Hong
Kong Med J 2019 Jun;25(3):192–200 | Epub 10 Jun 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Parental consanguinity in Hong Kong
KH Siong, MB, BS, FHKAM (Obstetrics and
Gynaecology)1; Sidney KC Au Yeung, MB, BS, FRCOG1;
TY Leung, MD, FRCOG2
1 Department of Obstetrics and
Gynaecology, Tuen Mun Hospital, Tuen Mun, Hong Kong
2 Department of Obstetrics and
Gynaecology, The Chinese University of Hong Kong, Prince of Wales
Hospital, Shatin, Hong Kong
Corresponding author: Dr KH Siong (skh664@ha.org.hk)
Abstract
Introduction: Consanguineous
union increases the risk of genetic disorders in offspring. The present
study aimed to evaluate the prevalence and characteristics of parental
consanguinity in Hong Kong, and its effects on pregnancy, perinatal, and
child health outcomes.
Methods: Pregnant women in
consanguineous unions attending an obstetrics unit at a public hospital
in Hong Kong were retrospectively studied. Their pregnancy, perinatal,
and child health outcomes were compared with an ethnicity-matched
control group of pregnant women in non-consanguineous unions.
Results: The overall prevalence
of parental consanguinity was 0.6% (first cousins or closer, 78.4%;
beyond first cousins, 21.6%). The majority were ethnic Pakistani
(85.0%). Women in consanguineous unions were more likely to have an
obstetric history of congenital abnormality (10.5%), unexplained
intrauterine fetal demise (4.2%) and unexplained neonatal death (4.6%),
or family history of congenital abnormality (4.6%). Offspring of
consanguineous parents had significantly higher risk of recessive
diseases (odds ratio [OR]=8.70, 95% confidence interval
[CI]=1.06-71.36), structural abnormalities (OR=4.55, 95% CI=2.17-9.53)
and developmental delay (OR=6.72, 95% CI=1.48-30.63), and significantly
higher incidence of autistic spectrum disorder (2.1%;
P=0.008).
Conclusions: It is essential
that information on the increased risks associated with parental
consanguinity is included in genetic counselling for consanguineous
couples, so that they can make informed decisions.
New knowledge added by this study
- The majority of consanguineous unions in Hong Kong are of Pakistani ethnicity.
- It is well known that, in addition to recessive genetic diseases, offspring of consanguineous unions have higher incidences of non–genetically confirmed structural abnormalities, developmental delay, and autism spectrum disorders. The present study confirms this in the Hong Kong population.
- Identification of consanguineous couples is essential to ensure appropriate referral for genetic counselling and diagnosis.
- Health education and information about availability of carrier screening should be provided for consanguineous couples to make informed choices.
Introduction
‘Consanguinity’ is a term derived from the Latin
word ‘consanguineus’, meaning ‘of the same blood’. In medical genetics,
consanguineous union is generally referred as a union between couples
related as second cousins or closer.1
The prevalence of consanguinity varies significantly worldwide, depending
on cultural background, religious belief, and geography. The highest rates
are estimated in the Near and Middle East and in Northern Africa, where
20% to 50% of marriages are consanguineous.1
2 The prevalence in Southern
Europe, South America, and Japan is about 1% to 5%, whereas Western
European countries, North America, and Oceania have the lowest prevalence
of <1%.1 2
Consanguineous union increases the risk of genetic
disorders in offspring, especially for autosomal recessive diseases.
However, recent studies suggest that parental consanguinity is also a risk
factor for other adverse outcomes, even in developed multi-ethnic
countries where the prevalence of consanguineous marriages is perceived as
lower. For example, in Vienna where the background consanguinity rate was
<1%, Posch et al3 reported that
39.7% of consanguineous couples had obstetric history of congenital
malformations or genetic disorders. Becker et al4
reported that 6.1% of consanguineous couples were referred to a specialist
centre in Germany for a history of major fetal anomalies. A 10-year
retrospective analysis conducted in Australia, where the consanguinity
rate is 5.5%, concluded that parental consanguinity was associated with
higher rates of threatened premature labour, fetal congenital abnormality,
stillbirth, and perinatal mortality.5
In that study, consanguinity was also found to be an independent risk
factor of nearly 3-fold for stillbirth.
In Hong Kong, parental consanguinity is more
frequent among non-Chinese ethnic minorities, which account for 8% of the
total population.6 Internationally, healthcare workers lack knowledge on
the risks of consanguinity.7 8 9
Inconsistencies in information provided during genetic counselling and
screening has been observed.10
Consanguineous couples are often unaware of the potential health hazards
in their offspring.11 12 13 The level
of concern and awareness of the adverse effects of parental consanguinity
among patients and physicians is low, and available data on consanguinity
in Hong Kong are limited. Therefore, in the present study, we aimed to
clarify the prevalence and characteristics of pregnancies from
consanguineous unions in Hong Kong, and to assess the related effects on
maternal, perinatal, and child health outcomes.
Methods
The Prenatal Diagnosis Clinic in Tuen Mun Hospital
is responsible for counselling consanguineous couples. Dating ultrasound
and counselling sessions for Down syndrome screening are arranged for all
pregnant women who have their booking appointment in our locality. At the
booking appointment, patients are also asked about consanguinity.
