© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
COMMENTARY
Transcatheter arterial embolisation can be the
standard treatment for non-variceal upper gastrointestinal bleeding
refractory to endoscopy
HF Chan, FHKAM (Radiology)1; KW Lai,
FHKAM (Surgery)2; Alfred WT Yung, FHKAM (Radiology)1;
WH Luk, FHKAM (Radiology)1; LF Cheng, FHKAM (Radiology)1;
Johnny KF Ma, FHKAM (Radiology)1
1 Department of Radiology, Princess
Margaret Hospital, Laichikok, Hong Kong
2 Department of Surgery, Princess
Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr HF Chan (chf178@ha.org.hk)
Introduction
Endoscopic treatment is the standard first-line
management for non-variceal upper gastrointestinal (GI) bleeding.
Recurrent bleeding occurs in about 10% to 15% of patients after the first
endoscopy and is associated with significant mortality.1 Surgery has hitherto been the traditional treatment for
non-variceal upper GI bleeding refractory to endoscopy. In recent years,
transcatheter arterial embolisation (TAE) has been shown to be equally
effective in control of bleeding and associated with fewer complications.2 3
4 5
6 Moreover, TAE avoids the risks
associated with general anaesthesia. In our centre, TAE is the standard
next step for endoscopy-refractory non-variceal upper GI bleeding. We
believe that this practice can also be adopted in other centres where
interventional radiology expertise and facilities are available.
Exemplar case
We experienced an 89-year-old woman with multiple
medical co-morbidities including hypertension, mitral valve regurgitation,
congestive heart failure, diabetes mellitus, and chronic obstructive
airway disorder. The patient was admitted to Princess Margaret Hospital on
29 March 2017 with massive haematemesis. On admission her blood pressure
was 105/60 mm Hg, heart rate 100 beats per minute, and oxygen saturation
97% in room air. Pallor was noted on physical examination and per rectal
exam was empty. Blood results revealed a haemoglobin level 8.1 g/dL,
platelet count 225 × 109/L, international normalised ratio 1.0, creatinine
level 144 μmol/L, and urea level 17 mmol/L. First
oesophagogastroduodenoscopy (OGD) arranged on the same day immediately
following admission revealed a 3-cm roll-edged ulcer (Forrest IIa) at the
fundus of the greater curvature. Biopsy was taken. Haemostasis was
achieved by endoscopic application of one haemoclip and injection of 4 mL
adrenaline 1:10000. An intravenous proton pump inhibitor was started but
the patient developed a further massive haematemesis a few hours later.
Repeat OGD revealed a large adherent clot over the same ulcer. After
removing the clot, spurting was noted adjacent to the haemoclip (Fig
1). Endoscopic interventions included adrenaline injection (totally
13 mL 1:10000 adrenaline) and application of haemoclips and haemospray.
Despite these measures the patient became haemodynamically unstable during
the procedure and required urgent intubation by an anaesthetist for airway
protection. The patient also required large-volume blood product
transfusion and high-dose inotrope.
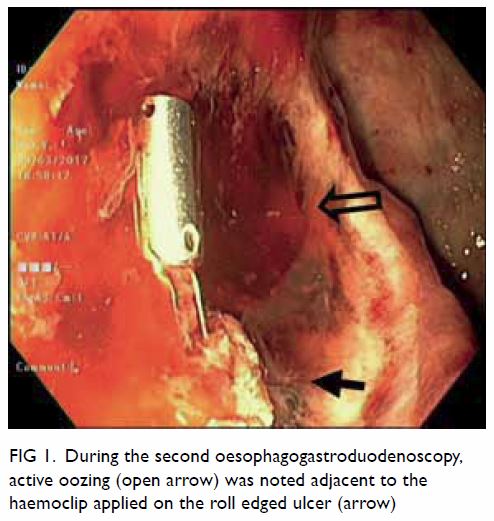
Figure 1. During the second oesophagogastroduodenoscopy, active oozing (open arrow) was noted adjacent to the haemoclip applied on the roll edged ulcer (arrow)
The intervention radiologist was consulted urgently
and the patient directly transferred from the endoscopy centre to the
interventional radiology suite about 1 hour after completion of the second
OGD. Arterial access was obtained at the right common femoral artery.
Celiac artery angiogram revealed a pseudoaneurysm at the mid splenic
artery, corresponding to the site of haemoclip on digital subtraction
angiogram image. Selective angiogram of the splenic artery confirmed the
finding of pseudoaneurysm and active extravasation from the pseudoaneurysm
was demonstrated. Exclusion of the pseudoaneurysm was achieved using a
sandwich embolisation technique at distal, and across and proximal to the
neck of the pseudoaneurysm, blocking the efferent (back door) and afferent
splenic artery (front door). Seven fibered platinum coils were used.
Post-embolisation angiogram confirmed the pseudoaneurysm had been
obliterated. Normal blood flow to the spleen was also obliterated and the
spleen was perfused by collaterals from the pancreas (Fig
2). The total procedure time for angiogram and embolisation was
about 45 minutes. The patient’s haemodynamic status and haemoglobin level
rapidly stabilised after the procedure. During resuscitation, the patient
received a total of 1 L gelofusine, 7 units of packed cells, 4 units of
fresh frozen plasma, and 4 units of platelet concentrate.
