Hong
Kong Med J 2019 Apr;25(2):94–101 | Epub 27 Mar 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Enhanced recovery after surgery for liver resection
Charing CN Chong, FHKAM (Surgery)1; WY
Chung, MSc (Nursing)1; YS Cheung, FHKAM (Surgery)1;
Andrew KY Fung, FHKAM (Surgery)1; Anthony KW Fong, FHKAM
(Surgery)1; HT Lok, FHKAM (Surgery)1; John Wong,
FHKAM (Surgery)1; KF Lee, FHKAM (Surgery)1; Simon KC
Chan, FHKAM (Anaesthesia)2; Paul BS Lai, FHKAM (Surgery)1
1 Department of Surgery, Prince of Wales
Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Anaesthesia, Prince of
Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Prof Charing CN Chong (chongcn@surgery.cuhk.edu.hk)
Abstract
Introduction: Enhanced recovery
after surgery (ERAS) reduces postoperative length of
hospital stay and patient stress response to liver surgery. The aim of
the present study was to evaluate the efficacy and feasibility of an
ERAS programme for liver resection.
Methods: A multidisciplinary
ERAS protocol was implemented for both open and laparoscopic liver
resection in a tertiary hospital in Hong Kong. The clinical outcomes of
patients who underwent liver resection and underwent the ERAS
perioperative programme were compared with those who received a
conventional perioperative programme between September 2015 and July
2016. Propensity score matching analysis was used to minimise background
differences.
Results: A total of 20 patients
who underwent liver resection were recruited to the ERAS programme.
Their clinical outcomes were compared with another 20 patients who
received hepatectomy under a conventional perioperative programme after
propensity score matching. The ERAS programme was associated with a
significantly shorter length of hospital stay (P=0.033) without an
increase in complication rates in patients who underwent open liver
resection. There was no such significant association in patients who
underwent laparoscopic liver resection. No patients required readmission
in this cohort.
Conclusions: The ERAS
perioperative programme for liver resection is safe and feasible. It
significantly shortened the hospital stay after open liver resection but
not after laparoscopic liver resection.
New knowledge added by this study
- Enhanced recovery after surgery (ERAS) for liver resection is safe and feasible in Hong Kong.
- The ERAS programme significantly shortened hospital stays after open liver resection, but not after laparoscopic liver resection.
- The ERAS programme can be safety implemented for liver resection in Hong Kong.
Introduction
Enhanced recovery after surgery (ERAS) is a
multimodal pathway developed to improve recovery after major surgery.
Since its formal introduction in the 1990s, ERAS has been adopted quickly
because of the cost efficiency derived from its reduction in length of
hospital stays, an important issue in the context of current rapidly
increasing healthcare costs and the consequent need for optimisation.1 2 Application
of ERAS integrates various medical interventions involving surgeons,
anaesthetists, physiotherapists, dieticians, and nurses.3 The benefits of ERAS have been well proven in
colectomy.4 5 6 7 Liver cancer is the fourth leading cause of cancer
death in both sexes worldwide.8
Liver resection remains the mainstay of curative treatment for liver
cancer. Liver resection is associated with a high rate of postoperative
morbidity ranging from 15% to 48%9
10 and a postoperative hospital
stay of 9 to 15 days.11 The high
rates of complications lead to prolonged hospital stay and increase costs
of hospitalisation.
An ERAS programme combines a number of elements
that aim to enhance postoperative recovery, facilitate earlier discharge,
and reduce surgical stress response.3
4 It mainly focuses on minimising
the impact of surgery on patient homeostasis.12
The reduction of postoperative physiological stress by attenuation of the
neurohormonal response to the surgical intervention not only provides the
basis for a faster recovery but also diminishes the risk of organ
dysfunction and complications.13
Programmes for ERAS consist of well-organised pathways of clinical
interventions that begin with out-patient preoperative information,
counselling, and physical optimisation; proceed to pre-, intra-, and
post-operative protocol-driven actions; and end with patient discharge
following pre-established criteria.14
The main pillars of ERAS are extensive preoperative counselling, no bowel
preparation, no sedative premedication, no preoperative fasting,
preoperative carbohydrate loading, tailored anaesthesiology, perioperative
intravenous fluid restriction, non-opioid pain management, no routine use
of drains and nasogastric tubes, early removal of the urinary catheter,
and early postoperative feeding and mobilisation.15
16 Several major studies have
suggested that ERAS is feasible and significantly reduces complications
and the length of hospital stay for patients undergoing colonic resection.4 5
6 7
17 Furthermore, ERAS has been
successfully applied to urological,18
cardiovascular,19 gynaecological,20 orthopaedic,21 and thoracic surgeries.22
However, the literature on ERAS after liver resection is limited. The aim
of the present study was to evaluate the safety and efficacy of an ERAS
programme for open or laparoscopic liver resection.
