© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Rosai-Dorfman disease presenting as a solitary
soft-tissue mass in the thigh: a case report
Alice KY Au, FHKCR, FHKAM (Radiology)1;
HM Cheng, FHKCR, FHKAM (Radiology)1; KY Cho, FHKCR, FHKAM
(Radiology)1; CW Tam, FHKCR, FHKAM (Radiology)1;
Jennifer LS Khoo, FHKCR, FHKAM (Radiology)1; Joshua HY Ng, MB,
BS2; Vincent TW Hau, MB, ChB, FHKAM (Orthopaedic Surgery)3
1 Department of Radiology, Pamela Youde
Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 Department of Clinical Pathology,
Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
3 Department of Orthopaedics and
Traumatology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong
Kong
Corresponding author: Dr Alice KY Au (augar520@gmail.com)
Case report
A 46-year-old man presented to the Department of
Orthopaedics and Traumatology, Pamela Youde Nethersole Eastern Hospital in
February 2004 with a 3-month history of self-detected left thigh mass. It
was of spontaneous onset with no history of trauma, associated pain,
weakness, or numbness. The patient had full range of movement and no
lymphadenopathy was noted. Magnetic resonance imaging (MRI) [Fig
1] revealed a large area of infiltrative soft-tissue thickening at
the medial aspect of the left distal thigh and involved the subcutaneous
layer. The lesion measured 8.4 × 3.4 × 11.2 cm (anteroposterior ×
transverse × longitudinal) and was characterised by T1-weighted (T1W)
hypointense to isointense and T2-weighted (T2W) fat-suppressed
hyperintense signals with internal heterogeneity. Internal foci of
hypointensity in the T2W fat-suppressed images were noted. An internal
reticular pattern of septal thickening was also found. There was
enhancement after gadolinium contrast administration. The margin of the
lesion was well delineated from the underlying vastus medialis and
sartorius muscles with no features of muscular invasion or destruction.
The knee joint was unremarkable and bone marrow signal was normal. The
neurovascular bundle was also intact. Overall features were non-specific
for either inflammatory or neoplastic pathology.
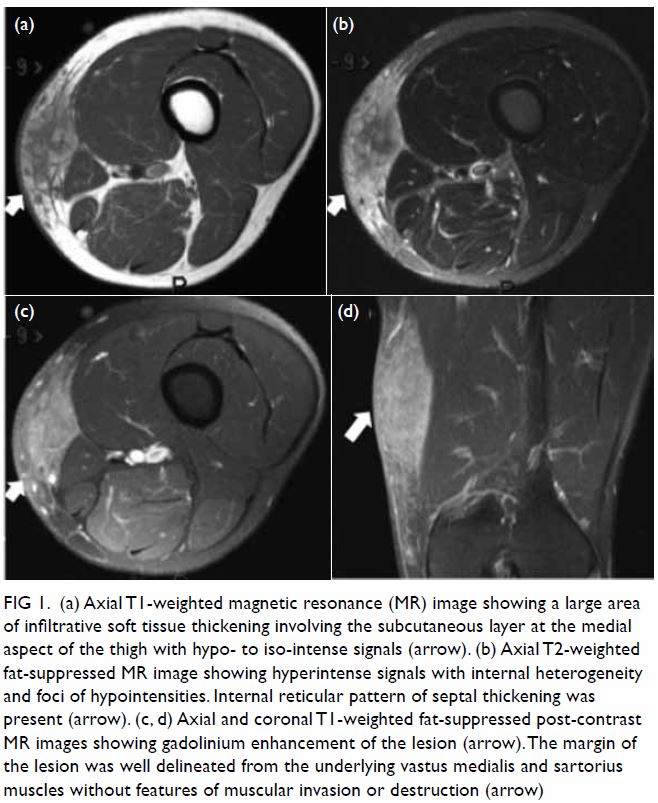
Figure 1. (a) Axial T1-weighted magnetic resonance (MR) image showing a large area of infiltrative soft tissue thickening involving the subcutaneous layer at the medial aspect of the thigh with hypo- to iso-intense signals (arrow). (b) Axial T2-weighted fat-suppressed MR image showing hyperintense signals with internal heterogeneity and foci of hypointensities. Internal reticular pattern of septal thickening was present (arrow). (c, d) Axial and coronal T1-weighted fat-suppressed post-contrast MR images showing gadolinium enhancement of the lesion (arrow). The margin of the lesion was well delineated from the underlying vastus medialis and sartorius muscles without features of muscular invasion or destruction (arrow)
Microscopic examination of an incisional biopsy
over the left vastus medialis with a wedge of skin and subcutaneous tissue
revealed infiltrate in the subcutis and to a lesser extent the deep
dermis. The infiltrate consisted of lymphocytes and a low number of plasma
cells. Immunohistochemical stains showed mainly T-cells and some B-cells.
Occasional areas with aggregates of paler histiocytic cells were present
and suggested granuloma formation. Stains for acid-fast bacilli and fungus
were negative. The paler histiocytic cells were S100-positive and showed
lymphophagocytosis (Fig 2). Molecular study by polymerase chain reaction
showed no clonal T-cell proliferation. The overall features were
suggestive of Rosai-Dorfman disease (RDD).