Hospital-accredited interpreters are arranged for couples who are not
fluent in Cantonese or English. Identification of consanguineous cases
depends on self-reporting by couples. A pedigree chart is constructed for
each case. Couples are counselled about the possible effects of parental
consanguinity on pregnancy outcomes, and advised to attend antenatal care
regularly.
A retrospective cohort study of all parental
consanguinity cases over a 10-year period from 1 January 2007 to 31
December 2016 was conducted. The antenatal records of these cases were
reviewed. Details were gathered about pregnancy loss, fetal congenital
abnormalities, pregnancy and perinatal outcomes, and neonatal and
childhood development in the preceding pregnancy. The family history of
each case was also collected from patient records, including known genetic
or congenital anomalies, or intellectual or developmental disabilities. A
morphology scan was arranged for consanguineous cases. Each family
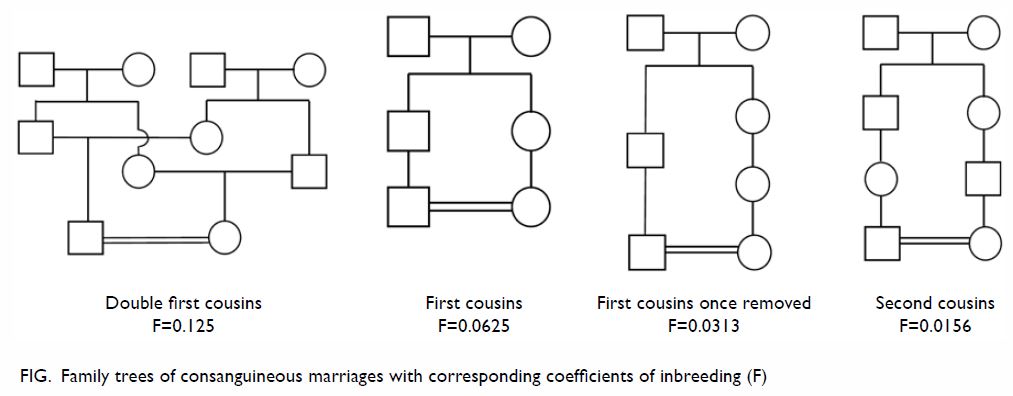
pedigree was studied to determine the degree of parental consanguinity (Fig). Only couples fulfilling the definition of
consanguineous unions (second cousins or closer) were included for
analysis in the present study.
Socio-demographic characteristics were collected,
including ethnicity, maternal and paternal age, religious beliefs, working
status, education level, and occupation. Maternal antepartum and
peripartum characteristics, and fetal and perinatal information were
available. Information about the neonatal, infancy, and childhood outcomes
of the offspring were retrieved from the public sector electronic record
system.
The relationship between consanguinity and fetal,
neonatal, infant, or childhood diseases that required long-term paediatric
management was evaluated and categorised into one of three categories:
Category A—Improbable association with
consanguinity: cases known to be caused by numerical or structural
chromosomal abnormalities, or not to have an autosomal recessive mode of
inheritance;
Category B—Probable association with consanguinity:
cases known to have an autosomal recessive mode of inheritance,
particularly when both parents were found to be the carriers of genetic
disorders; and
Category C—Possible/unclear association with
consanguinity: cases where the mode of inheritance was unclear, or when
genetic testing was unremarkable.
The characteristics and outcomes of consanguineous
cases were compared with a control group of non-consanguineous unions. The
next record of a non-consanguineous case of the same ethnicity after that
of a case of consanguineous union was selected as the control. This
ensured the similar composition of ethnicity which might have
socio-economic effects on the maternal and fetal outcomes within the study
and control groups.14 As some
consanguineous couples might have contributed more than one pregnancies in
our database, only adverse past obstetric outcome in the immediately
preceding pregnancy was counted in the analysis, and any positive family
history reported by such couples was counted as one case only, in order to
prevent duplicated entries for multigravida women. Most previous studies
have not evaluated the effects of closer consanguinity that might increase
risks of hereditary disorders.5 15 16
To evaluate the effect of degree of inbreeding, comparisons were made
among ‘first cousin or closer’ (including first cousin and double first
cousin), ‘beyond first cousin’ (including first cousin once removed and
second cousin), and non-consanguineous relationships.
Approval of this study was granted by the research
and ethics committee of the study hospital. Guidelines for reporting
observational studies according to the Strengthening the Reporting of
Observational Studies in Epidemiology (STROBE) statement were followed.
Statistical analysis was performed using SPSS
(Windows version 22.0; IBM Corp, Armonk [NY], US). Cross-tabulation
between degrees of consanguinity and the different variables was performed
in order to evaluate the characteristics of the study population.
Differences in continuous variables were compared using t test or
one-way analysis of variance. Differences in categorical variables were
analysed with Chi squared test or Fisher’s exact test. Linear regression
was carried out to adjust the collinearity among variables. Multivariate
logistic regression analysis was used to determine the risk of
consanguinity for adverse pregnancy and perinatal outcomes, with
adjustment of significant confounders. Adjusted odds ratio (OR) with 95%
confidence interval (CI) were calculated. Statistical significance was
established for P<0.05.
