Hong
Kong Med J 2019 Jun;25(3):228–34 | Epub 10 Jun 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
Peanut allergy and oral immunotherapy
TH Lee, ScD, FRCP1; June KC Chan, MSc,
RD (US)1; PC Lau, RN, BNurs1; WP Luk, MPhil2;
LH Fung, MPhil2
1 Allergy Centre, Hong Kong Sanatorium
& Hospital, Happy Valley, Hong Kong
2 Medical Physics and Research, Hong
Kong Sanatorium & Hospital, Happy Valley, Hong Kong
Corresponding author: Dr TH Lee (takhong.lee@hksh.com)
Abstract
Peanut allergy is the commonest cause of
food-induced anaphylaxis in the world, and it can be fatal. There have
been many recent improvements to achieve safe methods of peanut
desensitisation, one of which is to use a combination of
anti–immunoglobulin E and oral immunotherapy. We have treated 27
patients with anti–immunoglobulin E and oral immunotherapy, and report
on the outcomes and incidence of adverse reactions encountered during
treatment. The dose of peanut protein tolerated increased from a median
baseline of 5 to 2000 mg after desensitisation, which is substantially
more than would be encountered through accidental ingestion. The
incidence of adverse reactions during the escalation phase of oral
immunotherapy was 1.8%, and that during the maintenance phase was 0.6%.
Most adverse reactions were mild; three episodes were severe enough to
warrant withdrawal from oral immunotherapy, but none required
epinephrine injection. Preliminary data suggest that unresponsiveness is
lost when daily ingestion of peanuts is stopped after the maintenance
period.
Introduction
Peanut is a leading food allergen alongside
shellfish, eggs, milk, beef, and tree nuts.1
Strict peanut avoidance is difficult and stressful for patients and
families. The incidence rates of accidental ingestion can be as high as
50%,2 3
and it can cause anaphylaxis, which is sometimes fatal. Therefore, new
management strategies for peanut allergy are required, such as oral
immunotherapy (OIT).
Peanut oral immunotherapy without anti–immunoglobulin E
Most trials on peanut OIT have been conducted in
the absence of anti–immunoglobulin E (anti-IgE) pretreatment.4 5 6 7 8 9 10 These studies involve gradually increasing small
doses of peanut (escalation phase) up to a maintenance dose of 300 to 4000
mg peanut protein (PP), with or without a phase of rush immunotherapy when
several doses were given on the same day at the start of OIT. The daily
maintenance dose was then sustained for 6 months to 3 years. Peanut
tolerance in subjects increased over time, and the tolerance to peanut in
open food challenge (OFC) at completion of the treatment was often more
than 2-fold greater than the daily maintenance intake. Efficacy of peanut
OIT was high, where 67 % to 93 % of subjects were successfully
desensitised to the maintenance dose. These studies have also been
considered to demonstrate an acceptable degree of safety although there
were dropouts in all the trials. Adverse reaction (AR) rates were 1.2% for
build-up doses and 3.7% to 6.3% for home doses. Most ARs were
oropharyngeal symptoms but there were some cases of anaphylaxis requiring
epinephrine injection. In addition, eosinophilic gastroenteritis was a
complication in some patients. In a recent peanut allergy OIT study using
defatted slightly roasted peanut flour for desensitisation, 4.3% of
children receiving peanut experienced severe ARs compared with <1% of
those receiving placebo; 21% of the peanut group withdrew from the study.10 Further, 14% of those ingesting
peanut required epinephrine injection, including one child who experienced
anaphylaxis and required three epinephrine injection, compared with 3.2%
on placebo.