Methods
Patients
This was a prospective feasibility study carried
out in a tertiary academic hospital. The inclusion criteria recruited all
consecutive patients undergoing elective liver resection who were aged 18
to 70 years, with American Society of Anesthesiologists (ASA) grade I or
II, with no severe physical disabilities, who required no assistance with
activities of daily living, and with informed consent available. Patients
undergoing emergency surgery, who had received preoperative portal vein
embolisation, who were expected to receive concomitant procedures other
than cholecystectomy, who were mentally incapable of written consent, and
women who were pregnant were excluded.
During the same period, 42 patients who fulfilled
the same inclusion criteria underwent liver resection and a conventional
perioperative programme, as the On-Q Pain Buster system (I-Flow
Corporation, Lake Forest [CA], US) was not available for financial
reasons. None of the control patients were assigned to that group because
they refused the ERAS programme. Propensity score matching analysis was
used to minimise bias and confounding factors in patient selection, and 20
matched pairs of patients were generated for comparison.
Surgery
The same team of hepatobiliary surgeons experienced
in both laparoscopic and liver surgery performed all operations. Our open
and laparoscopic techniques have been described previously.23 In brief, open hepatectomy was performed via right
subcostal incisions with upward midline extensions and in some cases with
left subcostal extensions. In most cases, the liver was mobilised in
standard fashion before parenchymal transection, whereas in the rest, we
adopted the anterior approach or the hanging technique. Liver transection
was performed with a cavitron ultrasonic surgical aspirator (Valleylab,
Boulder [CO], US) and TissueLink (TissueLink Medical Inc, Dover [DE], US).
For laparoscopic hepatectomy, a combination of TissueLink and LigaSure
(Valleylab) were used for liver transection. The Pringle manoeuvre was not
routinely applied during liver resection. Endovascular staplers (Tyco
Healthcare, Norwalk [CT], US) were used to divide larger vascular
pedicles.
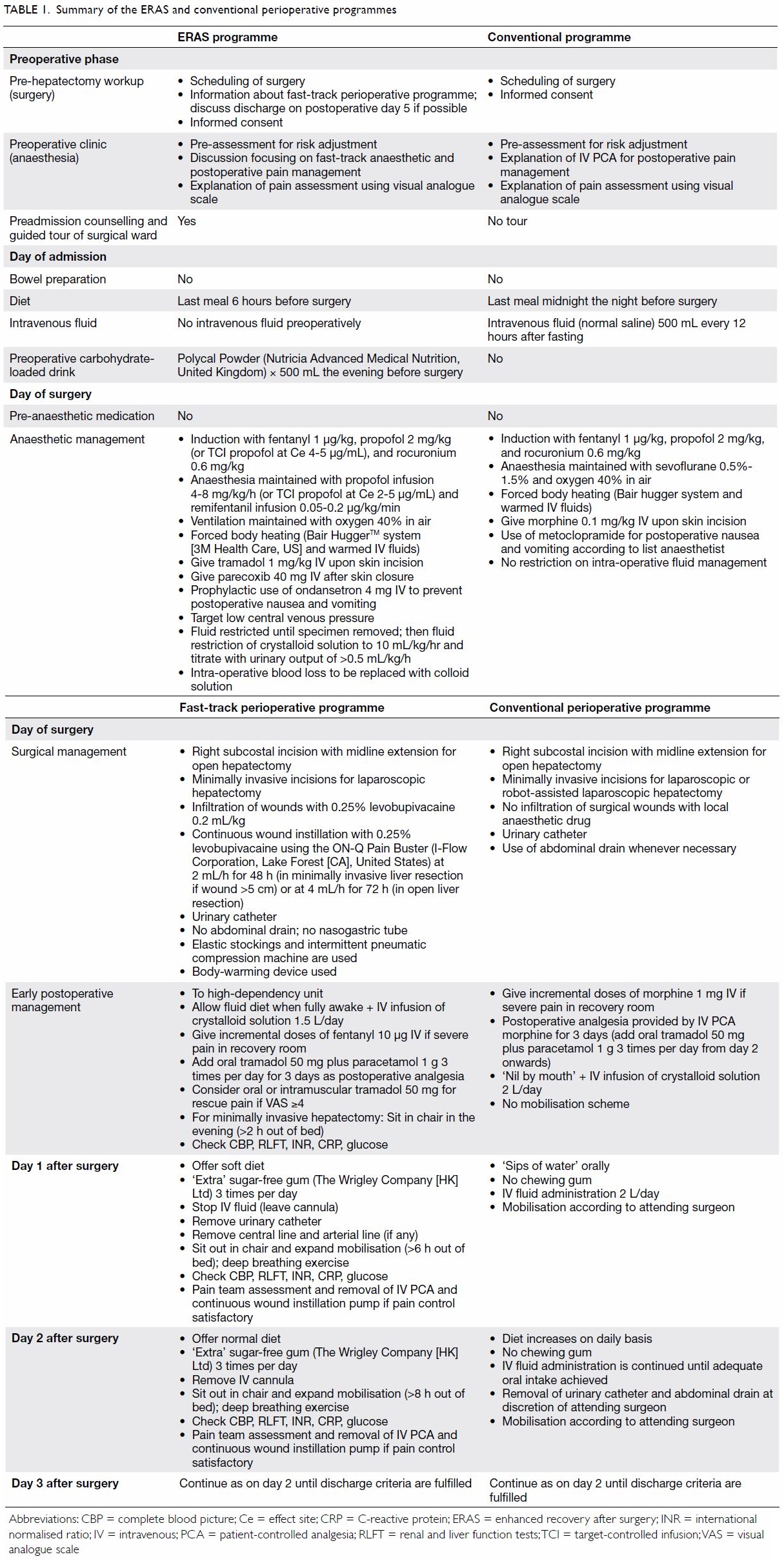
Fast-track perioperative programmes