Results
Of 56 657 fetuses, 334 (0.6%) were conceived by
consanguineous parents; of these, the majority (85.0%, 284 of 334) were
ethnic Pakistani (among whom the prevalence of consanguineous union is
highest, at 30.5%), followed by Indian (6.2%), Nepalese (2.7%), Filipino
(0.4%), and Chinese (0.04%) [Table 1]. Of all consanguineous unions, the majority
were first cousin consanguineous unions (76.6%) and double first cousin
unions (1.8%); together, these were categorised as first cousin or closer
(≤1C) unions. The remainder were categorised as beyond first cousin
(>1C) unions, and included first cousin once removed unions (5.1%), and
second cousin unions (16.5%). Comparison of background variables including
maternal and paternal age, education level, religion, length of stay in
Hong Kong, marital status, working status, occupation, parity, and body
mass index showed no significant differences between the consanguineous
group and the non-consanguineous control group (Table 2).
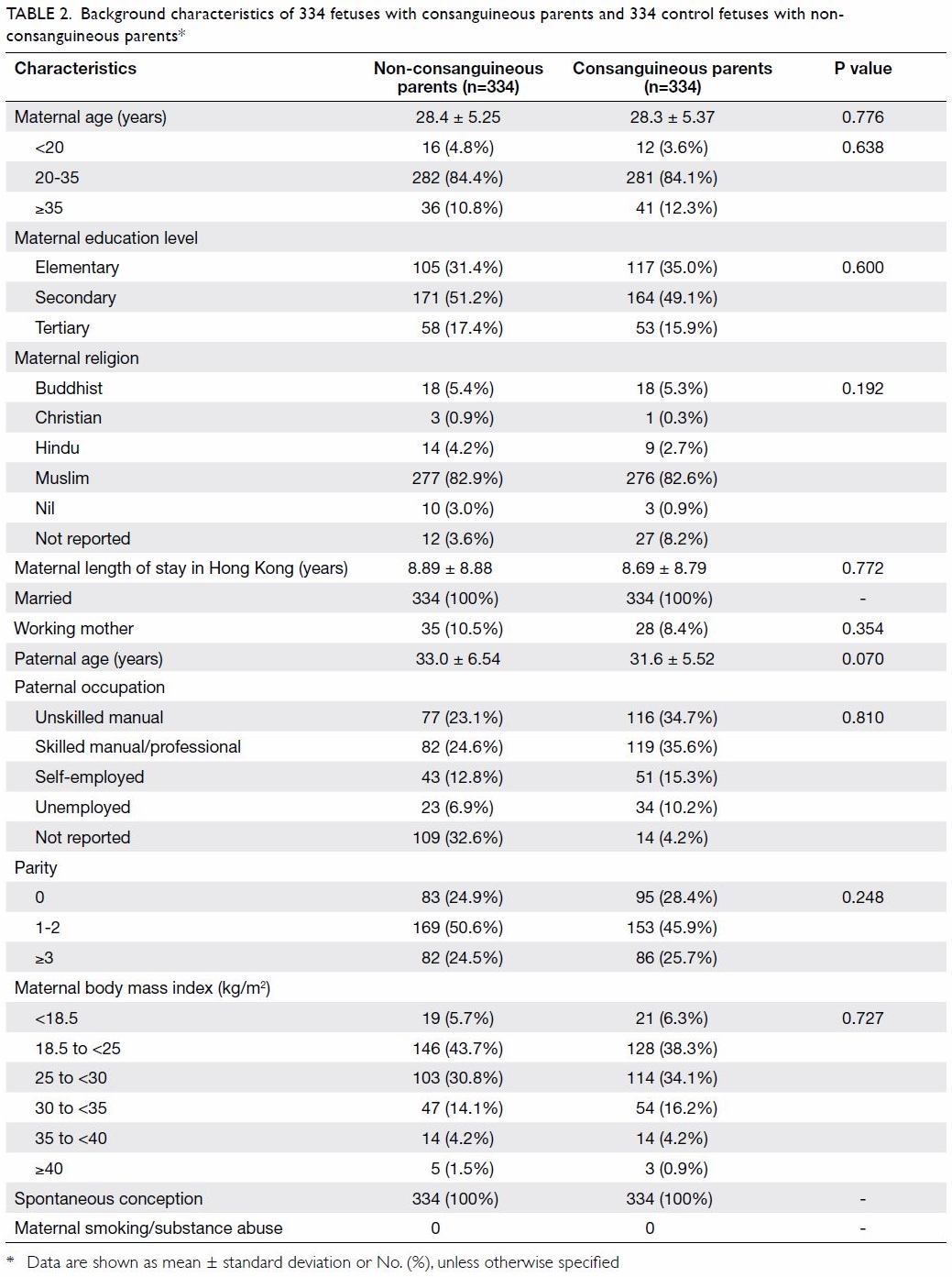
Table 2. Background characteristics of 334 fetuses with consanguineous parents and 334 control fetuses with nonconsanguineous parents
Women in consanguineous unions were significantly
more likely to have experienced congenital abnormality (10.5% vs 0.4%;
P<0.001), unexplained intrauterine fetal demise (4.2% vs 0.4%; P=0.005)
and neonatal death (4.6% vs 1.2%; P=0.024) in the preceding pregnancy, and
family history of congenital abnormality (4.6% vs 0%; P<0.001) than
were non-consanguineous controls (Table 3). Down syndrome screening was offered to all
women, but the attendance was only about one-fifth for all groups.