To sustain non-responsiveness following OIT, Tang
et al7 used a combined therapy of
probiotics and peanut OIT. The majority (89.7%) of the probiotics and
peanut OIT group were desensitised, and sustained unresponsiveness (SU)
was achieved in 87.1% of the children, who could then consume peanuts ad
libitum. A related follow-up study indicated that 58% of the probiotics
and peanut OIT group subjects achieved 8-week SU at 4 years.8
Peanut oral immunotherapy with anti–immunoglobulin E
Prior studies that have combined anti-IgE
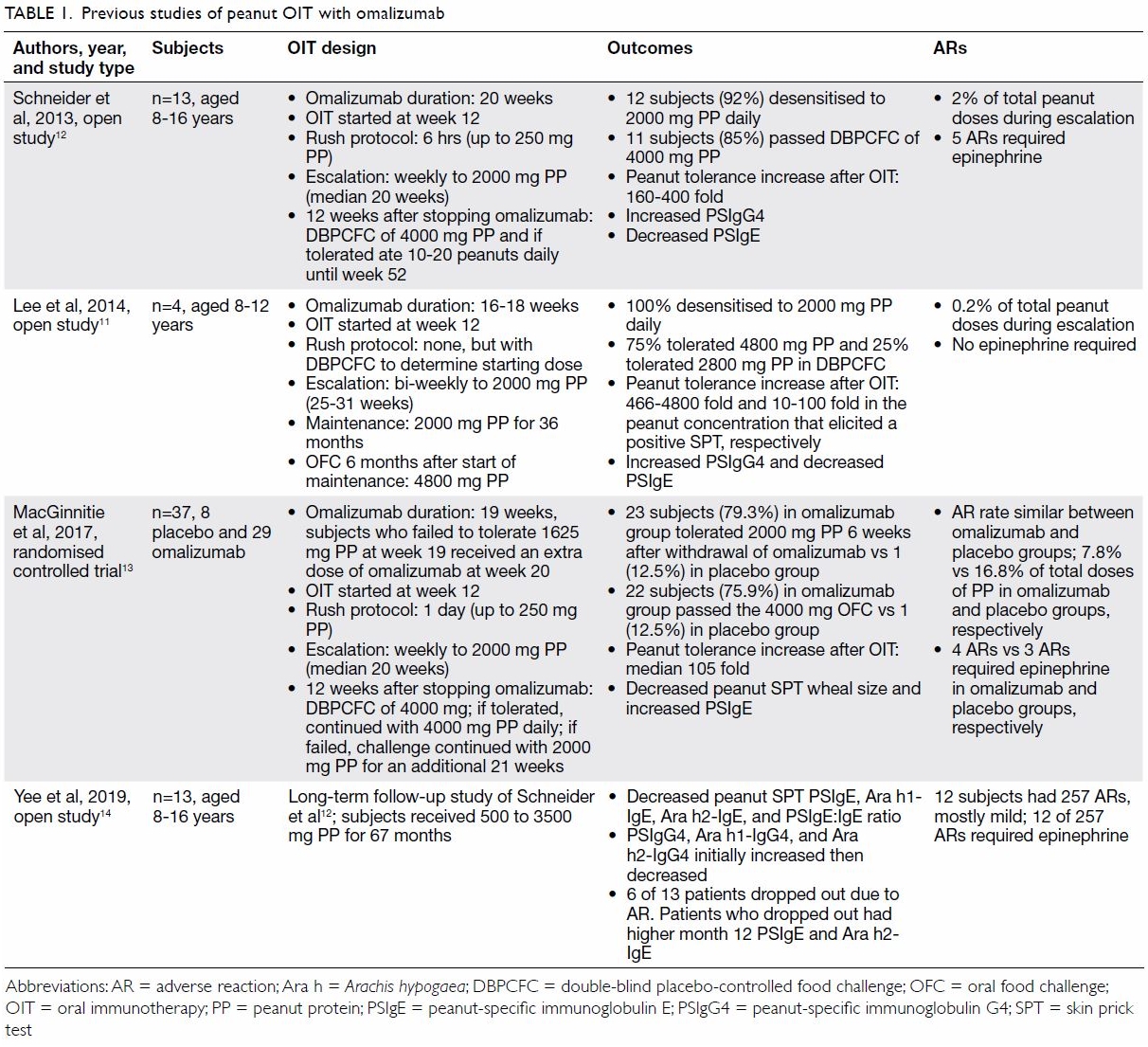
premedication with OIT are summarised in Table 1.11 12 13
14 In contrast to other studies, a
study conducted in Hong Kong by Lee et al11
did not have a rush immunotherapy phase (when several doses of peanuts
were administered on day 1); instead, peanut dose was increased more
gradually at 2-week intervals. Despite differences in study design, the
outcomes from all the studies were similar.11
12 13
14 Lee et al11 found four children tolerated 466 to 4800-fold more
PP on OFC than before OIT; their threshold in peanut-specific skin prick
tests increased by 10- to 100-fold; and each subject’s peanut
allergen-specific IgG4 level increased after OIT. The prevalence of ARs in
the study by Lee et al11 appeared
to be lower than that first reported by Schneider et al12 using anti-IgE combined with OIT which included a
rush immunotherapy step; however, the Hong Kong population included in the
Lee et al study was small.
