetol 2015;14:142.
Crossref
7. Kilov G, Leow S, Thomas M. SGLT2
inhibition with dapagliflozin—a novel approach for the management of type
2 diabetes. Aust Fam Physician 2013;42:706-10.
8. Ishihara H, Yamaguchi S, Nakao I, Okitsu
A, Asahina S. Efficacy and safety of ipragliflozin as add-on therapy to
insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a
multi-centre, randomized, placebo-controlled, double-blind study. Diabetes
Obes Metab 2016;18:1207-16. Crossref
9. Rosenstock J, Jelaska A, Zeller C, et
al. Impact of empagliflozin added on to basal insulin in type 2 diabetes
inadequately controlled on basal insulin: a 78-week randomized,
double-blind, placebo-controlled trial. Diabetes Obes Metab
2015;17:936-48. Crossref
10. Neal B, Perkovic V, de Zeeuw D, et al.
Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose
cotransporter 2, when used in conjunction with insulin therapy in patients
with type 2 diabetes. Diabetes Care 2015;38:403-11. Crossref
11. Wilding JP, Norwood P, T’Joen C,
Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with
type 2 diabetes receiving high doses of insulin plus insulin sensitizers:
applicability of a novel insulin-independent treatment. Diabetes Care
2009;32:1656-62. Crossref
12. Thewjitcharoen Y, Yenseung N,
Malidaeng A, et al. Effectiveness of long-term treatment with SGLT2
inhibitors: real-world evidence from a specialized diabetes center.
Diabetol Metab Syndr 2017;9:96. Crossref
13. Fala L. Jardiance (Empagliflozin), an
SGLT2 inhibitor, receives FDA approval for the treatment of patients with
type 2 diabetes. Am Health Drug Benefits 2015;8(Spec Feature):92-5.
14. Handelsman Y. Potential place of SGLT2
inhibitors in treatment paradigms for type 2 diabetes mellitus. Endocr
Pract 2015;21:1054-65. Crossref
15. Yamada T, Shojima N, Noma H, Yamauchi
T, Kadowaki T. Sodium-glucose co-transporter-2 inhibitors as add-on
therapy to insulin for type 1 diabetes mellitus: Systematic review and
meta-analysis of randomized controlled trials. Diabetes Obes Metab
2018;20:1755-61. Crossref
16. Rosenstock J, Marquard J, Laffel LM,
et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes:
the EASE trials. Diabetes Care 2018;41:2560-9. Crossref
17. Herman ME, O’Keefe JH, Bell DS,
Schwartz SS. Insulin therapy increases cardiovascular risk in type 2
diabetes. Prog Cardiovasc Dis 2017;60:422-34. Crossref
18. Wu JH, Foote C, Blomster J, et al.
Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular
events, death, and major safety outcomes in adults with type 2 diabetes: a
systematic review and meta-analysis. Lancet Diabetes Endocrinol
2016;4:411-9. Crossref
19. Tang H, Cui W, Li D, et al.
Sodium-glucose co-transporter 2 inhibitors in addition to insulin therapy
for management of type 2 diabetes mellitus: a meta-analysis of randomized
controlled trials. Diabetes Obes Metab 2017;19:142-7. Crossref
20. Min SH, Yoon JH, Hahn S, Cho YM.
Comparison between SGLT2 inhibitors and DPP4 inhibitors added to insulin
therapy in type 2 diabetes: a systematic review with indirect comparison
meta-analysis. Diabetes Metab Res Rev 2017;33(1). Epub 2016 Jun 8. Crossref
21. Dandona P, Mathieu C, Phillip M, et
al. Efficacy and safety of dapagliflozin in patients with inadequately
controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre,
double-blind, phase 3, randomised controlled trial. Lancet Diabetes
Endocrinol 2017;5:864-76. Crossref
22. Bonora BM, Avogaro A, Fadini GP.
Sodium-glucose co-transporter- 2 inhibitors and diabetic ketoacidosis: an
updated review of the literature. Diabetes Obes Metab 2018;20:25-33. Crossref
23. Mathieu C, Dandona P, Gillard P, et
al. Efficacy and safety of dapagliflozin in patients with inadequately
controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a
randomized controlled trial. Diabetes Care 2018;41:1938-46. Crossref
24. Buse JB, Garg SK, Rosenstock J, et al.
Sotagliflozin in combination with optimized insulin therapy in adults with
type 1 diabetes: The North American inTandem1 Study. Diabetes Care
2018;41:1970-80. Crossref
25. Garg SK, Henry RR, Banks P, et al.
Effects of sotagliflozin added to insulin in patients with type 1
diabetes. N Engl J Med 2017;377:2337-48. Crossref
26. Al-Hayek AA, Robert AA, Braham RB,
Turki AS, Al-Sabaan FS. Frequency and associated risk factors of recurrent
diabetic ketoacidosis among Saudi adolescents with type 1 diabetes
mellitus. Saudi Med J 2015;36:216-20. Crossref
27. Giessmann LC, Kann PH. Risk and
relevance of insulin pump therapy in the aetiology of ketoacidosis in
people with type 1 diabetes. Exp Clin Endocrinol Diabetes 2018 Jul 26.
Epub ahead of print. Crossref
28. Fortin K, Pries E, Kwon S. Missed
medical appointments and disease control in children with type 1 diabetes.
J Pediatr Health Care 2016;30:381-9. Crossref
29. Terauchi Y, Tamura M, Senda M, Gunji
R, Kaku K. Efficacy and safety of tofogliflozin in Japanese patients with
type 2 diabetes mellitus with inadequate glycaemic control on insulin
therapy (J-STEP/INS): results of a 16-week randomized, double-blind,
placebo-controlled multicentre trial. Diabetes Obes Metab
2017;19:1397-407. Crossref
30. Ott C, Jumar A, Striepe K, et al. A
randomised study of the impact of the SGLT2 inhibitor dapagliflozin on
microvascular and macrovascular circulation. Cardiovasc Diabetol
2017;16:26. Crossref
31. Araki E, Onishi Y, Asano M, Kim H,
Yajima T. Efficacy and safety of dapagliflozin over 1 year as add-on to
insulin therapy in Japanese patients with type 2 diabetes: the DAISY
(Dapagliflozin Added to patients under InSulin therapY) trial. Diabetes
Obes Metab 2017;19:562-70. Crossref
32. Weber MA, Mansfield TA, Cain VA, Iqbal
N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of
dapagliflozin versus placebo in patients with type 2 diabetes on
combination antihypertensive therapy: a randomised, double-blind,
placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol
2016;4:211-20. Crossref
33. Leiter LA, Cefalu WT, de Bruin TW,
Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in
individuals with type 2 diabetes mellitus with preexisting cardiovascular
disease: a 24-week, multicenter, randomized, double-blind,
placebo-controlled study with a 28-week extension. J Am Geriatr Soc
2014;62:1252-62. Crossref
34. Wilding JP, Woo V, Rohwedder K, Sugg
J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with
type 2 diabetes receiving high doses of insulin: efficacy and safety over
2 years. Diabetes Obes Metab 2014;16:124-36. Crossref
35. Devineni D, Morrow L, Hompesch M, et
al. Canagliflozin improves glycaemic control over 28 days in subjects with
type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab
2012;14:539-45. Crossref
36. Garg SK, Peters AL, Buse JB, Danne T.
Strategy for mitigating DKA risk in patients with type 1 diabetes on
adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes
Technol Ther 2018;20:571-5. Crossref
37. Goldenberg RM, Berard LD, Cheng AY, et
al. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and
recommendations for prevention and diagnosis. Clin Ther 2016;38:2654-64. Crossref