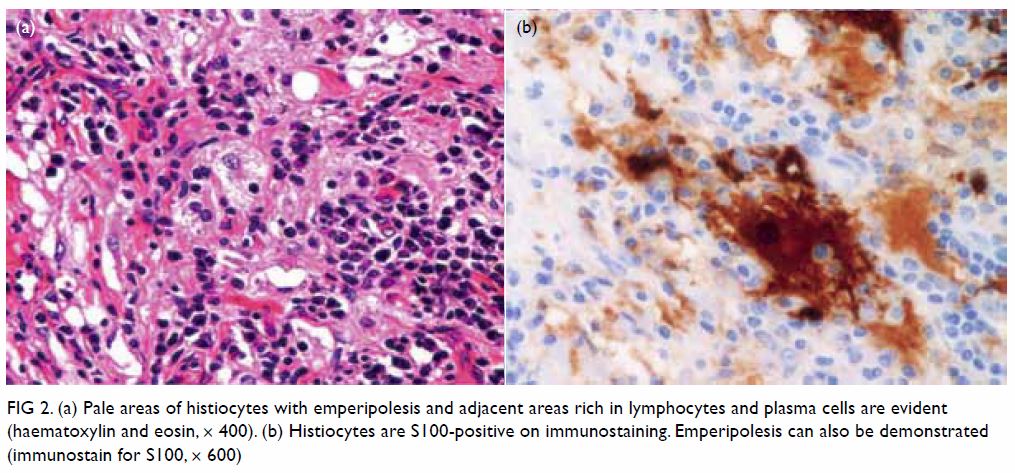
Figure 2. (a) Pale areas of histiocytes with emperipolesis and adjacent areas rich in lymphocytes and plasma cells are evident (haematoxylin and eosin, × 400). (b) Histiocytes are S100-positive on immunostaining. Emperipolesis can also be demonstrated (immunostain for S100, × 600)
Radical excision of the lesion was performed
subsequently and included the epimysium of the gracilis, sartorius and
aponeurosis of the vastus medialis. The excision margin in the radial
excision of the lesion was 2 cm. Microscopic examination revealed that the
mass in the subcutis was composed of nodules or aggregates of
lymphohistiocytic cells separated by areas of fibrosis. The cellular
aggregates were composed of dark areas with plasma cells and lymphocytes
and pale areas with clusters of histiocytes. The histiocytes showed round
vesicular nuclei, distinct nucleoli, and abundant foamy cytoplasm with
presence of emperipolesis (phagocytosis of plasma cells and lymphocytes).
The histiocytes showed positive immunostaining for S100. Special stains
for acid-fast bacilli and fungus were again negative. The overall features
were consistent with RDD. The resection margins were unremarkable. The
patient recovered well postoperatively.
Eight years after the operation, the patient
detected a nodular swelling over the inferior margin of the surgical site.
Serial MRI showed a static nodular T1W hypointense, and T2W isointense to
mildly hyperintense soft-tissue lesion with contrast enhancement,
measuring approximately 8 mm in diameter (Fig 3). Features could represent postoperative
change or tumour recurrence. The patient was otherwise asymptomatic and he
opted for follow-up scans to monitor the lesion instead of surgical
excision.
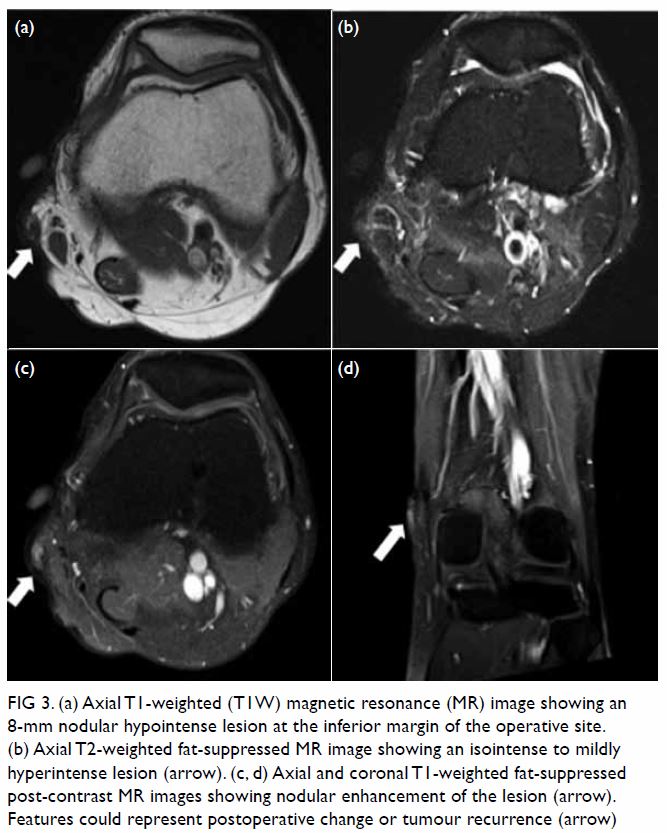
Figure 3. (a) Axial T1-weighted (T1W) magnetic resonance (MR) image showing an 8-mm nodular hypointense lesion at the inferior margin of the operative site. (b) Axial T2-weighted fat-suppressed MR image showing an isointense to mildly hyperintense lesion (arrow). (c, d) Axial and coronal T1-weighted fat-suppressed post-contrast MR images showing nodular enhancement of the lesion (arrow). Features could represent postoperative change or tumour recurrence (arrow)
Discussion
Rosai-Dorfman disease is also known as sinus
histiocytosis with massive lymphadenopathy and was first described by
Rosai and Dorfman in 1969.1 It is a
rare non-malignant histiocytic proliferative disorder. Although the
disease may develop at any age, it is more common in young adults with a
mean age of onset of 20 years and a slight male predominance (1.4:1).2 3
The aetiology of RDD is unknown, although previous
studies have attempted to relate RDD to infectious agents including
Epstein-Barr virus, human herpesvirus 6, herpes simplex virus, Brucella,
Klebsiella rhinoscleromatis, and Nocardia.4 The disease involves a wide distribution and can affect
a multitude of organ systems, including nodal involvement and extranodal
involvement. The majority of patients present with painless massive
cervical lymphadenopathy. Most patients have a complete and spontaneous
remission, but some may experience recurrent or persistent albeit stable
lymphadenopathy. In rare cases, the disease may follow an aggressive
course and be fatal.