Sublingual immunotherapy
Comparisons between studies on sublingual
immunotherapy (SLIT) are difficult because different doses and durations.15 16
17 18
19 However, tentative conclusions
can be drawn: in many instances SLIT achieved at least a 10-fold increase
in peanut tolerance from baseline after several years of treatment. The
ARs experienced during SLIT treatment were mild and consisted mainly of
oropharyngeal symptoms. Although SLIT had a better safety profile, OIT
appeared to be more efficacious overall.19
Epicutaneous immunotherapy
The early trials of epicutaneous immunotherapy
(EPIT) were encouraging with at least a 10-fold improvement in tolerated
dose following 8 weeks of treatment.20
21 The safety level was high. The
ARs were mostly local and mild and epinephrine injection was not required.
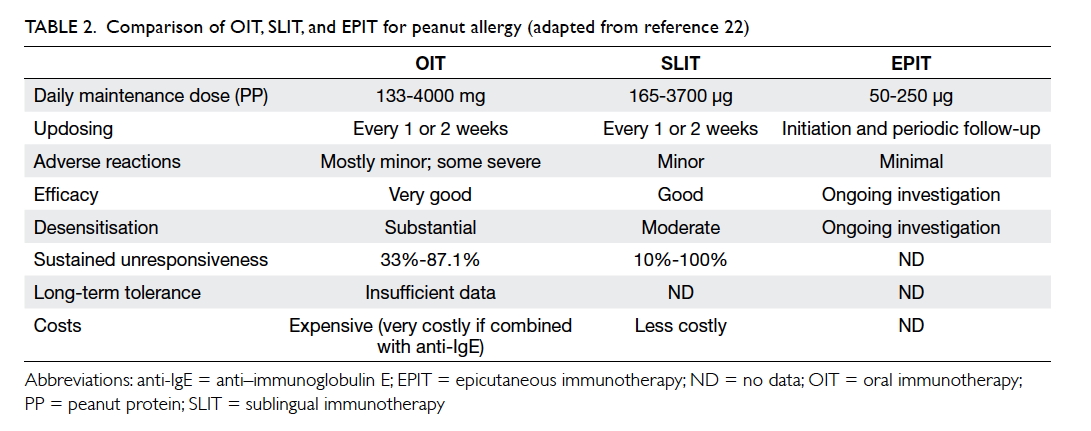
The efficacy, safety, and costs of OIT, SLIT, and
EPIT are compared in Table 2.22
Although it is more efficacious, OIT has greater potential for ARs and is
the most costly option, especially if combined with anti-IgE treatment.
Update on the Hong Kong experience
Our centre has now treated 27 peanut-allergic
patients aged 6 to 16 years (22 male, 5 female) with anti-IgE and OIT,
including the four children previously reported.11
Patients were considered for anti-IgE and OIT treatment if they were: aged
≥6 years with a history of allergic symptoms developing within 60 minutes
of peanut ingestion; serum total IgE between 30 and 1500 IU/mL; a positive
skin prick test and/or presence of peanut-specific IgE, and positive oral
peanut challenge. They were of good general health with no prior exposure
to monoclonal antibodies. Asthma must have been under control, with a
forced expiratory volume in 1 second of at least 80% of the predicted
value. Systemic glucocorticoids, beta blockers, and angiotensin-converting
enzyme inhibitors were prohibited before screening and throughout the
study. Aspirin, antihistamines, and antidepressants were not permitted for
3 days, 1 week, and 2 weeks, respectively, before skin testing or oral
food challenge. If potential subjects had poorly controlled asthma, poorly
controlled atopic dermatitis, or inability to discontinue antihistamines
or other medication for skin testing and oral challenges, they were
excluded. They were also ineligible if it seemed unlikely that they would
comply with the treatment protocol.
