Hong
Kong Med J 2019 Aug;25(4):279–86 | Epub 5 Aug 2019
© Hong
Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Local infiltration analgesia in primary total knee
arthroplasty
YY Fang1, MB, BS; QJ Lee2,
FCSHK, FHKCOS; Esther WY Chang2, MSc; YC Wong2,
FHKCOS
1 Department of Orthopaedics and
Traumatology, Princess Margaret Hospital, Laichikok, Hong Kong
2 Department of Orthopaedics and
Traumatology, Yan Chai Hospital, Tsuen Wan, Hong Kong
Corresponding author: Dr YY Fang (yingyan.f.mbbs@gmail.com)
Abstract
Introduction: Postoperative pain
in total knee arthroplasty (TKA) can hinder rehabilitation and cause
morbidity. Local infiltration analgesia (LIA), comprising an anaesthetic
drug, non-steroidal anti-inflammatory drug, and adrenaline, has been
introduced to reduce pain and systemic side-effects. This study
evaluated the efficacy of LIA in TKA with respect to morphine
consumption and postoperative pain score.
Methods: This single-centre
retrospective cohort study recruited patients with knee osteoarthritis
who were scheduled for primary TKA during the period from January 2017
to December 2017. Patients with chronic inflammatory joint disease,
contra-indications for LIA, or dementia were excluded. Patients in the
LIA group were administered single-dose LIA intra-operatively, while
those in the control group were not. Primary outcomes were postoperative
pain score, morphine demand, and morphine consumption; secondary
outcomes were range of motion, quadriceps power, and postoperative
length of stay.
Results: In total, 136 patients
were recruited (68 per group). Total postoperative morphine demand and
consumption, as well as pain scores from postoperative day (POD) 1 to
POD 4, were lower in the LIA group than in the control group. The range
of motion from POD 1 to POD 4 and quadriceps power on POD 1 were higher
in the LIA group than in the control group. Quadriceps power from POD 2
to POD 4 and postoperative length of stay were not significantly
different between groups.
Conclusions: Intra-operative
single-dose LIA can effectively reduce postoperative pain, morphine
demand, and morphine consumption. Therefore, the use of LIA is
recommended during TKA.
New knowledge added by this study
- After total knee arthroplasty (TKA), postoperative morphine demand and consumption, as well as pain scores from postoperative day (POD) 1 to POD 4, were lower in the local infiltration analgesia (LIA) group than in the control group.
- The range of motion from POD 1 to POD 4 and quadriceps power on POD 1 were greater in the LIA group than in the control group.
- Quadriceps power from POD 2 to POD 4 and postoperative length of stay were not significantly different between groups.
- Intra-operative administration of LIA effectively reduced postoperative patient pain and consumption of morphine.
- Routine use of LIA in TKA protocols may facilitate more rapid recovery from surgery through earlier return of range of motion and quadriceps power.
Introduction
Total knee arthroplasty (TKA) is a common
orthopaedic procedure to relieve the problem of end-stage degenerative
knee osteoarthritis, particularly in the context of the ageing population,
increasing incidence of degenerative joint diseases, and modern emphasis
on quality of life. However, TKA is associated with significant
postoperative pain, which can hinder rehabilitation and cause morbidity.1 Various methods for pain relief
have been introduced, including epidural analgesia, peripheral nerve
blocks, local infiltration analgesia (LIA), intravenous patient-controlled
analgesia, and oral analgesia. Spinal anaesthesia has been associated with
severe complications, such as postoperative headache, intra-operative
hypotension, and risk of spinal infection.1
In addition, intravenous or oral narcotics can cause nausea, vomiting,
somnolence, respiratory depression, and urinary retention.1 Thus, LIA has become increasingly popular for its
potential to avoid these complications.
Local infiltration analgesia was first described by
Kerr and Kohan2 in Australia in
2008. It involves use of a mixture of an anaesthetic drug and a
non-steroidal anti-inflammatory drug, to which adrenaline or a
corticosteroid can be added.3 Local
infiltration analgesia is administered intra-operatively through injection
into the posterior capsule of the knee, as well as the soft tissues around
the surgical field.3 4 There is increasing evidence to support the use of LIA
in TKA.4 5
6 7
8 However, other studies have shown
that the efficacy of LIA during TKA is not superior to that of previously
available methods.9 10 11 In
addition, the use of LIA is reportedly safe,1
12 13
14 15
but has only recently been adopted in medical centres in Hong Kong. To the
best of our knowledge, there have been no studies of the efficacy of LIA
in patients undergoing TKA in Hong Kong.