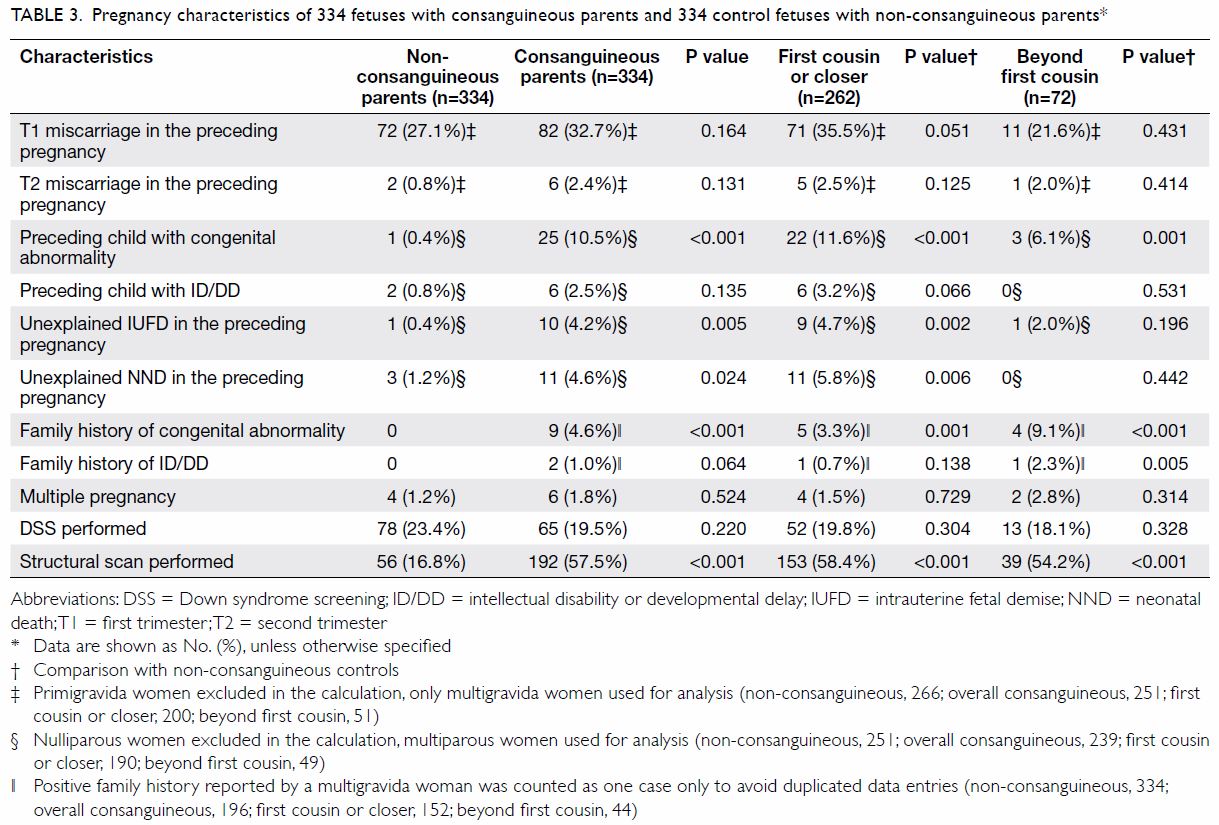
Table 3. Pregnancy characteristics of 334 fetuses with consanguineous parents and 334 control fetuses with non-consanguineous parents
In terms of major maternal and perinatal
complications, there were no significant differences between the
non-consanguineous control group and the overall consanguineous group or
the subgroups, except that pregnancies of ≤1C unions were more often
complicated with pre-eclampsia (4.2% vs 1.2%; P=0.02) than were those of
the non-consanguineous control group (Table 4).