Details of the ERAS programme and the conventional
perioperative programme are summarised in Table 1. The design of the ERAS programme was based
on consensus between our surgeons, anaesthetists, physiotherapists,
dieticians and nurses, who reviewed the relevant literature and made
appropriate adjustments to suit the local situation. Patients who were to
undergo elective hepatectomy were first screened in an out-patient clinic
or in wards for eligibility for the ERAS programme. Patients who fulfilled
the inclusion and exclusion criteria were interviewed by the principal
investigator or co-investigators for the recruitment and preoperative
counselling. A guided tour on surgical ward led by a trained nurse and an
information booklet about the preoperative guidance were given to each
patient. The booklet described the method used for respiratory
rehabilitation, daily medical events after admission, daily mobilisation
goals, and nutritional goals after the operation. The patient was seen at
a preoperative anaesthesia clinic for preoperative assessment of risk
adjustment and education about the fast-track anaesthetic and
postoperative pain management, especially during mobilisation.
All patients received a 20-mL local infiltration of
local anaesthesia (0.25% levobupivacaine) followed by continuous wound
instillation at 4 mL/h for 72 h using the On-Q Pain Buster System balloon
pump (I-Flow Corporation). Pain control was supplemented using
opioid-sparing multimodal analgesia, including oral paracetamol and
non-steroidal anti-inflammatory drugs. For minimally invasive liver
resection, continuous infiltration of the wound with local anaesthetic
agents was used and early mobilisation started on postoperative day 0. The
principal investigator held regular audit meetings with the research team
and medical/nursing staff to ensure protocol compliance.
Discharge criteria
Patients could be discharged if they fulfilled the
discharge criteria, which consisted of (1) adequate pain control with oral
analgesics, (2) absence of nausea, (3) ability to tolerate solid food, (4)
liver function on an improving trend, (5) mobilisation and self-support as
compared to the preoperative level, and (6) acceptance of discharge by the
patient.