We aimed to investigate the efficacy of LIA in
patients with knee osteoarthritis undergoing TKA. The primary outcomes of
this study were pain scores and morphine consumption from postoperative
day (POD) 1 to POD 4. The secondary outcomes of this study were range of
motion, quadriceps power, and postoperative length of stay.
Methods
Study design
This was a single-centre, retrospective cohort
study based in Yan Chai Hospital, a joint centre in Hong Kong.
Patients and study population
This study was approved by the Kowloon West Cluster
Research Ethics Committee. The study cohort consisted of Chinese patients
aged ≥18 years with knee osteoarthritis who were scheduled to undergo
primary TKA during the period from January 2017 to December 2017 in Yan
Chai Hospital in Hong Kong. Exclusion criteria were the presence of
chronic inflammatory joint disease (eg, rheumatoid arthritis or Charcot
arthropathy); current use of other medications or measures that may alter
pain tolerance (eg, regular steroid or opioid use, nerve blocks, or
epidural anaesthesia); presence of dementia; presence of conditions
precluding the use of LIA (eg, allergy or intolerance to a drug used in
LIA, renal insufficiency, bleeding disorder, or prolonged QT interval).
The use of LIA in TKA began on 14 June 2017. Therefore, there were two
matched cohorts in this study: the control group was recruited before 14
June 2017, when LIA was not yet used; the LIA group was recruited on or
after 14 June 2017, when LIA was routinely administered if not
contra-indicated.
Study procedures
Baseline assessments were performed for all
patients in this study, including preoperative blood tests and relevant
X-rays. Written informed consent for TKA was provided by each patient. As
noted above, the use of LIA in the centre began on 14 June 2017;
therefore, patients who underwent TKA on or after that date also gave
written informed consent to receive LIA, provided that they did not have
any contra-indications to LIA. Antibiotic prophylaxis was administered to
each patient prior to operation.
All TKA procedures were performed by surgeons in
Yan Chai Hospital, using the medial parapatellar approach. A tourniquet
was applied to the operated limb with pressure 2 times the systolic blood
pressure; the tourniquet was released after wound closure. Cemented
prostheses were used in all cases.
Intra-operative single-dose LIA was administered to
patients in the LIA group. The LIA mixture consisted of 30 mg ketorolac,
100 mg levobupivacaine, and 0.5 mg adrenaline; these components were
diluted in normal saline to a final volume of 100 mL, using sterile
technique. The LIA mixture was prepared in two 50-mL syringes with
19-gauge needles for injection, and injection was performed at three time
points. The first injection was performed before prosthesis cementation
and implantation. The posterior capsule was infiltrated with approximately
20% of the total volume of LIA. During infiltration, the midpoint of the
posterior capsule was avoided, due to the close proximity of the
neurovascular bundle. The second injection was performed after prosthesis
implantation: 60% of the total volume of LIA was infiltrated into the
released collateral ligaments, both gutters, anterior supracondylar soft
tissue, quadriceps cut ends, and retinaculum. The third injection was
performed immediately before skin closure: the remaining 20% of the total
LIA volume was injected subcutaneously. For the control group, no LIA was
administered. A suction drain at 200 mm Hg was inserted in all patients,
and was removed on POD 1.
In both control and LIA groups, the same
postoperative protocol was followed. Immediately postoperatively, each
patient received instruction from a nurse regarding the use of
patient-controlled anaesthesia (PCA), which comprised 1 mg/mL morphine.
When patients experienced pain, they could self-administer 1 mg of
morphine intravenously. To prevent overdose, the lockout interval was set
at 6 minutes, and the 4-hour maximum morphine dose was 30 mg.
Patient-controlled anaesthesia was discontinued on POD 1 or 2, in
accordance with the anaesthetist’s assessment. In addition to intravenous
morphine, oral analgesics were administered; these included 1 g
acetaminophen 4 times daily for 6 days and 50 mg tramadol 4 times daily
for 4 days. After the administration period of oral analgesics (6 days for
acetaminophen and 4 days of tramadol), these oral analgesics were
administered only when necessary. Physiotherapy to achieve full
weight-bearing walking was offered to all patients on POD 1. Routine deep
vein thrombosis screening was performed once, on or after POD 3 by Doppler
ultrasound in the Radiology Department of Yan Chai Hospital.