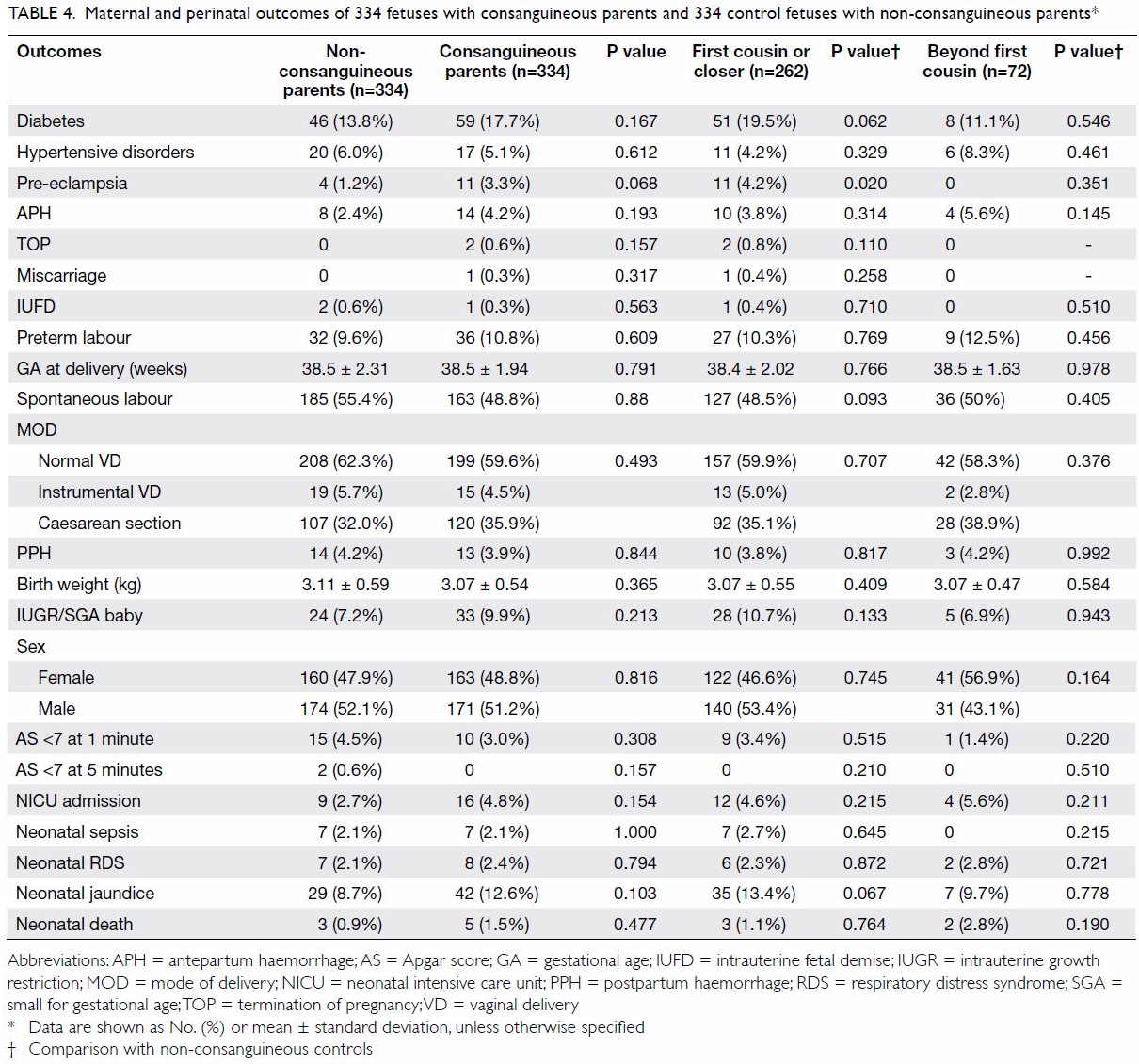
Table 4. Maternal and perinatal outcomes of 334 fetuses with consanguineous parents and 334 control fetuses with non-consanguineous parents
Altogether there were 58 fetuses and 14 fetuses
having different abnormalities, from 55 consanguineous and 14 control
couples respectively (Table 5). Offspring of consanguineous couples had a
higher risk of having category C disorders (OR=4.60; 95% CI=2.35-9.00) or
category B disorders (OR=8.70; 95% CI=1.06-71.36), compared with those of
non-consanguineous couples. The overall prevalence of category C disorders
(14.7%) was higher than that of category B disorders (2.4%). Compared with
the non-consanguineous control group, the prevalence of category C
disorders was significantly higher in the ≤1C subgroup (OR=5.59; 95%
CI=2.83-11.06); it was lower in the >1C subgroup, but the difference
was not significant.
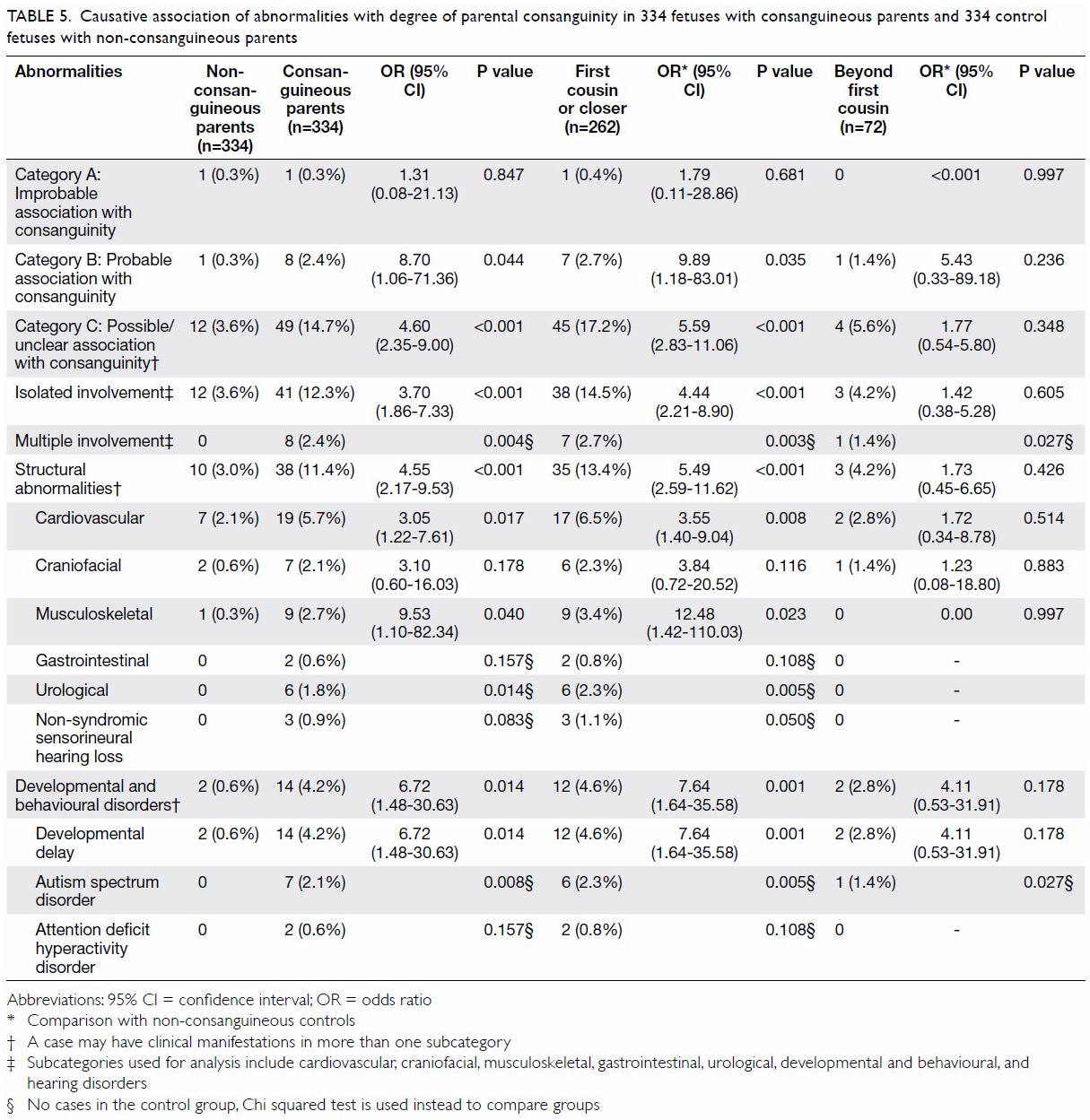
Table 5. Causative association of abnormalities with degree of parental consanguinity in 334 fetuses with consanguineous parents and 334 control fetuses with non-consanguineous parents
The prevalence of structural malformations was
higher in the consanguineous group than that in the non-consanguineous
control group, especially for those abnormalities involving
cardiovascular, musculoskeletal, and urological systems (Table
5). Parental consanguinity also significantly increased the risk of
developmental delay in offspring of consanguineous couples (OR=6.72, 95%
CI=1.48-30.63) and in those of ≤1C couples (OR=7.64, 95% CI=1.64-35.58).
Autism spectrum disorder was more prevalent in offspring of consanguineous
couples (2.1%) than in those of non-consanguineous couples (0%) [P=0.008].
The diseases recorded in the consanguineous group and in the control group
are detailed in online supplementary Appendices 1 and 2, respectively.
Discussion
To the best of our knowledge, this is the first
comprehensive study in Hong Kong describing the prevalence of parental
consanguinity. Our results support those of previous studies that revealed
a higher prevalence of parental consanguinity in certain ethnic groups,
and the higher prevalence of known genetic disorders (category B) among
their offspring. In addition, our study has revealed that the prevalence
of fetal structural abnormalities, developmental delay, and autism