Main outcome measures
The primary outcome of the study was total
postoperative hospital stay, including that of patients readmitted within
30 days after surgery. The secondary outcomes of the study included the
readmission rate and morbidity and mortality within 30 days.
Propensity score matching analysis
The clinical outcomes of patients who underwent
liver resection and received the ERAS programme were compared with those
who received a conventional perioperative programme in the same period.
Propensity score matching analysis was performed to control for potential
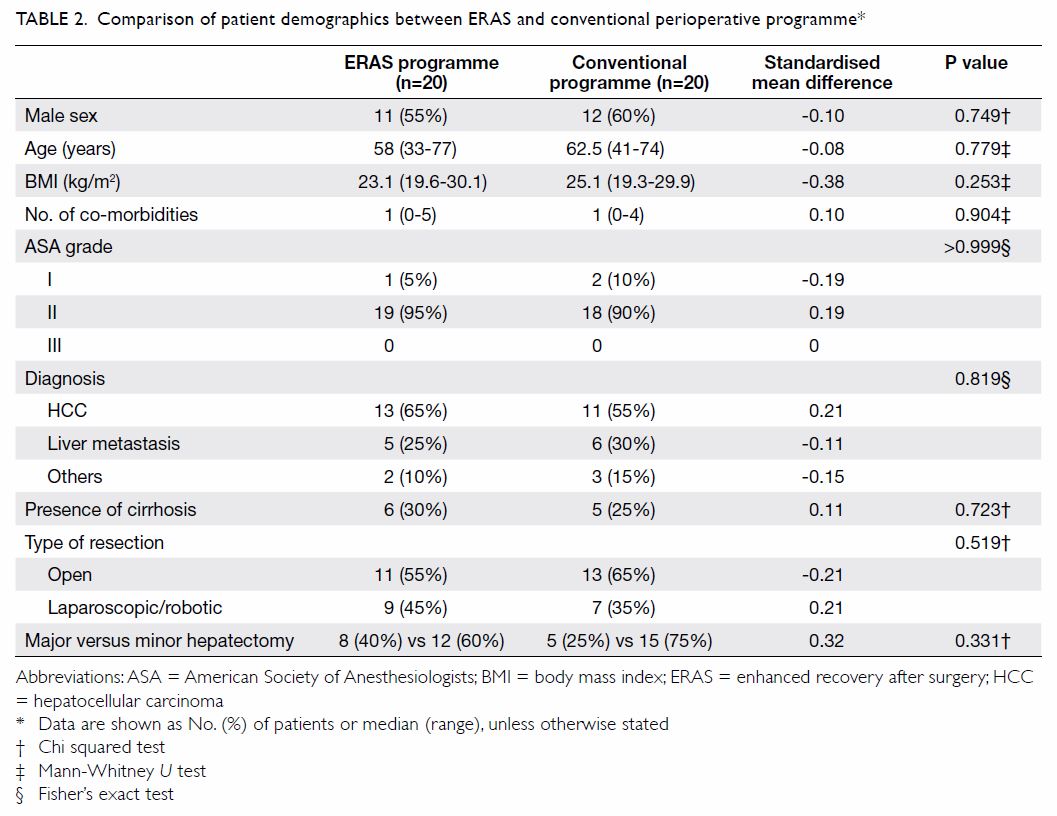
bias. Sex, age, number of co-morbidities, ASA grade, diagnosis, presence
of cirrhosis, and type of resection were chosen as our baseline covariates
to calculate each patient’s propensity score. The propensity scores were
estimated by fitting a logistic regression model with the above
covariates. The patients were then matched by their propensity scores
using one-to-one nearest neighbour matching without replacement.
Statistical analyses
Statistical analyses and propensity score matching
calculations were performed using SPSS (Windows version 20.0; IBM Corp,
Armonk [NY], US). Chi squared tests (or Fisher’s exact tests, when
appropriate) were used to compare categorical data. Mann-Whitney U
tests were used to compare continuous, non-normally distributed outcomes
between treatment groups. A two-sided P<0.05 was considered to be
statistically significant.