Outcomes
Primary outcomes were visual analogue scale (VAS)
pain score during the period from POD 1 to POD 4 and total morphine use.
Visual analogue scale pain scores were rated by patients using a scale of
0 to 10, where 0 was no pain and 10 was the highest pain imaginable. The
amounts (in milligrams) of morphine demanded and consumed by each patient
were recorded; there may be a discrepancy between these two values because
a lockout interval and maximum dose of morphine were set in the PCA
machine to avoid patient overdose. As noted above, PCA was discontinued on
either POD 1 or POD 2, in accordance with the anaesthetist’s assessment.
Secondary outcomes were range of motion (ie,
degrees of active flexion) during the period from POD 1 to POD 4,
quadriceps power during the period from POD 1 to POD 4, and postoperative
length of stay. Degrees of active flexion and quadriceps power were used
because both have been shown to positively influence rehabilitation and
functional ability.16 17 Degrees of active flexion was measured by the
attending physician during daily ward rounds, using a goniometer;
measurements were corrected to the nearest 5 degrees. Quadriceps power was
also rated by the attending physician during daily ward rounds, using the
Medical Research Council rating scale of 0 to 5.18
Quadriceps power ≥3 was used as a cut-off in the present study; the
percentage of patients in each group with quadriceps power ≥3 was assessed
during the period from POD 1 to POD 4. Postoperative length of stay was
recorded as the number of days that patients remained in the hospital
after TKA.
Sample size
The primary outcomes were postoperative VAS pain
score and total morphine consumption. Previous studies assessed VAS pain
score using scales of 0 to 10 (where 0=no pain and 10=extreme pain) or 0
to 100 mm (where 0=no pain and 100=extreme pain) with 10-mm increments.19 20
21 22
23 24
25 In previous studies that have
used a 10-point VAS pain score scale, mean (standard deviation)
postoperative VAS pain score was 6.1 (1.1) in the control group.2 19 20 26
Therefore, a reduction of 1 point in the VAS pain score was considered to
be a clinically relevant difference. The sample size for the present study
was calculated using an alpha level of 0.05 and 80% power. With these
assumptions, a sample size of 19 patients per group was needed to detect a
1-point reduction in VAS pain score (ClinCalc.com; clincalc.com/stats/
samplesize.aspx). In addition, a reduction of 40% in morphine usage was
considered to be a clinically relevant difference.27 Based on previous studies, the mean (standard
deviation) of total morphine usage was 20.6 (6.8) mg.2 28 Using the
above alpha and power values, a sample size of 11 patients per group was
needed to detect a 40% reduction in morphine usage.
To allow for analysis of secondary outcomes and
attrition due to missing data, a more conservative sample size estimation
was adopted. The estimated sample size for range of motion was 57 patients
per group, based on the report published by Zhang et al,29 and a 5% increase in degree of flexion being
considered clinically relevant. To allow 15% attrition due to missing
data, a sample size of 68 patients per group was used.
Statistical analysis
Statistical analyses were performed with SPSS
(Windows version 23.0; IBM Corp, Armonk [NY], United States). The Chi
squared test was used to analyse categorical variables between two groups
(LIA and control). The Shapiro-Wilk test was used to determine whether
data followed a normal distribution. The independent samples t
test and Mann-Whitney U test were used to compare respective
parametric and non-parametric continuous data between the two groups.
Differences with P<0.05 were considered to be statistically
significant.
Results
A total of 136 knees were recruited (68 per group).
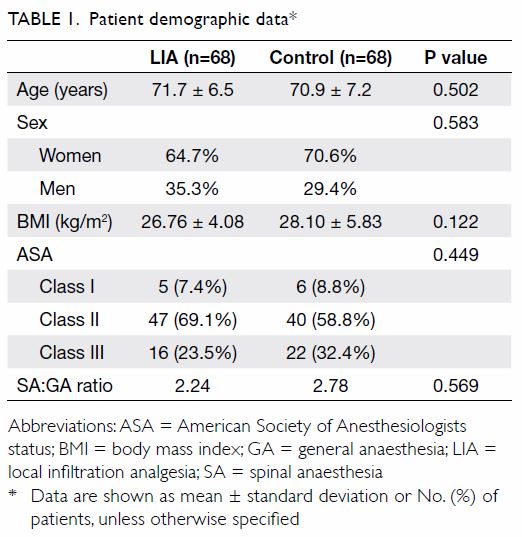
There were no significant differences between the groups with respect to
baseline demographic data (Table 1). The results of the Shapiro-Wilk test
showed that the following data were not normally distributed: VAS pain
score, morphine consumption, degrees of active flexion, and postoperative
length of stay.