spectrum disorders (category C) are also high. This has implications for
prenatal counselling and diagnosis, and related healthcare services.
Our comparison of maternal age and parity showed no
significant difference between the consanguineous group and control group.
This is in contrast to findings by Islam et al16
and Hosseini-Chavoshi et al,17 who
found that women in consanguineous unions were younger and of higher
parity in Iran and Oman, where the consanguinity rate was more than 30%.
Studies in India and Pakistan populations also showed that mothers in
consanguineous relationships were more likely to be socially and
economically disadvantaged.11 18 The similarity in the
socio-economic characteristics between the consanguineous and
non-consanguineous unions of our study indicates that socio-economic
factors are unlikely to be causes of the poorer fetal outcomes, both in
the index pregnancy and the preceding pregnancy, found in our
consanguineous group.
We identified eight offspring with autosomal
recessive diseases in the consanguineous group, including three cases of
beta-thalassaemia major and five cases of other rarer diseases (online
supplementary Appendix 1). Although the carrier status of
thalassaemia can be screened by low mean corpuscular volume of red blood
cells, the carrier status of other recessive disorders can be more
complex. For some disorders, comprehensive genetic carrier screening using
exome sequencing is required.4 19 20
21 Our data provide useful
information for preconception counselling for consanguineous couples.
However, exome sequencing is expensive, and this screening test is not yet
available in public hospitals. Health education and information about the
availability of carrier screening should be provided to all pregnant
women, regardless of cultural, religious, or socio-economic background.
Once a consanguineous couple is diagnosed to be the carrier of a genetic
disease, they should be encouraged to discuss carrier screening with their
siblings, who may also carry the same recessive gene and be in
consanguineous union. Access to obstetric care and genetic counselling
services in prenatal diagnosis clinics allows couples to make informed
choices. Knowledge on various cultural, religious, or socio-economic
issues allows healthcare workers to provide appropriate support and to
best advise patients.