Pure cutaneous RDD is a distinct clinical entity
that has an older age of onset (median 43.5 years) and a male-to-female
ratio of 1:2.3 In contrast to
systemic RDD that is commonly seen in blacks and rarely reported in
Asians, most patients with purely cutaneous RDD are Asians or whites. The
lesion remains localised to the skin even after long-term follow-up.5
Histologically, RDD is characterised by sheets of
large pale histiocytes with large, round, vesicular nuclei. Phagocytosis
of lymphoid cells or neutrophils by histiocytes may be found
(“emperipolesis”). Immunohistochemical stains are useful when diagnosing
RDD and the most consistent and reliable phenotype for RDD is S100
positive and CD1a negative.
Relative to the wide disease spectrum, there are
variable radiographic features. Although no specific imaging
characteristics allow differentiation of lymphadenopathy in RDD from the
myriad other disease processes, massive painless bilateral cervical lymph
node enlargement, particularly when it occurs in children and adolescents,
should prompt consideration of RDD as a differential diagnosis. Nodal
involvement may be evidenced as lymphadenopathy. In computed tomography
scan of the sinuses and brain, polypoid masses, mucosal thickening,
soft-tissue lesion of the paranasal sinuses or nasal cavity with or
without associated osseous erosion can be seen. Features of brain
involvement include a hyperattenuating meningeal-based mass showing
contrast enhancement or parenchymal oedema surrounding the lesion. In MRI
of the sinuses and brain, sinus lesions may also demonstrate hypointensity
on T2W images. Meningeal-based mass lesions may demonstrate T1W
isointensity to grey matter, T2W hyperintensity to grey matter and
homogeneous contrast enhancement.6
Gallium scanning may show increased uptake and increased metabolism with
fluorodeoxyglucose positron emission tomography. The differential
diagnosis is broad and includes infectious (granulomatous) disease,
Wegener’s granulomatosis, other histiocytosis, Hodgkin’s and non-Hodgkin’s
lymphoma, and fibroinflammatory lesions. In general, RDD does not show
bone or soft-tissue destruction as in cases of Wegener’s granulomatosis
and T-cell lymphoma.
Rosai-Dorfman disease usually follows a benign and
self-limiting course with treatment largely targeted at controlling local
manifestations. Surgical options may be warranted for symptomatic control.
Author contributions
All authors contributed to the concept or design,
acquisition of data, analysis or interpretation of data, drafting of the
manuscript, and critical revision for important intellectual content. All
authors had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was conducted in accordance with the
Declaration of Helsinki. The patient provided written informed consent.
References
1. Rosai J, Dorfman RF. Sinus histiocytosis
with massive lymphadenopathy. A newly recognized benign clinical
pathologic entity. Arch Pathol 1969;87:63-70.
2. Annessi G, Giannetti A. Purely cutaneous
Rosai-Dorfman disease. Br J Dermatol 1996;134:749-53. Crossref
3. Brenn T, Calonje E, Granter SR, et al.
Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J
Dermatopathol 2002;24:385-91. Crossref
4. Lu CI, Kuo TT, Wong WR, Hong HS.
Clinical and histopathologic spectrum of cutaneous Rosai-Dorfman disease
in Taiwan. J Am Acad Dermatol 2004;51:931-9. Crossref
5. Farooq U, Chacon A, Vincek V, Elgart GW.
Purely cutaneous Rosai-Dorfman disease with immunohistochemistry. Indian J
Dermatol 2013;58:447-50. Crossref
6. Symss NP, Cugati G, Vasudevan MC,
Ramamurthi R, Pande A. Intracranial Rosai Dorfman disease: report of three
cases and literature review. Asian J Neurosurg 2010;5:19-30.