Complications
There were no cases of wound infection, delayed
wound healing, or prolonged wound drainage. One patient in the LIA group
experienced medial tibial plateau fracture intra-operatively; the fracture
was repaired using a screw. One patient in the LIA group had an incidental
finding of popliteal vein aneurysm during routine postoperative Doppler
ultrasound screening for deep vein thrombosis.
Primary outcomes
Visual analogue scale pain score
As noted above, VAS pain score data followed a
non-normal distribution. Thus, the Mann-Whitney U test was used
for comparison between the two groups. Patients in the LIA group had
significantly lower pain scores during the period from POD 1 to POD 4,
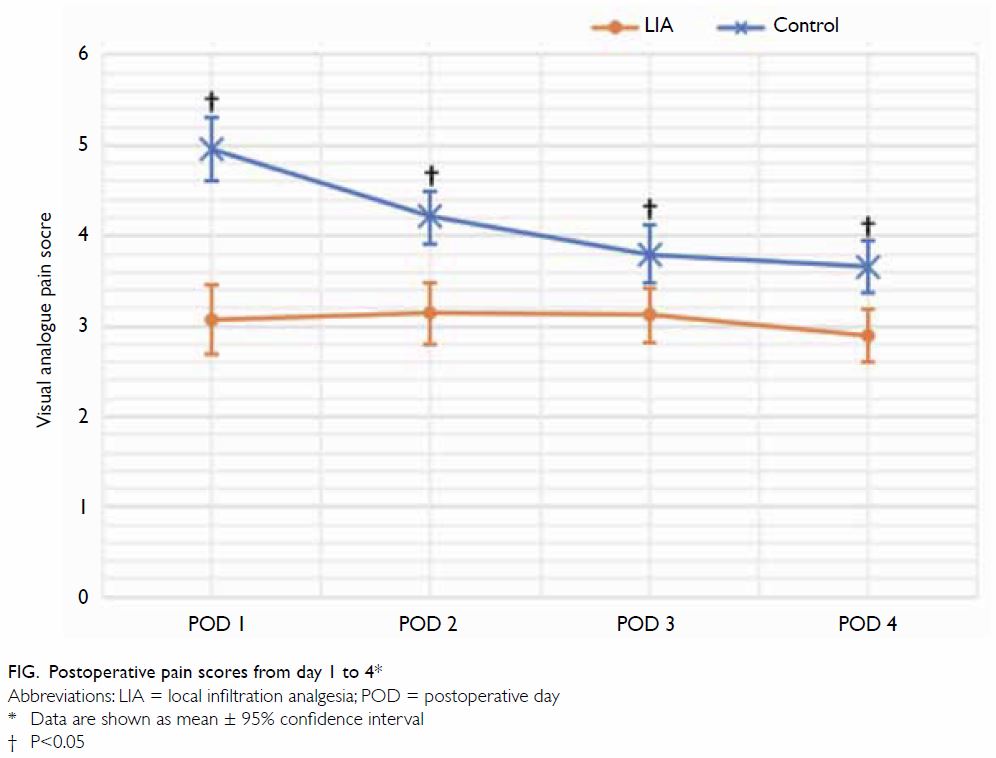
compared with patients in the control group (Fig). On POD 1, the mean VAS pain score was 3.07 in
the LIA group, compared with 4.96 in the control group (P<0.001); on
POD 2, the LIA group had a pain score of 3.14, compared with 4.21 in the
control group (P<0.001). Differences in pain score on POD 3 and POD 4
were smaller, but remained statistically significant. On POD 3, the pain
score in the LIA group was 3.12, while that in the control group was 3.79
(P=0.001); on POD 4, the pain score in the LIA group was 2.89, while that
in the control group was 3.66 (P<0.001) [Fig].
Morphine consumption
The mean amount of morphine demanded by patients
through PCA in the LIA group was 20.10 mg, whereas that in the control
group was 29.85 mg (P<0.001, Mann-Whitney U test). The mean
amount of morphine consumed by patients in the LIA group was 11.85 mg,
while that in the control group was 19.54 mg (P<0.001, Mann-Whitney U
test).