The subjects received between 150 and 600 (median
375) mg of anti-IgE for a median of 18 weeks, as determined by baseline
serum IgE concentration and body weight.11
From about 12 weeks after beginning anti-IgE pretreatment, peanuts were
eaten daily at home at an initial dose determined by OFC according to our
previously reported protocol.11
Updosing was supervised at bi-weekly intervals in the clinic for 12 to 28
(median 16) weeks (escalation phase) until an oral intake of 2000 mg PP
daily was achieved, as previously described in detail.11 The parents of one child requested to stop escalation
after 800 mg of PP because they felt that he was already protected from
accidental ingestion and had a strong taste aversion to peanuts. He
continued on 800 mg during his maintenance phase. If a patient had a major
AR on an updosing visit, the next daily dose was reduced to a previously
tolerated dose (often halved), and escalation proceeded more slowly (3-4
weeks) until higher doses were tolerated or the patient withdrew.
Successful escalation was followed by a maintenance phase, when patients
normally ingested 2000 mg PP daily.
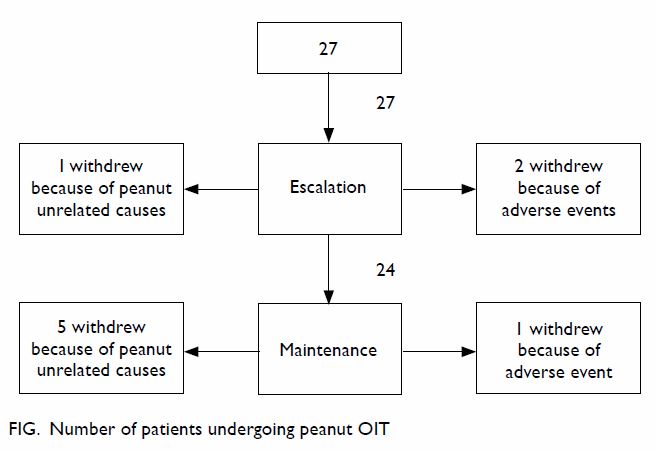
Twenty-three of the 27 peanut allergic children
completed the escalation phase according to protocol (85%). There were
three dropouts, of which two were caused by peanut-related AR, and the
third moved away from Hong Kong for family reasons. Another child stopped
updosing at 800 mg, as described already, but continued into the
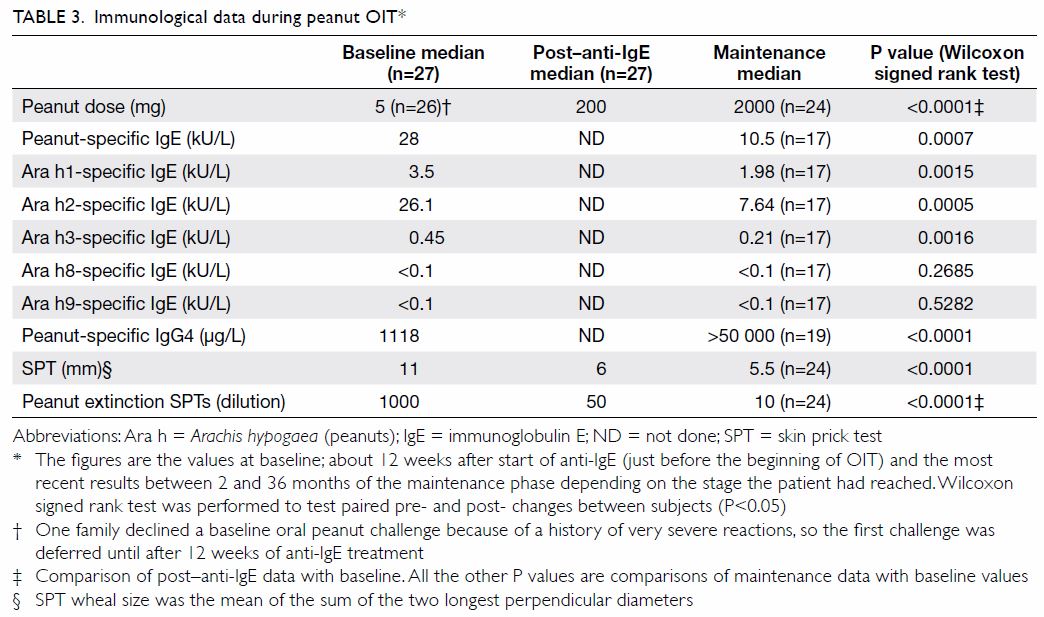
maintenance phase (Fig). The dose of PP tolerated at OFC increased from
a median of 5 mg at baseline to 200 mg after anti-IgE treatment and
subsequently to a median of 2000 mg in the maintenance phase. There was a
400-fold improvement in the median tolerated peanut dose (Table
3), yielding a final tolerance greater than the amount of peanuts
likely to be encountered through inadvertent ingestion.