Results
A total of 20 patients who underwent liver
resection at Prince of Wales Hospital, Hong Kong, from September 2015 to
July 2016, were recruited into the ERAS programme. Their median age was 58
years (range, 33-77 years). The majority (n=19, 95%) of the patients were
in ASA grade II. Hepatocellular carcinoma (n=13, 65%) and colorectal liver
metastasis (n=5, 25%) were the main indications for operation. All
patients had Child-Pugh score class A. Major and minor hepatectomy were
performed in eight (40%) and 12 (60%) patients, respectively. Minimally
invasive hepatectomy (laparoscopic or robotic) were performed in nine
patients, and the remaining 11 (55%) patients received open hepatectomy.
There were no major complications as defined by the Clavien-Dindo
classification of surgical complications, and no patients required
readmission.24 25 Only two (10%) patients developed minor
complications, which were wound seroma (n=1, 5%) and urinary retention
(n=1, 5%).
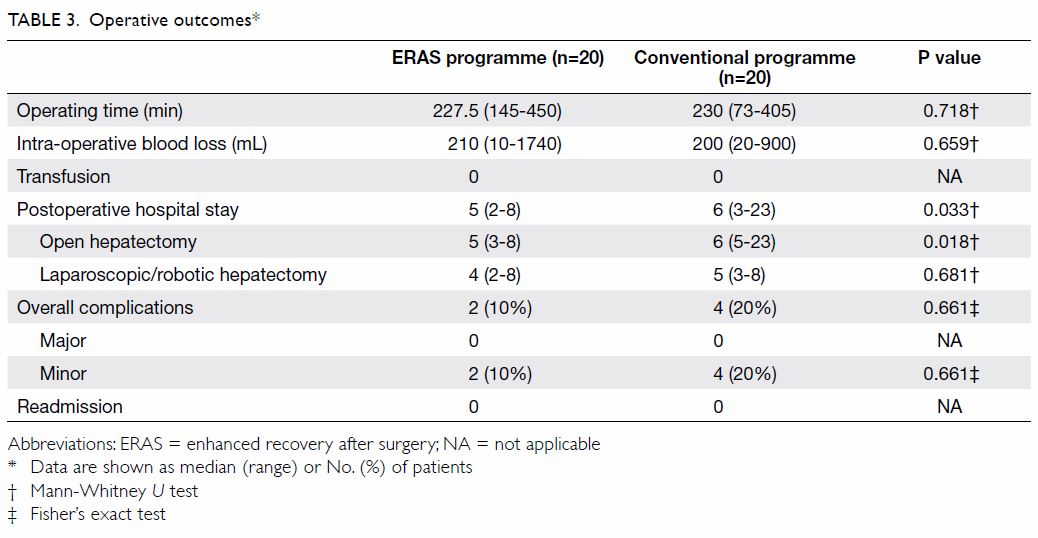
The demographics of patients in the ERAS and
conventional perioperative programme groups were comparable (Table
2). Perioperative outcomes are summarised in Table 3. There were no significant differences in
patient demographics, liver function, tumour characteristics, or surgical
techniques between the two groups. When compared with the conventional
perioperative programme, the ERAS programme was associated with a
significantly shorter postoperative hospital stay (5 vs 6 days, P=0.033).
There was no significant difference in rates of postoperative
complications or readmission.
Discussion
Results from the present study indicate that the
ERAS programme is safe and feasible in both open and laparoscopic liver
resections in Hong Kong. There was a significant reduction in the length
of postoperative hospital stay in the ERAS group.
Although ERAS programmes are not new, their
development in liver resection has been relatively slow because of the
operation’s high complexity and the high frequency of underlying liver
cirrhosis in this group of patients. Patients with liver cirrhosis who
undergo liver resection have special concerns that require special
attention. Because the ERAS principles for liver resection were adapted
from colonic surgery, more evidence is needed to prove the benefits of
ERAS in liver resection and to tailor the elements of ERAS to liver
resection.
For example, most ERAS programmes in open liver
surgery use thoracic epidural analgesia. However, patients who undergo
liver surgery experience transient coagulopathy after the operation, which
may increase the risk of spinal hematoma if epidural analgesia is used.