Secondary outcomes
Range of motion
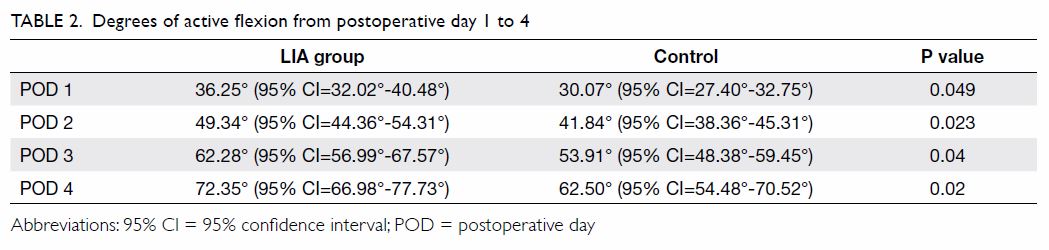
The range of motion (degrees of active flexion) in
the LIA group was significantly greater than that in the control group
during the period from POD 1 to POD 4 (P<0.05 for all comparisons,
Mann-Whitney U test) [Table 2].
Quadriceps power
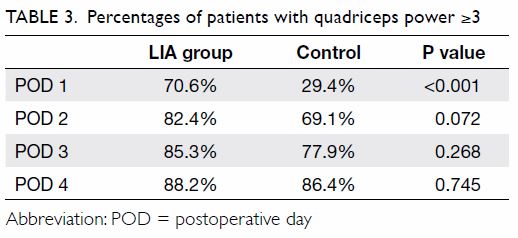
The percentage of patients with quadriceps power ≥3
was compared between the two groups using the Chi squared test. On POD 1,
70.6% of patients in the LIA group had quadriceps power ≥3, compared with
29.4% of patients in the control group (P<0.001). On POD 2, POD 3, and
POD 4, there was a trend suggestive of a higher percentage of patients in
the LIA group with quadriceps power ≥3, but the difference was not
statistically significant (Table 3).
Postoperative length of stay
The postoperative length of stay did not
significantly differ between LIA and control groups (5.49 days vs 6.29
days; P=0.092, Mann-Whitney U test).
Discussion
Pain is an important concern during and immediately
after TKA, as it affects patients’ quality of life and can hinder
rehabilitation progress. A single intra-operative dose of LIA consisting
of a mixture of levobupivacaine, ketorolac, and adrenaline improved
postoperative pain control, as evidenced by reduced VAS pain scores during
the period from POD 1 to POD 4 in the present study. Some previous studies2 29
demonstrated no significant differences in pain score between LIA and
control groups from POD 1 onwards. In more recent studies by Vaishya et al8 and Fan,30 pain-relieving effects
of LIA were observed through POD 3, which was similar to the findings of
significantly lower pain scores through POD 4 in our study. In addition,
differences in pain scores between groups appeared to be greater on POD 1
and POD 2 than on POD 3 and POD 4.
There is no gold standard for LIA. Briefly, it
consists of a local anaesthetic, non-steroidal anti-inflammatory drug, and
adrenaline; some authors have added morphine and/or steroid to the
mixture.24 30 31 32 Most studies have used ropivacaine as the local
anaesthetic, while some used bupivacaine. The only previous study
performed in Hong Kong30 and the
present study both used levobupivacaine. According to Casati and Putzu,15 ropivacaine and levobupivacaine
were developed to avoid bupivacaine-related severe toxicity. Compared with
bupivacaine, ropivacaine and levobupivacaine have slightly lower
anaesthetic potency; however, they exhibit lower central nervous system
and cardiovascular toxicity. There is an increasing trend for using
ropivacaine or levobupivacaine in LIA, rather than bupivacaine.15 Because of the variations in LIA mixtures, it is
difficult to identify the ‘most effective’ component or components. Thus,
further studies are needed to support standardisation of LIA.
Both morphine demand and consumption were lower in
the LIA group. Because PCA in this study included the use of a lockout
interval to avoid morphine overdose, we analysed morphine demand, which
more accurately reflected the need for pain control in each patient.
Previous studies have reported convincing evidence for lower morphine
consumption in patients who had received LIA during TKA.1 2 21 22 23 27 28 30 31 33 However,
none of the previous studies assessed morphine demand. In the present
study, the reduction of both morphine demand and consumption in the LIA
group further support the conclusion that the use of LIA improved pain
control after TKA.
