© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Hashimoto’s encephalopathy with partial
response to steroid therapy: a case report
Bahar Kaymakamzade, MD1; Senem Ertugrul
Mut, MD1; Amber Eker, MD1; Hanife Özkayalar, MD2
1 Department of Neurology, Near East
University Faculty of Medicine, Nicosia, Cyprus
2 Department of Pathology, Near East
University Faculty of Medicine, Nicosia, Cyprus
Corresponding author: Dr Senem Ertugrul Mut (senemertugrul@yahoo.com)
Case report
Hashimoto’s encephalopathy (HE), also termed as
steroid responsive encephalopathy associated with autoimmune thyroiditis,
is a rare and highly variable clinical spectrum. The clinical presentation
includes seizures, stroke-like episodes, cognitive decline,
neuropsychiatric symptoms, and myoclonus.1
We report a rare and unusual case of HE in which there was a partial
response to steroid therapy.
A 76-year-old man was admitted to the Department of
Neurology of the Near East University Hospital, Cyprus, in October 2016
with the chief complaint of myoclonic jerks and walking difficulty for the
past 6 weeks. The patient’s family had observed no significant cognitive
or behavioural change. He did not experience any seizure. His medical
history included hypertension and diabetes. On neurological examination he
was alert and fully oriented. Motor weakness was noted at the left lower
extremity with Babinski sign. He had myoclonus in all limbs and bilateral
postural tremor, which was predominantly left sided. Magnetic resonance
imaging (MRI) scan of the brain revealed widespread T2 hyperintensities
mainly in the juxtacortical areas (Fig 1). Blood studies including complete blood count
and electrolyte count; liver, renal and thyroid function tests; tumour
markers; and paraneoplastic antibody analysis (anti-Hu, -Yo, -Ri, -Ma,
-CV2) were all normal. Vasculitis markers including antinuclear
antibodies, anti-ds DNA, anticardiolipin immunoglobulin (Ig) M, IgG
antibodies, antiphosphatidylserine IgM, IgG antibodies, perinuclear
antineutrophil cytoplasmic antibodies, cytoplasmic antineutrophil
cytoplasmic antibodies, anti-La and anti-Ro antibodies, and rheumatoid
factor were within normal limits. He tested negative for human
immunodeficiency virus. His cognitive status worsened during his first
week of hospitalisation and he rapidly developed delusions and aggressive
behaviour. He was not able to cooperate with the neurocognitive
assessment. Electroencephalogram showed 5 to 6 Hz diffuse slow-wave
activity without any epileptiform discharge (Fig 2). Cerebrospinal fluid (CSF) analysis showed a
very high protein content (1.95 g/L). The CSF/serum glucose ratio was
normal. Cerebrospinal fluid investigation and culture was negative,
excluding central nervous system infection. Whole-body positron emission
tomography was performed to exclude paraneoplastic processes and the
result was normal. Consequently, steroid-responsive encephalopathy and
associated autoimmune thyroiditis was suspected, and antithyroglobulin
antibody (anti-TG-Ab) and antithyroperoxidase antibody (anti-TPO-Ab)
levels were studied. Serum level of both was increased (431 IU/mL and 40
IU/mL respectively). He was not taking any medication (eg, lithium,
amiodarone, etc) that could account for the positivity of thyroid
antibodies. Thyroid ultrasonography did not show any pathological
findings. Intravenous pulse steroid treatment (IVPS, methylprednisolone 1
g/day) was started. Myoclonus resolved on the fourth day of treatment.
Because the cognitive status of the patient was not adequately changed,
IVPS was extended to 10 days. A partial response was obtained in cognition
and Mini-Mental State Examination score was 11/30 after IVPS. Another
electroencephalogram showed mild improvement. Consequently, oral
methylprednisolone was continued at a dosage of 1 mg/kg/day. Afterwards,
intravenous Ig treatment was given at a dose of 0.4 g/kg for 5 days. No
additional improvement was seen. Lumbar puncture and thyroid autoantibody
testing were repeated. The anti-TPO-Ab and anti-TG-Ab levels were
normalised, and CSF was acellular at that time and protein content
decreased (1.45 g/L). Azathioprine 100 mg/day was gradually added to his
treatment. The patient was discharged from the hospital with oral steroid
and azathioprine treatment. His neurological status was stable. He died 3
months later due to a lung infection.