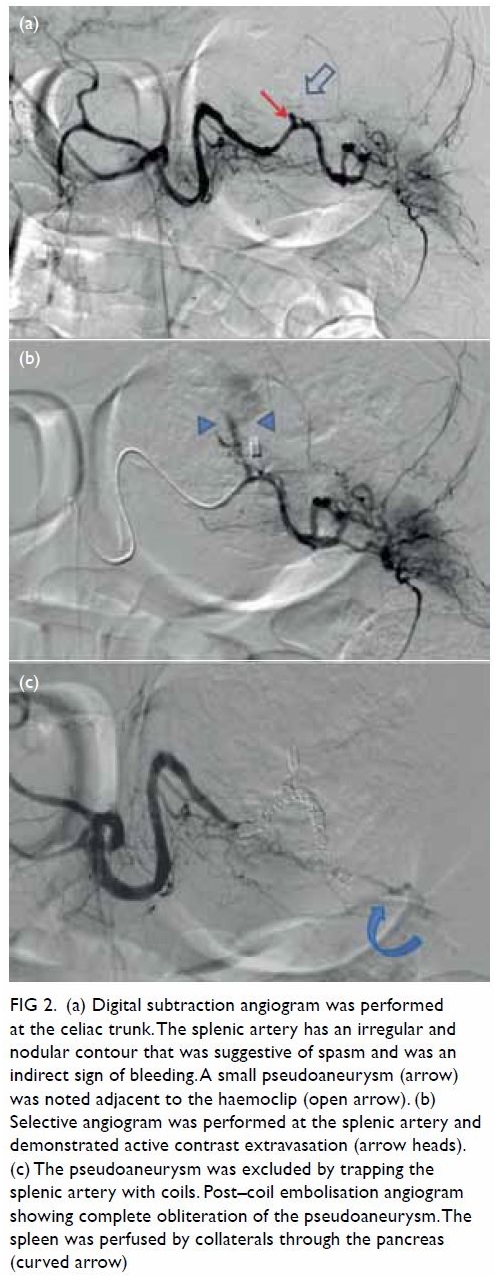
Figure 2. (a) Digital subtraction angiogram was performed at the celiac trunk. The splenic artery has an irregular and nodular contour that was suggestive of spasm and was an indirect sign of bleeding. A small pseudoaneurysm (arrow) was noted adjacent to the haemoclip (open arrow). (b) Selective angiogram was performed at the splenic artery and demonstrated active contrast extravasation (arrow heads). (c) The pseudoaneurysm was excluded by trapping the splenic artery with coils. Post–coil embolisation angiogram showing complete obliteration of the pseudoaneurysm. The spleen was perfused by collaterals through the pancreas (curved arrow)
The patient was extubated the next day. Her
condition continued to improve, and the patient was discharged to a
convalescence hospital 2 weeks later. She did not present with any signs
or symptoms of splenic infarct. The pathology report of the gastric ulcer
confirmed it as benign. To date, the patient has not presented with any
signs or symptoms of upper GI bleeding or significant haemoglobin drop.
Follow-up OGD was not arranged in view of the patient’s advanced age and
because it would be unlikely to influence subsequent management. The
splenic artery pseudoaneurysm appeared to be caused by a benign gastric
ulcer at the greater curvature eroding into the splenic artery. Fewer than
10 such cases have been reported in the English literature. The successful
management of this case of rare massive upper GI bleeding highlights the
importance of collaboration between endoscopist, anaesthetist, and
interventional radiologist. Timely involvement of an interventional
radiologist after failed endoscopic haemostasis not only allows rapid
diagnosis of the underlying aetiology but also allows embolisation to stop
bleeding at the same time.
Transcatheter arterial embolisation versus surgery
To date, there has been no prospective controlled
trial to compare TAE with surgery in non-variceal upper GI bleeding
refractory to endoscopic treatment. Nevertheless the efficacy of TAE in
terms of technical success (62%-100%) and clinical success (44%-99%) has
been demonstrated in several case series.7
8 9
10 11
12 More importantly, a number of
retrospective studies comparing the two strategies favour the use of TAE.2 3
4 5
6 They show that TAE is equally
effective in bleeding control, has a low complication rate and does not
increase mortality, even though patients in the TAE group tended to have a
higher surgical risk.2 3 4 5 6
Embolisation technique
The source of bleeding is often first identified at
endoscopy, guiding subsequent angiography, and TAE. The principle of TAE
is to selectively obstruct or reduce blood flow to the bleeding point and
minimise ischaemia in the rest of the GI tract. Generally speaking, the
risk of ischaemia in upper GI bleeding is small because of the rich
collateral circulation in the stomach and duodenum.13 The rich collateral arterial network in the upper GI
tract nonetheless also causes treatment failure as blood flow to the
bleeding point may persist through retrograde flow from collaterals.13 This problem can be overcome by superselective
embolisation of the bleeder branch using a small microcatheter.12 When contrast extravasation or pseudoaneurysm is
detected in a large vessel such as the gastroduodenal artery or left
gastric artery, the artery can be trapped by coils placed across the point
of extravasation or pseudoaneurysm, so as to close the “front door” and
“back door”.13 Recently,
impressive results have been reported with superselective embolisation
using N-butyl cyanoacrylate glue.12
13 N-butyl cyanoacrylate
embolisation has a shorter procedure time and occludes the target vessel
independent of the patient’s coagulation system. It is particularly useful
in patients who are haemodynamically unstable or coagulopathic.