One previous study indicated that epidural analgesia increases the risk of
bleeding and prolongs prothrombin time after liver resection.26 Furthermore, the majority of patients with liver
cancer in our locality also had co-existing liver cirrhosis. This group of
patients is coagulopathic even before liver resection, and the risk of
bleeding complications related to the epidural analgesic is a particular
concern.27 In the present study,
we used an infusion pump for continuous infiltration of the wound with
local anaesthetic agents for pain control in patients who underwent open
liver resection. The acute pain service provided regular ward rounds to
review pain control. Other analgesics would be added if pain control was
unsatisfactory. We have previously studied the analgesic efficacy of this
infusion pump in open liver surgery and found that total morphine
consumption was reduced in patients who received continuous wound
instillation of local anaesthetic after open liver surgery. This technique
also effectively reduced pain at rest and after spirometry.28 The small size of the device can facilitate early
mobilisation during the postoperative period. Recent recommendations of
ERAS guidelines for liver surgery suggest that routine thoracic epidural
analgesia is not recommended and that a wound infusion catheter is a good
alternative.29
Restrictive use of surgical site drains after
operation is one of the key elements of most ERAS protocols to support
early mobilisation and reduce postoperative pain and discomfort.30 Recent meta-analyses did not recommend routine
abdominal drainage in elective uncomplicated hepatectomy.31 However, cirrhotic patients are at risk of developing
ascites and bleeding after liver resection. Therefore, according to the
ERAS society recommendations for perioperative care for liver surgery, the
available evidence is inconclusive, and no recommendation can be given
either for or against the use of prophylactic drainage after hepatectomy.29 Data from larger studies are
needed to evaluate the role of intra-abdominal drains in this specific
group of patients.
Nevertheless, ERAS protocols might still be
beneficial to cirrhotic patients, particularly in regard to the omission
of overnight fasting and carbohydrate loading. Cirrhotic patients have
decreased hepatic glycogen storage and impaired gluconeogenesis: an
overnight fast is equivalent to a fast of 2 to 3 days in a healthy person.
Omission of overnight fasting and carbohydrate loading may lessen the
nutritional stress for these patients.
Shorter hospital stays have been reported after
minimally invasive liver resection.23
32 33
Whether a similar decrease in hospital stay can be achieved by open
surgery with an optimised fast-track programme remains unclear. In the
current series, length of hospital stay was reduced by 1 day in both the
minimally invasive and open surgery groups. However, only the difference
in the open surgery group reached statistical significance. The major
limitation of our study is its small sample size. Therefore, it did not
have enough power to demonstrate statistical significance in small
differences. Early reports on ERAS in liver surgery have demonstrated a
significant reduction in hospital stay by 2 to 6 days.34 35 36 Some might contend that it was careful selection of
patients that resulted in the reduction of length of stay. However,
diverse groups publishing on consecutive series with ERAS principles have
shown consistent results.30 It is
highly likely that the ERAS protocol can shorten hospital stays. However,
whether it can lead to a reduction in healthcare costs will be the focus
of future studies in this field. Another limitation of this study is the
uncertainty of balance of characteristics between the two groups.
Standardised mean differences showed imbalances of some demographics (eg,
body mass index and extent of hepatectomy) between the treatment groups,
but the P values did not reach statistical significance. Again this is
caused by the small sample size, which yields a model that is not
sensitive enough to detect small differences.
Conclusion
The ERAS programme for liver resection is safe and
feasible. It resulted in a reduction in hospital stay without an increase
in morbidity and mortality. Larger-scale studies are needed to optimise
the programme’s elements and study its cost-effectiveness.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept and design of study: CCN Chong, SKC Chan,
PBS Lai.
Acquisition of data: WY Chung, YS Cheung, AKY Fung, AKW Fong, HT Lok.
Analysis or interpretation of data: CCN Chong.
Drafting of the article: CCN Chong.
Critical revision for important intellectual content: PBS Lai, SKC Chan, KF Lee, J Wong.
Acquisition of data: WY Chung, YS Cheung, AKY Fung, AKW Fong, HT Lok.
Analysis or interpretation of data: CCN Chong.
Drafting of the article: CCN Chong.
Critical revision for important intellectual content: PBS Lai, SKC Chan, KF Lee, J Wong.
Acknowledgement
We would like to thank Mr Philip Ip for his
statistical support in this project.
Conflicts of interest
As an editor of the journal, PBS Lai was not
involved in the peer review process. Other authors have no conflicts of
interest to disclose.