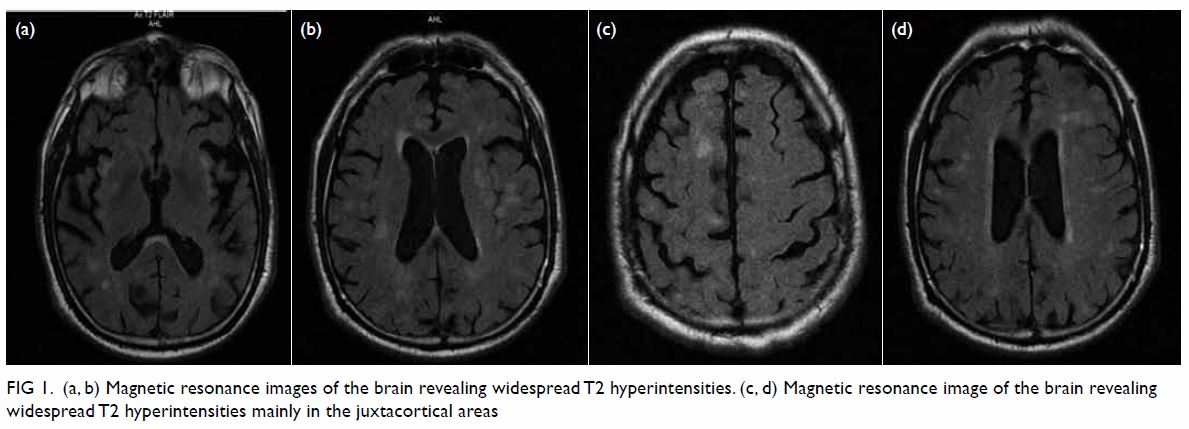
Figure 1. (a, b) Magnetic resonance images of the brain revealing widespread T2 hyperintensities. (c, d) Magnetic resonance image of the brain revealing widespread T2 hyperintensities mainly in the juxtacortical areas
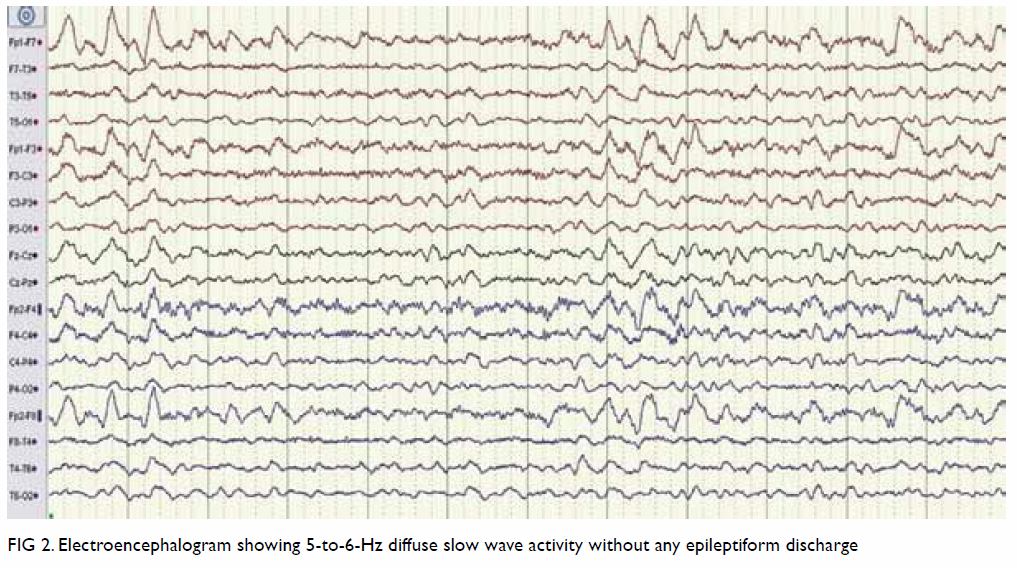
Figure 2. Electroencephalogram showing 5-to-6-Hz diffuse slow wave activity without any epileptiform discharge
Discussion
The clinical findings, CSF analysis, and MRI of our
patient were compatible with HE. The challenging conditions we faced were
the lack of prominent cognitive or psychiatric change at the beginning and
normal thyroid functions. The differential diagnosis included vasculitis,
paraneoplastic limbic encephalitis, and Creutzfeldt-Jakob disease (CJD).