Practical considerations
Successful performance of TAE relies on centre
expertise. The published studies of TAE were performed in high-volume
centres with a specialist interventional radiology service. The results
might not be reproducible in smaller centres. Another consideration is the
availability of an interventional radiologist and angiography suite
out-of-hours. Longer time to angiography was found to predict early
rebleeding and every effort should be made to perform TAE early, even
outside office hours. Lack of access to a 24-hour interventional radiology
service in smaller centres is a major barrier to the routine use of TAE.
Conclusion
Transcatheter arterial embolisation is an effective
and safe treatment and compares favourably with surgery in the management
of endoscopy-refractory non-variceal upper GI bleeding. In centres where
expertise and facilities are available, TAE can be considered the routine
next step management when endoscopy fails to control bleeding.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept and design: All authors.
Acquisition of data: HF Chan.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision for important intellectual content: All authors.
Acquisition of data: HF Chan.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision for important intellectual content: All authors.
Conflicts of interest
The authors have no conflicts of interest to
disclose.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the
Declaration of Helsinki. The patient provided informed consent for all
procedures.
References
1. Jairath V, Kahan BC, Logan RF, et al.
National audit of the use of surgery and radiological embolization after
failed endoscopic haemostasis for non-variceal upper gastrointestinal
bleeding. Br J Surg 2012;99:1672-80. Crossref
2. Ang D, Teo EK, Tan A, et al. A
comparison of surgery versus transcatheter angiographic embolization in
the treatment of nonvariceal upper gastrointestinal bleeding uncontrolled
by endoscopy. Eur J Gastroenterol Hepatol 2012;24:929-38. Crossref
3. Wong TC, Wong KT, Chiu PW, et al. A
comparison of angiographic embolization with surgery after failed
endoscopic hemostasis to bleeding peptic ulcers. Gastrointest Endosc
2011;73:900-8. Crossref
4. Venclauskas L, Bratlie SO, Zachrisson K,
Maleckas A, Pundzius J, Jönson C. Is transcatheter arterial embolization a
safer alternative than surgery when endoscopic therapy fails in bleeding
duodenal ulcer? Scand J Gastroenterol 2010;45:299-304. Crossref
5. Ripoll C, Bañares R, Beceiro I, et al.
Comparison of transcatheter arterial embolization and surgery for
treatment of bleeding peptic ulcer after endoscopic treatment failure. J
Vasc Interv Radiol 2004;15:447-50. Crossref
6. Eriksson LG, Ljungdahl M, Sundbom M,
Nyman R. Transcatheter arterial embolization versus surgery in the
treatment of upper gastrointestinal bleeding after therapeutic endoscopy
failure. J Vasc Interv Radiol 2008;19:1413-8. Crossref
7. Loffroy R, Guiu B, D’Athis P, et al.
Arterial embolotherapy for endoscopically unmanageable acute
gastroduodenal hemorrhage: predictors of early rebleeding. Clin
Gastroenterol Hepatol 2009;7:515-23. Crossref
8. Aina R, Oliva VL, Therasse E, et al.
Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome
assessment. J Vasc Interv Radiol 2001;12:195-200. Crossref
9. Toyoda H, Nakano S, Takeda I, et al.
Transcatheter arterial embolization for massive bleeding from duodenal
ulcers not controlled by endoscopic hemostasis. Endoscopy 1995;27:304-7. Crossref
10. De Wispelaere JF, De Ronde T, Trigaux
JP, de Canniére L, De Geeter T. Duodenal ulcer hemorrhage treated by
embolization: results in 28 patients. Acta Gastroenterol Belg
2002;65:6-11.
11. Dempsey DT, Burke DR, Reilly RS,
McLean GK, Rosato EF. Angiography in poor-risk patients with massive
nonvariceal upper gastrointestinal bleeding. Am J Surg 1990;159:282-6. Crossref
12. Hur S, Jae HJ, Lee H, Lee M, Kim HC,
Chung JW. Superselective embolization for arterial upper gastrointestinal
bleeding using N-butyl cyanoacrylate: a single-center experience in 152
patients. J Vasc Interv Radiol 2017;28:1673-80. Crossref
13. Loffroy R, Favelier S, Pottecher P, et
al. Transcatheter arterial embolization for acute nonvariceal upper
gastrointestinal bleeding: Indications, techniques and outcomes. Diagn
Interv Imaging 2015;96:731-44. Crossref