Our results revealed that category C disorders are
more prevalent among offspring of consanguineous couples, especially in
the ≤1C subgroup. Fetal structural ultrasonographic examination should be
offered to ≤1C couples, especially for the cardiovascular, urological, and
skeletal systems.22 23 24 25 26 Detailed
genetic counselling and investigation services must be offered to ≤1C
couples if fetal abnormalities are detected.3
4
Our results revealed increased risk of
developmental and behavioural disorders for offspring of consanguineous
couples. However, disorders such as developmental delay and autism
spectrum disorder are not diagnosable before birth. Preconception and
prenatal counselling should be offered to consanguineous couples, who
should also be reminded about regular postnatal follow-up examinations, in
order to avoid any delay in diagnosing any developmental or behavioural
disorders.27
Pakistani ethnicity accounted for only 1.6% of all
fetuses but 85% of consanguineous couples in our study. According to the
Hong Kong 2016 population by-census, 0.25% of the total Hong Kong
population was of Pakistani ethnicity.6
However, the majority of this local Pakistani population is within
potentially reproductive age-groups (15-24 years, 19.2%; 25-34 years,
14.9%; 35-44 years, 21.3%), and they tend to have more children per couple
than do ethnic Chinese couples.6 It
is essential to include information about the increased risks of parental
consanguinity during the antenatal care and provide appropriate genetic
counselling once a consanguineous couple is identified.
In addition to poor fetal outcomes, we also found a
3-fold increased risk of pre-eclampsia among women in ≤1C unions. Familial
aggregation and possible genetic correlation of pre-eclampsia have been
observed, but the exact effect of consanguinity remains controversial.28 29 Mumtaz et
al15 suggested that parental
consanguinity is a risk factor of 1.6-fold for preterm birth at less than
33 weeks of gestation. Low birth weight has also been associated with
first-cousin relationships, but the risk increase was found to be marginal
(OR=1.36)30. Our study did not
confirm higher incidences of antepartum, peripartum, neonatal and
perinatal complications in overall consanguinity. Findings on the effect
of consanguinity on various complications are inconsistent, especially
when these complications are multifactorial in pathogenesis.5 15 27 29 30
One limitation of our study is the retrospective
nature that might have led to incompleteness of information for analysis,
especially when previous pregnancies were not in Hong Kong. Another
limitation is that some of the fetal abnormalities classified under
category C may in fact be category B disorders, as some of them recurred
in the same couples (online supplementary Appendix 1); the majority of category C disorders
did not receive genetic investigations. However, there is a high
dependence on public health service in our locality, and this facilitated
data retrieval of postnatal, infancy, and childhood outcomes of the
offspring. Different types of parental consanguinity were also included in
our analysis to provide the stratified risks according to the degree of
inbreeding. Collection of socio-economic characteristics was also
comprehensive. The same composition of ethnicity in both the
consanguineous and control groups further minimised the socio-economic
confounding effects in the analysis. Another limitation is that the
genetic data were often incomplete or not up-to-date for the studied
cases, which were recorded from 2007 to 2016.
It is recommended that a territory-wide prospective
study is conducted on consanguineous couples to further delineate their
healthcare needs in Hong Kong.
Conclusions
Identification of consanguineous couples is
essential to ensure appropriate referral for preconception or prenatal
counselling and diagnosis. Our study showed the majority of consanguineous
unions in Hong Kong are of Pakistani ethnicity. International studies have
reported that in addition to recessive genetic diseases, offspring of
consanguineous unions have higher incidences of non–genetically confirmed
structural abnormalities, developmental delay, and autism spectrum
disorders. The present study confirms this in the Hong Kong population.
Information on the increased risks associated with parental consanguinity
should be included in genetic counselling for consanguineous couples, so
that they can make informed decisions.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design of the study: All authors.
Acquisition of data: KH Siong.
Analysis or interpretation of data: KH Siong, TY Leung.
Drafting of the manuscript: KH Siong, TY Leung.
Critical revision for important intellectual content: All authors.
Acquisition of data: KH Siong.
Analysis or interpretation of data: KH Siong, TY Leung.
Drafting of the manuscript: KH Siong, TY Leung.
Critical revision for important intellectual content: All authors.
Conflicts of interest
The authors have no conflicts of interest to
disclose.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval was obtained from New Territories
West Cluster Clinical Research Ethics Committee (Ref NTWC/CREC/18012).
References
1. Bittles A. Consanguinity and its
relevance to clinical genetics. Clin Genet 2001;60:89-98. Crossref
2. Consang.net. Global prevalence of
consanguinity. Available from:
http://www.consang.net/index.php/Global_prevalence. Accessed 1 Apr 2018.
3. Posch A, Springer S, Langer M, Blaicher
W, Streubel B, Schmid M. Prenatal genetic counseling and consanguinity.
Prenat Diagn 2012;32:1133-8. Crossref
4. Becker R, Keller T, Wegner RD, et al.
Consanguinity and pregnancy outcomes in a multi-ethnic, metropolitan
European population. Prenat Diagn 2015;35:81-9. Crossref
5. Kapurubandara S, Melov S, Shalou E,
Alahakoon I. Consanguinity and associated perinatal outcomes, including
stillbirth. Aust N Z J Obstet Gynaecol 2016;56:599-604. Crossref
6. Hong Kong SAR Government. Hong Kong 2016
Population By-census. Available from:
https://www.bycensus2016.gov.hk/en/bc-articles.html. Accessed 1 Apr 2018.