The MRI was very helpful for differential diagnosis. The pattern of
isolated cortical hyperintensity with concomitant combined cortical and
deep grey matter (basal ganglia) hyperintensity on fluid attenuation
inversion recovery along with restricted diffusion can differentiate CJD
from other rapidly progressive dementias with a high sensitivity and
specificity.2 Because the
consecutive diffusion-weighted images of the patient were not compatible
with CJD, 14-3-3 assay in the CSF was not studied. In addition, it is not
specific for CJD and its positivity is also reported in HE.3 The laboratory and imaging findings were not compatible
with limbic encephalitis. We assessed his objective clinical recovery
(dramatic disappearance of myoclonus and partial cognitive-behavioural
improvement) following pulse steroid therapy. Response to treatment may
also exclude the diagnosis of CJD.
The pathophysiology of HE is not well understood.
Autoimmune cerebral vasculitis and antibody-mediated neuronal reaction are
the most accepted mechanisms. Most patients are euthyroid at the time of
diagnosis.4 Antithyroperoxidase
antibody is known as a positive predictor of responsiveness to steroid
therapy and higher titres are associated with a more favourable outcome.5 Most cases in the literature
treated with steroids make a complete recovery.5
Since it is a rare condition, the optimum treatment for steroid-resistant
cases is unknown. Response to intravenous Ig or plasmapheresis treatments
in steroid non-responsive cases has also been reported.5 Despite the normalisation of anti-TPO-Ab and anti-TG-Ab
levels and somewhat improved inflammatory findings of CSF after treatment,
we observed a complete response in myoclonus and only a partial
improvement in cognition in our patient. This supports the hypothesis that
thyroid autoantibodies are not the only pathogenic mechanism in HE. Other
causes, the role of the thyroid gland, and other antibodies should be
clarified by future studies.
Author contributions
Concept or design: B Kaymakamzade, S Ertugrul Mut.
Acquisition of data: H Özkayalar, A Eker, S Ertugrul Mut.
Analysis or interpretation of data: B Kaymakamzade.
Drafting of the manuscript: All authors.
Critical revision for important intellectual content: S Ertugrul Mut, B Kaymakamzade.
Acquisition of data: H Özkayalar, A Eker, S Ertugrul Mut.
Analysis or interpretation of data: B Kaymakamzade.
Drafting of the manuscript: All authors.
Critical revision for important intellectual content: S Ertugrul Mut, B Kaymakamzade.
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Acknowledgements
We thank Dr Mustafa Canatan and Dr Fehim Türktan
for their contribution to the editing of the manuscript.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was conducted in accordance with the
principles outlined in the Declaration of Helsinki. The patient provided
written informed consent.
References
1. Mocellin R, Walterfang M, Velakoulis D.
Hashimoto’s encephalopathy: epidemiology, pathogenesis and management. CNS
Drugs 2007;21:799-811. Crossref
2. Vitali P, Maccagnano E, Caverzasi E, et
al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from
other rapid dementias. Neurology 2011;76:1711-9. Crossref
3. Hernández Echebarría LE, Saiz A, et al.
Detection of 14-3-3 protein in the CSF of a patient with Hashimoto’s
encephalopathy. Neurology 2000;54:1539-40. Crossref
4. Oide T, Tokuda T, Yazaki M, et al.
Anti-neuronal autoantibody in Hashimoto’s encephalopathy:
neuropathological, immunohistochemical, and biochemical analysis of two
patients. J Neurol Sci 2004;217:7-12. Crossref
5. Litmeier S, Prüss H, Witsch E, Witsch J.
Initial serum thyroid peroxidase antibodies and long-term outcomes in
SREAT. Acta Neurol Scand 2016;134:452-7. Crossref