The immunological data are shown in Table
3. There was a marked decrease in biomarkers such as peanut-specific
IgE and Ara h1, 2, and 3 (but not in Ara h 8 and 9, which were very low at
baseline). Skin prick testing (SPT) and the dilution of peanut extract in
extinction titration SPT also showed improvements. The level of peanut
sIgG4 increased substantially, consistent with the recruitment of an
IL-10/Treg pathway.
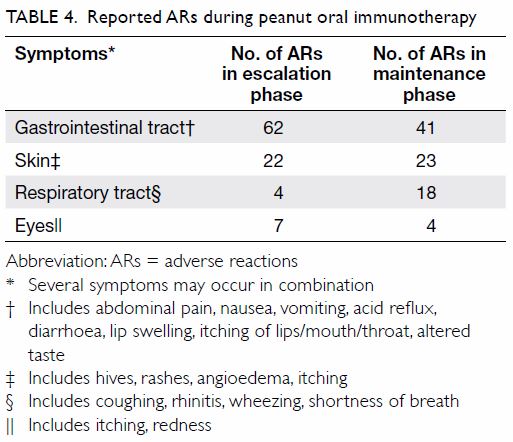
Side-effects during peanut oral immunotherapy
Escalation phase
The ARs during updosing in hospital were directly
observed; those ARs experienced at home were self-reported by patients’
parents. There were 18 observed and 46 reported episodes of AR to 3560
administered doses of peanut (1.8%). Thus, 71.9% of all ARs during the
escalation phase occurred at home. One episode could comprise one or more
symptoms (Table 4). Most ARs were minor (Table
4) and resolved spontaneously or after administration of an
antihistamine. One subject had 12 minor episodes but still completed
escalation. There were four major episodes, which involved development of
asthma, repeated vomiting, and angioedema (0.1%), and they occurred in two
patients who dropped out (Fig).
The frequent occurrence of
gastrointestinal symptoms (n=62) is consistent with that reported previously.14 23
Maintenance phase
Twenty-four patients entered the maintenance phase
of OIT (Fig). The duration of their maintenance phases so
far has ranged from 2 to 42 (median 24) months. One child planned to study
overseas and therefore continued on the maintenance doses for 6 months
longer than planned (42 months in total) until he returned to Hong Kong
for holidays. All parents and patients were asked to report any AR.
To date, there have been 80 reported episodes of AR
from 14 350 administered doses (0.6%). The majority of subjects had no
ARs, and 85% of all the ARs reported were experienced by seven (29.2%)
patients. Six of these patients were able to continue with OIT, but one
patient withdrew because of severe eczema.
Forty-one, 23, and 18 side-effects reported during
the maintenance phase were related to the gastrointestinal tract, skin,
and respiratory system, respectively; thus, gastrointestinal symptoms
predominated again (Table 4). The gastrointestinal symptoms were mostly
mild and resolved either spontaneously or after antihistamine
administration. Occasionally, it was also necessary to administer an oral
anti-spasmodic drug.