Declaration
The results of this project were presented in the
12th Biennial E-AHPBA Congress 2017 (23-26 May 2017, Mainz, Germany) and
in the RCSEd/CSHK Conjoint Scientific Congress 2018 (15-16 September 2018,
Hong Kong).
Funding/support
This project was supported by a Direct Grant from
the Chinese University of Hong Kong (Ref No: MD14705).
Ethics approval
The study was approved by the Joint Chinese
University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (CREC 2015.024).
References
1. Kehlet H, Wilmore DW. Evidence-based
surgical care and the evolution of fast-track surgery. Ann Surg
2008;248:189-98. Crossref
2. Basse L, Raskov HH, Hjort Jakobsen D.
Accelerated postoperative recovery programme after colonic resection
improves physical performance, pulmonary function and body composition. Br
J Surg 2002;89:446-53. Crossref
3. Kehlet H. Multimodal approach to
postoperative recovery. Curr Opin Crit Care 2009;15:355-8. Crossref
4. Lemmens L, van Zelm R, Borel Rinkes I,
van Hillegersberg R, Kerkkamp H. Clinical and organizational content of
clinical pathways for digestive surgery: a systematic review. Dig Surg
2009;26:91-9. Crossref
5. Spanjersberg WR, Reurings J, Keus F, van
Laarhoven CJ. Fast track surgery versus conventional recovery strategies
for colorectal surgery. Cochrane Database Syst Rev 2011;(2):CD007635. Crossref
6. Khoo CK, Vickery CJ, Forsyth N, Vinall
NS, Eyre-Brook IA. A prospective randomized controlled trial of multimodal
perioperative management protocol in patients undergoing elective
colorectal resection for cancer. Ann Surg 2007;245:867-72. Crossref
7. Gatt M, Anderson AD, Reddy BS,
Hayward-Sampson P, Tring IC, MacFie J. Randomized clinical trial of
multimodal optimization of surgical care in patients undergoing major
colonic resection. Br J Surg 2005;92:1354-62. Crossref
8. Bray F, Ferlay J, Soerjomataram I,
Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN
estimates of incidences and mortality worldwide for 36 cancers in 185
countries. CA Cancer J Clin 2018;68:394-424. Crossref
9. Benzoni E, Molaro R, Cedolini C, et al.
Liver resection for HCC: analysis of causes and risk factors linked to
postoperative complications. Hepatogastroenterology 2007;54:186-9.
10. Karanjia ND, Lordan JT, Fawcett WJ,
Quiney N, Worthington TR. Survival and recurrence after neo-adjuvant
chemotherapy and liver resection for colorectal metastases: a ten year
study. Eur J Surg Oncol 2009;35:838-43. Crossref
11. Jensen LS, Mortensen FV, Iversen MG,
Jørgensen A, Kirkegaard P, Kehlet H. Liver surgery in Denmark 2002-2007
[in Danish]. Ugeskr Laeger 2009;171:1365-8.
12. Kehlet H. Fast-track surgery-an update
on physiological care principles to enhance recovery. Langenbecks Arch
Surg 2011;396:585-90. Crossref
13. Varadhan KK, Lobo DN, Ljungqvist O.
Enhanced recovery after surgery: the future of improving surgical care.
Crit Care Clin 2010;26:527-47,x. Crossref
14. Muller S, Zalunardo MP, Hubner M,
Clavien PA, Demartines N; Zurich Fast Track Study Group. A fast-track
program reduces complications and length of hospital stay after open
colonic surgery. Gastroenterology 2009;136:842-7. Crossref
15. Wind J, Hofland J, Preckel B, et al.
Perioperative strategy in colonic surgery; LAparoscopy and/or FAst track
multimodal management versus standard care (LAFA trial). BMC Surg
2006;6:16. Crossref
16. Polle SW, Wind J, Fuhring JW, Hofland
J, Gouma DJ, Bemelman WA. Implementation of a fast-track perioperative
care program: what are the difficulties? Dig Surg 2007;24:441-9. Crossref
17. Kehlet H. Fast-track colorectal
surgery. Lancet 2008;371:791-3. Crossref
18. Pruthi RS, Nielsen M, Smith A, Nix J,
Schultz H, Wallen EM. Fast track program in patients undergoing radical
cystectomy: results in 362 consecutive patients. J Am Coll Surg
2010;210:93-9. Crossref
19. Barletta JF, Miedema SL, Wiseman D,
Heiser JC, McAllen KJ. Impact of dexmedetomidine on analgesic requirements
in patients after cardiac surgery in a fast-track recovery room setting.