7. Barrett P. A review of consanguinity in
Ireland—estimation of frequency and approaches to mitigate risks. Ir J Med
Sci 2016;185:17-28. Crossref
8. Teeuw ME, Hagelaar A, ten Kate LP,
Cornel MC, Henneman L. Challenges in the care for consanguineous couples:
an exploratory interview study among general practitioners and midwives.
BMC Fam Pract 2012;13:105. Crossref
9. Aalfs CM, Smets EM, de Haes HC, Leschot
NJ. Referral for genetic counselling during pregnancy: limited alertness
and awareness about genetic risk factors among GPs. Fam Pract
2003;20:135-41. Crossref
10. Bennett RL, Hudgins L, Smith CO,
Motulsky AG. Inconsistencies in genetic counseling and screening for
consanguineous couples and their offspring: the need for practice
guidelines. Genet Med 1999;1:286-92. Crossref
11. Joseph N, Pavan KK, Ganapathi K,
Apoorva P, Sharma P, Jhamb JA. Health awareness and consequences of
consanguineous marriages: a community-based study. J Prim Care Community
Health 2015;6:121-7. Crossref
12. Jaber L, Romano O, Halpern GJ, Livne
I, Green M, Shohat T. Awareness about problems associated with
consanguineous marriages: survey among Israeli Arab adolescents. J Adolesc
Health 2005;36:530. Crossref
13. Teeuw ME, Loukili G, Bartels EA, ten
Kate LP, Cornel MC, Henneman L. Consanguineous marriage and reproductive
risk: attitudes and understanding of ethnic groups practising
consanguinity in Western society. Eur J Hum Genet 2014;22:452-7. Crossref
14. Khalil A, Rezende J, Akolekar R,
Syngelaki A, Nicolaides KH. Maternal racial origin and adverse pregnancy
outcome: a cohort study. Ultrasound Obstet Gynecol 2013;41:278-85. Crossref
15. Mumtaz G, Nassar AH, Mahfoud Z, et al.
Consanguinity: a risk factor for preterm birth at less than 33 weeks’
gestation. Am J Epidemiol 2010;172:1424-30. Crossref
16. Islam MM. Effects of consanguineous
marriage on reproductive behaviour, adverse pregnancy outcomes and
offspring mortality in Oman. Ann Hum Biol 2013;40:243-55. Crossref
17. Hosseini-Chavoshi M, Abbasi-Shavazi
MJ, Bittles AH. Consanguineous marriage, reproductive behaviour and
postnatal mortality in contemporary Iran. Hum Hered 2014;77:16-25. Crossref
18. Bhopal RS, Petherick ES, Wright J,
Small N. Potential social, economic and general health benefits of
consanguineous marriage: results from the Born in Bradford cohort study.
Eur J Public Health 2014;24:862-9. Crossref
19. Teebi AS, El-Shanti HI. Consanguinity:
implications for practice, research, and policy. Lancet 2006;367:970-1. Crossref
20. Fareed M, Afzal M. Genetics of
consanguinity and inbreeding in health and disease. Ann Hum Biol
2017;44:99-107. Crossref
21. Committee on Genetics. Committee
Opinion No. 690: Carrier screening in the age of genomic medicine. Obstet
Gynecol 2017;129:e35-40. Crossref
22. Stoll C, Alembik Y, Roth MP, Dott B.
Parental consanguinity as a cause for increased incidence of births
defects in a study of 238,942 consecutive births. Ann Genet 1999;42:133-9.
23. Sheridan E, Wright J, Small N, et al.
Risk factors for congenital anomaly in a multiethnic birth cohort: an
analysis of the born in Bradford study. Lancet 2013;382:1350-9. Crossref
24. Nabulsi MM, Tamim H, Sabbagh M, Obeid
MY, Yunis KA, Bitar FF. Parental consanguinity and congenital heart
malformations in a developing country. Am J Med Genet A 2003;116:342-7. Crossref
25. Becker SM, Al Halees Z, Molina C,
Paterson RM. Consanguinity and congenital heart disease in Saudi Arabia.
Am J Med Genet 2001;99:8-13. Crossref
26. Yunis K, Mumtaz G, Bitar F, et al.
Consanguineous marriage and congenital heart defects: a case-control study
in the neonatal period. Am J Med Genet A 2006;140:1524-30.
27. Abbas HA, Yunis K. The effect of
consanguinity on neonatal outcomes and health. Hum Hered 2014;77:87-92. Crossref
28. Berends AL, Steegers EA, Isaacs A, et
al. Familial aggregation of preeclampsia and intrauterine growth
restriction in a genetically isolated population in The Netherlands. Eur J
Hum Genet 2008;16:1437-42. Crossref
29. Sezik M, Ozkaya O, Sezik HT, Yapar EG,
Kaya H. Does marriage between first cousins have any predictive value for
maternal and perinatal outcomes in pre-eclampsia? J Obstet Gynaecol Res
2006;32:475-81.
30. Poorolajal J, Ameri P, Soltanian A,
Bahrami M. Effect of consanguinity on low birth weight: a meta-analysis.
Arch Iran Med 2017;20:178-84.