An incidental finding of popliteal vein aneurysm
was noted in one patient in the LIA group. Venous aneurysm is rare, but
can be a source of thromboembolism.34
Nearly all patients described in the literature were symptomatic, and the
most common symptoms were pulmonary embolism and post-thrombotic syndrome.35 The definition of venous
aneurysm remains controversial. According to Sadowska et al,36 the diameter of a normal popliteal vein varies from 5
to 12 mm in women and 7 to 13 mm in men; some authors have suggested that
the diameter of the venous aneurysm should be twice the normal diameter,
while other reports have suggested that it should be at least 3 times the
normal diameter.35 In the present
study, the patient had a fusiform dilatation (anteroposterior diameter=22
mm; length=20 mm) of the popliteal vein with reflux noted. The popliteal
vein aneurysm was in the distal portion of the popliteal fossa,
immediately proximal to the branching of the saphenous vein, which was not
involved; the popliteal vein was posterior and lateral to the popliteal
artery at that level, and there was no intraluminal thrombus. The patient
remained asymptomatic throughout and was referred to vascular specialists
in our hospital for further follow-up; repeated duplex ultrasound by the
vascular specialists at 4 months postoperatively showed no progression of
the aneurysm. The popliteal vein was fully compressible without any
thrombus. In addition, there was no aneurysm or pseudoaneurysm in the
popliteal artery; thus, the patient continues to receive conservative
treatment.
The pathogenesis of popliteal vein aneurysm is
uncertain. Possible causes include congenital weakness, trauma,
inflammation, and localised degenerative changes.35
A popliteal vein aneurysm has been reported as a result of
post-arthroscopy trauma,37 but has
not been associated with TKA. Nonetheless, popliteal artery pseudoaneurysm
is an uncommon complication of TKA that has been previously reported.38 39
Pseudoaneurysm implies that trauma to the artery may have occurred during
TKA, which may comprise direct incision, injury during the injection of
LIA, or blunt instrument trauma (eg, from an oscillating saw). With the
increasing use of LIA, it is important to consider the risk of vascular
complications during injection into the posterior capsule. The potential
for popliteal pseudoaneurysm after LIA is not yet known. We consider it to
be unlikely that the popliteal aneurysm in our patient was a complication
of TKA and/or LIA.
Conclusion
Intra-operative single-dose LIA can effectively
reduce postoperative pain during the period from POD 1 to POD 4, and can
reduce both the demand and consumption of morphine. Therefore, we
recommend the use of LIA in TKA. Further studies are warranted to evaluate
the impact of LIA on long-term functional outcome, as well as to establish
a gold standard for the administration of LIA.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: All authors.
Acquisition of data: YY Fang, QJ Lee, EWY Chang.
Analysis or interpretation of data: YY Fang, QJ Lee.
Drafting of the article: YY Fang.
Critical revision for important intellectual content: YY Fang.
Acquisition of data: YY Fang, QJ Lee, EWY Chang.
Analysis or interpretation of data: YY Fang, QJ Lee.
Drafting of the article: YY Fang.
Critical revision for important intellectual content: YY Fang.
Declaration
The study was presented in the 38th Annual Congress
of the Hong Kong Orthopaedic Association, 3-4 November 2018, Hong Kong.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Kowloon West Cluster
Research Ethics Committee (Ref KW/EX-18-118[128-02]).
References
1. Xu CP, Li X, Wang ZZ, Song JQ, Yu B.
Efficacy and safety of single-dose local infiltration of analgesia in
total knee arthroplasty: a meta-analysis of randomized controlled trials.
Knee 2014;21:636-46. Crossref
2. Kerr DR, Kohan L. Local infiltration
analgesia: a technique for the control of acute postoperative pain
following knee and hip surgery: a case study of 325 patients. Acta Orthop
2008;79:174-83. Crossref
3. Keijsers R, van Delft R, van den Bekerom
MP, de Vires DC, Brohet RM, Nolte PA. Local infiltration analgesia
following total knee arthroplasty: effect on post-operative pain and
opioid consumption—a meta-analysis. Knee Surg Sports Traumatol Arthrosc
2015;23:1956-63. Crossref
4. Affas F. Local infiltration analgesia in
knee and hip arthroplasty efficacy and safety. Scand J Pain 2016;13:59-66.