While the incidence of AR during the maintenance
phase of OIT was very low, repeated ARs still occurred in some subjects,
and one episode was severe enough to warrant withdrawal from the
programme. This highlights the importance of continued vigilance
throughout OIT.
Dropouts
Four patients left Hong Kong for family reasons.
Another two patients (twins) developed unexplained intermittent mild
neutropenia after 2 years of maintenance OIT, which was not caused by
peanuts. Nonetheless, although they stopped daily peanut consumption, they
continued to be monitored to assess for SU. Two children were withdrawn
during escalation, and one dropped out during maintenance because of
peanut allergy related to AR during OIT (Fig). Thus, overall, one-third of subjects dropped
out (9 of 27), but only one-third of the dropouts (3 patients; 11.1%)
withdrew because of AR caused by peanut ingestion.
The incidence of AR in our subjects was similar5 9 24 or even lower than that in previous reports.6 8 10 25 26 Baseline allergic rhinitis and peanut SPT wheal sizes
have been suggested to be significant predictors of higher overall rate of
AR during peanut OIT,23 but in our
series, baseline peanut SPT results; extinction dilution SPTs;
peanut-specific IgE; Arachis hypogaea 1-, 2-, 3-, 8-, and
9-specific IgE concentrations; and the presence of rhinitis and asthma
were not predictors of ARs (P>0.05 for all correlations).
Preliminary data on sustained unresponsiveness
A major concern regarding immunotherapy is whether
it can induce long-term tolerance. Seven of our patients have been
followed up after cessation of daily peanut consumption. Three of these
subjects discontinued peanut ingestion after maintenance treatment with
1600 to 2000 mg PP daily, and their sensitivity returned, as evidenced by
ARs to intentional or accidental ingestion of peanuts as well as ARs to
100 mg and 400 mg PP upon OFC at 6 months (n=2) and 12 months (n=1),
respectively. The other four subjects have continued to ingest their
maintenance doses of peanuts 3 times weekly after the maintenance phase
was completed and have not experienced any ARs after 4, 7, 8, and 24
months of observation, respectively.
Syed et al27
randomised 43 subjects aged 4 to 45 years to receive peanut OIT (n=23) or
placebo (n=20). Peanut doses were escalated to 4000 mg PP and maintained
for 24 months. Then, subjects avoided peanuts for 3 months, and their SU
was assessed. In all, 87% of the subjects were successfully desensitised
to 4000 mg PP, and 30% achieved SU after avoiding peanuts for 3 months. Of
the seven subjects who had SU at 3 months, only three of them (13% of the
treatment group) still achieved SU at 6 months of peanut avoidance.
Conclusions
Our protocol of combining anti-IgE with OIT is
efficacious and safe, with only minor side-effects encountered by most
patients. This is a retrospective record review and therefore is an audit
of our real-world experience. There is growing momentum behind the
development of commercial products for peanut desensitisation,9 10 21 and it is essential to compare their efficacy and
safety with existing techniques for peanut immunotherapy in a real-world
situation.28 29 30
Selection of suitable patients to undergo OIT is
critical, as it is a labour-intensive and expensive treatment that
requires time, patience, and compliance from everyone involved. We spend
much time explaining the procedure in detail to the family and child to
ascertain whether they are likely to complete the treatment. The patient
or the patient’s parent (if the patient is a child) signs an informed
consent form if they agree to proceed. If they have concomitant asthma, we
ensure that this is optimally controlled before embarking on OIT. Even
with careful selection, four of our subjects left Hong Kong for family
reasons before OIT was completed. This was unavoidable but nevertheless
undesirable for the continuity of their treatment. Any treatment that
takes years to complete will always be a challenge, especially for
families whose children relocate for study, work, or other reasons. While
some treatments can be continued by centres overseas, OIT expertise is not
so easily accessible, and it may be necessary to discontinue treatment.
This is regrettable, as all the parents and patients, who completed their
desensitisation programmes successfully reported that their quality of
life had been improved.
We recommend that this treatment only be offered by
specialists with the appropriate training within an environment with
immediate resuscitation facilities and support staff who are trained to
manage allergic emergencies and can undertake patient education.