Pharmacotherapy 2009;29:1427-32. Crossref
20. Hansen CT, Sørensen M, Møller C,
Ottesen B, Kehlet H. Effect of laxatives on gastrointestinal functional
recovery in fast-track hysterectomy: a double-blind, placebo-controlled
randomized study. Am J Obstet Gynecol 2007;196:311.e1-7. Crossref
21. Andersen LØ, Gaarn-Larsen L,
Kristensen BB, Husted H, Otte KS, Kehlet H. Subacute pain and function
after fast-track hip and knee arthroplasty. Anaesthesia 2009;64:508-13. Crossref
22. Das-Neves-Pereira JC, Bagan P,
Coimbra-Israel AP, et al. Fast-track rehabilitation for lung cancer
lobectomy: a five-year experience. Eur J Cardiothoracic Surg
2009;36:383-91. Crossref
23. Lee KF, Chong CN, Wong J, Cheung YS,
Wong J, Lai P. Long-term results of laparoscopic hepatectomy versus open
hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J
Surg 2011;35:2268-74. Crossref
24. Clavien PA, Barkun J, de Oliveira ML,
et al. The Clavien-Dindo classification of surgical complications:
five-year experience. Ann Surg 2009;250:187-96. Crossref
25. Dindo D, Demartines N, Clavien PA.
Classification of surgical complications: a new proposal with evaluation
in a cohort of 6336 patients and results of a survey. Ann Surg
2004;240:205-13. Crossref
26. Sakowska M, Docherty E, Linscott D,
Connor S. A change in practice from epidural to intrathecal morphine
analgesia for hepato-pancreato-biliary surgery. World J Surg
2009;33:1802-8. Crossref
27. Ho AM, Lee A, Karmakar MK, et al.
Hemostatic parameters after hepatectomy for cancer. Hepatogastroenterology
2007;54:1494-8.
28. Chan SK, Lai PB, Li PT, et al. The
analgesic efficacy of continuous wound instillation with ropivacaine after
open hepatic surgery. Anaesthesia 2010;65:1180-6. Crossref
29. Melloul E, Hübner M, Scott M, et al.
Guidelines for perioperative care for liver surgery: enhanced recovery
after surgery (ERAS) society recommendations. World J Surg
2016;40:2425-40. Crossref
30. Ljungqvist O, Scott M, Fearon KC.
Enhanced recovery after surgery: a review. JAMA Surg 2017;152:292-8. Crossref
31. Gavriilidis P, Hidalgo E, de’Angelis
N, Lodge P, Azoulay D. Re-appraisal of prophylactic drainage in
uncomplicated liver resections: a systematic review and meta-analysis. HPB
(Oxford) 2017;19:16-20. Crossref
32. Nguyen KT, Laurent A, Dagher I, et al.
Minimally invasive liver resection for metastatic colorectal cancer: a
multi-institutional, international report of safety, feasibility, and
early outcomes. Ann Surg 2009;250:842-8. Crossref
33. Kazaryan AM, Pavlik Marangos I,
Rosseland AR, et al. Laparoscopic liver resection for malignant and benign
lesions: ten-year Norwegian single-center experience. Arch Surg
2010;145:34-40. Crossref
34. Lee A, Chiu CH, Cho MW, et al. Factors
associated with failure of enhanced recovery protocol in patients
undergoing major hepatobiliary and pancreatic surgery: a retrospective
cohort study. BMJ Open 2014;4:e005330. Crossref
35. van Dam RM, Hendry PO, Coolsen MM, et
al. Initial experience with a multimodal enhanced recovery programme in
patients undergoing liver resection. Br J Surg 2008;95:969-75. Crossref
36. Stoot JH, van Dam RM, Busch OR, et al.
The effect of a multimodal fast-track programme on outcomes in
laparoscopic liver surgery: a multicentre pilot study. HPB (Oxford)
2009;11:140-4. Crossref