Crossref
5. de Jonge T, Görgényi S, Szabó G, Torkos
MB. Local infiltration analgesia in total joint replacement [in
Hungarian]. Orv Hetil 2017;158:352-7. Crossref
6. Barastegui D, Robert I, Palau E, et al.
Can local infiltration analgesia increase satisfaction in postoperative
short-term pain control in total knee arthroplasty? J Orthop Surg (Hong
Kong) 2017;25:2309499017690461. Crossref
7. Seangleulur A, Vanasbodeekul P,
Prapaitrakool S, et al. The efficacy of local infiltration analgesia in
the early postoperative period after total knee arthroplasty: a systematic
review and meta-analysis. Eur J Anaesthesiol 2016;33:816-31. Crossref
8. Vaishya R, Wani AM, Vijay V. Local
infiltration analgesia reduces pain and hospital stay after primary TKA:
randomized controlled double blind trial. Acta Orthop Belg 2015;81:720-9.
9. Fan L, Yu X, Zan P, Liu J, Ji T, Li G.
Comparison of local infiltration analgesia with femoral nerve block for
total knee arthroplasty: a prospective, randomized clinical trial. J
Arthroplasty 2016;31:1361-5. Crossref
10. Albrecht E, Guyen O, Jacot-Guillarmod
A, Kirkham KR. The analgesic efficacy of local infiltration analgesia vs
femoral nerve block after total knee arthroplasty: a systematic review and
meta-analysis. Br J Anaesth 2016;116:597-609. Crossref
11. Mulford JS, Watson A, Broe D, Solomon
M, Loefler A, Harris I. Short-term outcomes of local infiltration
anaesthetic in total knee arthroplasty: a randomized controlled
double-blinded controlled trial. ANZ J Surg 2016;86:152-6. Crossref
12. Bonnette BA. Is local infiltration
analgesia (LIA) a safe and effective method for post-operative pain
management after a unilateral total knee arthroplasty (TKA)?
[dissertation]. US: Philadelphia College of Osteopathic Medicine; 2013.
13. Brydone AS, Souvatzoglou R, Abbas M,
Watson DG, McDonald DA, Gill AM. Ropivacaine plasma levels following
high-dose local infiltration analgesia for total knee arthroplasty.
Anaesthesia 2015;70:784-90. Crossref
14. Knudsen K, Beckman Suurküla M,
Blomberg S, Sjövall J, Edvardsson N. Central nervous and cardiovascular
effects of i.v. infusions of ropivacaine, bupivacaine and placebo in
volunteers. Br J Anaesth 1997;78:507-14. Crossref
15. Casati A, Putzu M. Bupivacaine,
levobupivacaine and ropivacaine: are they clinically different? Best Pract
Res Clin Anaesthesiol 2005;19:247-68. Crossref
16. Rowe PJ, Myles CM, Walker C, Nutton R.
Knee joint kinematics in gait and other functional activities measured
using flexible electrogoniometry: how much knee motion is sufficient for
normal daily life? Gait Posture 2000;12:143-55. Crossref
17. Moxley Scarborough D, Krebs DE, Harris
BA. Quadriceps muscle strength and dynamic stability in elderly persons.
Gait Posture 1999;10:10-20. Crossref
18. Medical Research Council. Aids to the
examination of the peripheral nervous system. Memorandum No 45
(superseding War Memorandum No. 7). London: Her majesty’s stationery
office. 1976. Available from:
https://mrc.ukri.org/documents/pdf/aids-to-the-examination-of-the-peripheral-nervous-system-mrc-memorandum-no-45-superseding-war-memorandum-no-7/.
Accessed Nov 2018.
19. Affas F, Nygårds EB, Stiller CO,
Wretenberg P, Olofsson C. Pain control after total knee arthroplasty: a
randomized trial comparing local infiltration anesthesia and continuous
femoral block. Acta Orthop 2011;82:441-7. Crossref
20. Rosen AS, Colwell CW, Pulido PA,
Chaffee, TL, Copp SN. A randomized controlled trial of intraarticular
ropivacaine for pain management immediately following total knee
arthroplasty. HSS J 2010;6:155-9. Crossref
21. Lamplot JD, Wagner ER, Manning DW.
Multimodal pain management in total knee arthroplasty: a prospective
randomized controlled trial. J Arthroplasty 2014;29:329-34. Crossref
22. Kurosaka K, Tsukada S, Seino D,
Morooka T, Nakayama H, Yoshiya S. Local infiltration analgesia versus
continuous femoral nerve block in pain relief after total knee
arthroplasty: a randomized controlled trial. J Arthroplasty 2016;31:913-7.Crossref
23. Tsukada S, Wakui M, Hoshino A.
Postoperative epidural analgesia compared with intraoperative
periarticular injection for pain control following total knee arthroplasty
under spinal anesthesia: a randomized controlled trial. J Bone Joint Surg
Am 2014;96:1433-8. Crossref
24. Tsukada S, Wakui M, Hoshino A. Pain
control after simultaneous bilateral total knee arthroplasty: a randomized
controlled trial comparing periarticular injection and epidural analgesia.