Our experience suggests that peanut sensitivity
will likely return after a few months when OIT is stopped, so regular
ingestion of peanut consumption is required to sustain the desensitised
state. We now advise patients to continue consuming the maintenance dose
of peanuts at least 3 times weekly to sustain desensitisation. They are
seen every 6 months for skin testing, and they undergo a formal peanut
challenge annually or more frequently, according to clinical judgment. We
also advise that they retain their epinephrine autoinjectors for emergency
treatment of unexpected events.
Alternative methods that hold promise for peanut
desensitisation are being developed, including SLIT,15 16 17 18 20 low-dose OIT without anti-IgE,6 9 31 co-administration of a probiotic7 8 with OIT to
promote longer-term tolerance, and EPIT.21
Thus additional transformative treatments for peanut and other food
allergies will be forthcoming in the near future.
Author contributions
All authors contributed to the design, acquisition
of data, analysis and interpretation of data, drafting of the manuscript,
and critical revision for important intellectual content. All authors also
had full access to the data, contributed to the study, approved the final
version for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Hong Kong Sanatorium
& Hospital Research Committee (Ref RC-2018-27). Patients provided
informed consent.
References
1. Ho MH, Lee SL, Wong WH, Ip P, Lau YL.
Prevalence of self-reported food allergy in Hong Kong children and teens—a
population survey. Asian Pac J Allergy Immunol 2012;30:275-84.
2. Michelsen-Huisman AD, van Os-Medendorp
H, Blom WM, et al. Accidental allergic reactions in food allergy: causes
related to products and patient’s management. Allergy 2018;73:2377-81. Crossref
3. Sicherer SH, Burks AW, Sampson HA.
Clinical features of acute allergic reactions to peanut and tree nuts in
children. Pediatrics 1998;102:e6. Crossref
4. Jones SM, Pons L, Roberts JL, et al.
Clinical efficacy and immune regulation with peanut oral immunotherapy. J
Allergy Clin Immunol 2009;124:292-300. Crossref
5. Varshney P, Jones SM, Scurlock AM, et
al. A randomized controlled study of peanut oral immunotherapy: clinical
desensitization and modulation of the allergic response. J Allergy Clin
Immunol 2011;127:654-60. Crossref
6. Anagnostou K, Islam S, King Y, et al.
Assessing the efficacy of oral immunotherapy for the desensitisation of
peanut allergy in children (STOP II): a phase 2 randomised controlled
trial. Lancet 2014;383:1297-304. Crossref
7. Tang ML, Ponsonby AL, Orsini F, et al.
Administration of a probiotic with peanut oral immunotherapy: a randomized
trial. J Allergy Clin Immunol 2015;135:737-44.e8. Crossref
8. Hsiao KC, Ponsonby AL, Axelrad C, Pitkin
S, Tang ML, PPOIT Study Team. Long-term clinical and immunological effects
of probiotic and peanut oral immunotherapy after treatment cessation:
4-year follow-up of a randomized, double-blind, placebo-controlled trial.
Lancet Child Adolesc Health 2017;1:97-105. Crossref
9. PALISADE Group of Clinical
Investigators, Vickery BP, Vereda A, et al. AR101 oral immunotherapy for
peanut allergy. N Engl J Med 2018;379:1991-2001. Crossref
10. Bird JA, Spergel JM, Jones SM, et al.
Efficacy and safety of AR101 in oral immunotherapy for peanut allergy:
results of ARC001, a randomized, double-blind, placebo-controlled phase 2
clinical trial. J Allergy Clin Immunol Pract 2018;6:476-85.e3.
11. Lee TH, Chan J, Lau VW, Lee WL, Lau
PC, Lo MH. Immunotherapy for peanut allergy. Hong Kong Med J
2014;20:325-30. Crossref
12. Schneider LC, Rachid R, LeBovidge J,
Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate
rapid oral desensitization in high-risk peanut-allergic patients. J
Allergy Clin Immunol 2013;132:1368-74. Crossref
13. MacGinnitie AJ, Rachid R, Gragg H, et
al. Omalizumab facilitates rapid oral desensitization for peanut allergy.
J Allergy Clin Immunol 2017;139:873-81. Crossref
14. Yee CS, Albuhairi S, Noh E, et al.
Long-term outcome of peanut oral immunotherapy facilitated initially by
omalizumab. J Allergy Clin Immunol Pract 2019;7:451-61.e7. Crossref
15. Kim EH, Bird JA, Kulis M, et al.
Sublingual immunotherapy for peanut allergy: clinical and immunologic
evidence of desensitization. J Allergy Clin Immunol 2011;127:640-6.e1. Crossref
16. Fleischer DM, Burks AW, Vickery BP, et
al. Sublingual immunotherapy for peanut allergy: a randomized,
double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol
2013;131:119-27.e1-7. Crossref
17. Burks AW, Wood RA, Jones SM, et al.
Sublingual immunotherapy for peanut allergy: long-term follow-up of a
randomized multicenter trial. J Allergy Clin Immunol 2015;135:1240-8.e1-3.
Crossref
18. Narisety SD, Frischmeyer-Guerrerio PA,
Keet CA, et al. A randomized, double-blind, placebo-controlled pilot study
of sublingual versus oral immunotherapy for the treatment of peanut
allergy. J Allergy Clin Immunol 2015;135:1275-82.e1-6. Crossref
19. Pajno GB, Fernandez-Rivas M, Arasi S,
et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food
allergy. Allergy 2018;73:799-815. Crossref
20. Sindher S, Fleischer DM, Spergel JM.
Advances in the treatment of food allergy: sublingual and epicutaneous
immunotherapy. Immunol Allergy Clin North Am 2016;36:39-54. Crossref
21. Jones SM, Sicherer SH, Burks AW, et
al. Epicutaneous immunotherapy for the treatment of peanut allergy in
children and young adults. J Allergy Clin Immunol 2017;139:1242-52. Crossref
22. Parrish CP. Management of peanut
allergy: a focus on novel immunotherapies. Available from:
https://www.ajmc.com/journals/supplement/2018/managed-care-perspective-peanut-allergy/management-of-peanut-allergy-a-focus-on-novel-immunotherapies.
Accessed 15 Feb 2019.
23. Virkud YV, Burks AW, Steele PH, et al.
Novel baseline predictors of allergic side effects during peanut oral
immunotherapy. J Allergy Clin Immunol 2017;139:882-8.e5. Crossref
24. Vickery BP, Berglund JP, Burk CM, et
al. Early oral immunotherapy in peanut-allergic preschool children is safe
and highly effective. J Allergy Clin Immunol 2017;139:173-81.e8. Crossref
25. Blumchen K, Ulbricht H, Staden U, et
al. Oral peanut immunotherapy in children with peanut anaphylaxis. J
Allergy Clin Immunol 2010;126:83-91.e1. Crossref
26. Yu GP, Weldon B, Neale-May S, Nadeau
KC. The safety of peanut oral immunotherapy in peanut-allergic subjects in
a single-center trial. Int Arch Allergy Immunol 2012;159:179-82. Crossref
27. Syed A, Garcia M, Lyu SC, et al.
Peanut oral immunotherapy results in increased antigen-induced regulatory
T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J
Allergy Clin Immunol 2014;133:500-10. Crossref
28. Wasserman RL, Hague AR, Pence DM, et
al. Real-world experience with peanut oral immunotherapy: lessons learned
from 270 patients. J Allergy Clin Immunol Pract 2019;7:418-26.e4. Crossref
29. Couzin-Franke J. A revolutionary
treatment for allergies to peanuts and other foods is going mainstream—but
do the benefits outweigh the risks? Available from: https://www.sciencemag.org/news/2018/10/revolutionary-treatment-allergies-peanuts-and-other-foods-going-mainstream-do-benefits. Accessed 16 May
2019.
30. Wasserman RL, Jones DH, Windom HH.
Oral immunotherapy for food allergy: The FAST perspective. Ann Allergy
Asthma Immunol 2018;121:272-5. Crossref
31. Nagakura KI, Yanagida N, Sato S, et
al. Low-dose oral immunotherapy for children with anaphylactic peanut
allergy in Japan. Pediatr Allergy Immunol 2018;29:512-8. Crossref