J Bone Joint Surg Am 2015;97:367-73. Crossref
25. Tammachote N, Kanitnate S, Manuwong S,
Yakumpor T, Panichkul P. Is pain after TKA better with periarticular
injection or intrathecal morphine? Clin Orthop Relat Res 2013;471:1992-9.
Crossref
26. Fajardo M, Collins J, Landa J, Adler
E, Meere P, Di Cesare PE. Effect of a perioperative intra-articular
injection on pain control and early range of motion following bilateral
TKA. Orthopedics 2011;34:354. Crossref
27. Niemeläinen M, Kalliovalkama J, Aho
AJ, Moilanen T, Eskelinen A. Single periarticular local infiltration
analgesia reduces opiate consumption until 48 hours after total knee
arthroplasty. A randomized placebo-controlled trial involving 56 patients.
Acta Orthop 2014;85:614-9. Crossref
28. Garcia JB, Barbosa Neto JO,
Vasconcelos JW, Ferro LS, Silva RC. Analgesic efficacy of the
intra-articular administration of high doses of morphine in patients
undergoing total knee arthroplasty [in English, Portuguese]. Rev Bras
Anestesiol 2010;60:1-12. Crossref
29. Zhang S, Wang F, Lu ZD, Li YP, Zhang
L, Jin QH. Effect of single-injection versus continuous local infiltration
analgesia after total knee arthroplasty: a randomized, double-blind,
placebo-controlled study. J Int Med Res 2011;39:1369-80. Crossref
30. Fan JC. Intra-operative periarticular
multimodal injection in total knee arthroplasty: a local hospital
experience in Hong Kong. Hong Kong Med J 2018;24:145-51. Crossref
31. Fu P, Wu Y, Wu H, Li X, Qian Q, Zhu Y.
Efficacy of intra-articular cocktail analgesic injection in total knee
arthroplasty—a randomized controlled trial. Knee 2009;16:280-4. Crossref
32. Busch CA, Shore BJ, Bhandari R, et al.
Efficacy of periarticular multimodal drug injection in total knee
arthroplasty. A randomized trial. J Bone Joint Surg Am 2006;88:959-63. Crossref
33. Essving P, Axelsson K, Kjellberg J,
Wallgren O, Gupta A, Lundin A. Reduced morphine consumption and pain
intensity with local infiltration analgesia (LIA) following total knee
arthroplasty. Acta Orthop 2010;81:354-60. Crossref
34. Aldridge SC, Comerota AJ, Katz ML,
Wolk JH, Goldman BI, White JV. Popliteal venous aneurysm: report of two
cases and review of world literature. J Vasc Surg 1993;18:708-15. Crossref
35. Sessa C, Nicolini P, Perrin M, Farah
I, Magne JL, Guidicelli H. Management of symptomatic and asymptomatic
popliteal venous aneurysms: a retrospective analysis of 25 patients and
review of the literature. J Vasc Surg 2000;32:902-12. Crossref
36. Sadowska A, Spodnik JH, Wójcik S.
Variations in popliteal fossa venous anatomy: implications for diagnosis
of deep-vein thrombosis. Folia Morphol (Warsz) 2013;72:51-6. Crossref
37. Jimenez F, Utrilla A, Cuesta C, et al.
Popliteal artery and venous aneurysm as a complication of arthroscopic
meniscectomy. J Trauma 1988;28:1404-5. Crossref
38. Boutchichi A, Ciornohac J, Daubresse
F. Pseudoaneurysm after total knee arthroplasty: a rare complication with
different possible clinical presentations. Acta Orthop Belg 2013;79:16-9.
39. Agarwala SR, Mohrir GS, Dotivala SJ.
Posttraumatic pseudoaneurysm of popliteal artery following total knee
arthroplasty. Indian J Orthop 2013;47:101-3. Crossref