© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Consensus statements on diagnosis and management
of chronic idiopathic constipation in adults in Hong Kong
Justin CY Wu, MB, ChB, MD1; Annie OO
Chan, MB, ChB, PhD2; TK Cheung, MB, BS, PhD3;
Ambrose CP Kwan, MB, BS3; Vincent KS Leung, MB, BS4;
WC Sze, MB, BS, GradDFM3; Victoria PY Tan, MB, BS5
1 Department of Medicine and
Therapeutics, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Gastroenterology and
Hepatology, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
3 Private Practice, Hong Kong
4 Department of Gastroenterology and
Hepatology, Hong Kong Baptist Hospital, Kowloon Tong, Hong Kong
5 Department of Medicine, The University
of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Prof Justin CY Wu (justinwu@cuhk.edu.hk)
Abstract
Objective: The estimated
prevalence of chronic idiopathic constipation in Hong Kong is 14%. An
expert panel of local gastroenterologists has created a set of consensus
statements with the aim of providing localised guidance on the diagnosis
and management of this common condition by primary care physicians.
Participants: An expert panel
consisting of seven local gastroenterologists convened in August 2018 in
Hong Kong.
Evidence: Published primary
research articles, meta-analyses, and guidelines and consensus
statements issued by different regional and international societies on
the diagnosis and management of chronic idiopathic constipation were
reviewed.
Consensus process: Draft
consensus statements were prepared prior to the meeting. The consensus
statements were finalised during the meeting with contributions from the
panel members based on their collective knowledge and clinical
experience.
Conclusions: A total of 11
consensus statements were created, including five concerning patient
assessment and diagnosis, two relating to non-pharmacological
management, and four on pharmacological management. These consensus
statements are intended to provide guidance to local general
practitioners and primary care physicians on managing patients with
chronic constipation in daily clinical practice.
Introduction
Chronic idiopathic constipation (CIC), also known
as functional constipation, is a common gastrointestinal disorder with an
estimated prevalence of 14% in the Hong Kong general population.1 Constipation is often perceived as a decrease in
frequency of bowel movements, but normal bowel function can range anywhere
from 3 times daily to 3 times weekly, and fewer than 50% of people
experience the conventional norm of once-daily bowel motion.2 In addition to infrequent bowel movements, patients
with chronic constipation also complain of straining, hard stools,
abdominal discomfort, and feelings of incomplete evacuation.3 In the primary care setting, the Rome IV criteria may
guide diagnosis of CIC, along with simple laboratory tests and physical
examination to rule out secondary causes.4
5
Management of CIC typically begins with
non-pharmacological measures including exercise and increased dietary
fibre and fluid intake. When these general measures have failed to relieve
constipation, therapies, such as bulking agents, osmotic laxatives,
stimulant laxatives, and stool softeners are used to promote regular bowel
movements. Newer pharmacological therapies include prokinetic agents (5-HT4
agonists) that promote gut motility and prosecretory agents (guanylate
cyclase C agonists) that increase intestinal secretion. In Hong Kong,
patients also seek Chinese herbal medicine and acupuncture for relief of
constipation.
Current management practices for CIC in Hong Kong
largely follow Western guidelines and principles. Given that there are
cultural differences among patients and physicians regarding symptom
perception, treatment practices, and goals, there is a need for localised
CIC management guidelines. Hence, these consensus statements were
developed with the aim of providing guidance to local primary care
physicians on diagnosis and management of CIC.
Methods
An expert panel consisting of seven local
gastroenterologists convened on 9 August 2018 in Hong Kong. Published
primary research articles, meta-analyses, and guidelines and consensus
statements issued by different regional and international societies on CIC
diagnosis and management were reviewed, and draft consensus statements
were prepared prior to the meeting. These statements were divided into
three sections: patient assessment and diagnosis, non-pharmacological
management, and pharmacological management. The consensus statements were
finalised during the meeting with contributions from the panel members
based on their collective knowledge and clinical experience.
Results
Patient assessment and diagnosis
Statement 1: Primary care doctors are the major care
providers for diagnosis and management of chronic idiopathic constipation
The Rome IV diagnostic criteria for CIC can be too
complex and impractical for daily use in the primary care setting.
Adopting a pragmatic approach that combines these symptom criteria with
other elements, such as a visual guide (Bristol Stool Scale) and quality
of life impairment, may enhance diagnosis of constipation and evaluation
of its severity. Higher vigilance about the patient’s regular use of
natural products or health supplements to promote bowel function is also
important, as it is often overlooked.
Patients with symptoms that are refractory to
second-line treatment should be referred for specialist assessment.
Failure to fulfil diagnostic criteria should not preclude referral to a
gastroenterologist, especially in patients with complications or
bothersome symptoms that affect their quality of life. Patients with
alarming features, such as anaemia, recent onset of symptoms after 50
years of their absence, rectal bleeding, significant weight loss, abnormal
physical examination, and family history of colon cancer should also be
referred for further assessment.4 5 6
Psychiatric co-morbidities are not uncommon in patients with chronic
constipation, and any feature of significant psychiatric co-morbidities
should prompt referral to a psychiatrist.7
Statement 2: Routine extensive diagnostic and
physiological testing is not recommended for chronic constipation
Thorough history taking pertaining to age,
symptomology, acuteness of symptoms, and medication history is important
to exclude various causes of constipation.5
8 Preliminary laboratory
investigations consisting of complete blood count, serum calcium, glucose
levels, and thyroid function tests are generally adequate to screen for
underlying metabolic or other organic pathology.5
8 9
Careful abdominal and digital rectal examinations are also important in
the primary care setting. A rectal exam can reveal rectal tumours,
haemorrhoids, impacted faeces, anal sphincter tone, presence of mucus, and
stool colour.5 8 9 Abdominal
X-ray is a simple, non-invasive investigation that may show faecal
impaction. These investigations have to be individualised according to
patient expectations and preferences.
Advanced diagnostic procedures, such as barium
enema, defaecography, colonic transit studies, magnetic resonance imaging,
manometry, and balloon expulsion test should be reserved for patients with
suspected slow transit constipation and defaecatory disorder in the
specialist care setting.
Statement 3: Differential diagnoses of chronic
idiopathic constipation include secondary causes of constipation, such as
medications, electrolyte imbalances, structural abnormalities and
metabolic (eg, hypothyroidism, hypercalcaemia, diabetes mellitus) or
pathological (eg, Parkinson’s disease, multiple sclerosis) disorders.
Alarming features suggestive of a serious gastrointestinal disorder should
prompt referral to a gastroenterologist or surgeon
Constipation is a common adverse reaction to many
medications (notably opioids, diuretics, antidepressants, antihistamines,
antispasmodics, anticonvulsants, aluminium antacids, and iron
supplements), and constipation may necessitate their discontinuation, if
appropriate.8 10 Intake of herbal supplements and traditional Chinese
medicines should also be considered as potential causes.
Any recent onset of constipation with alarming
symptoms should prompt the need for exclusion of colorectal cancer.
Although there is no evidence for an association between chronic
constipation and increased colorectal cancer risk, colon cancer screening
may be warranted in individuals >50 years with recent onset
constipation and/or other alarming features.5
11
Statement 4: The revised Rome IV criteria are useful
for diagnosing chronic idiopathic constipation but can be cumbersome to
use in clinical practice
The Rome IV criteria define CIC as the presence of
two or more of the following5:
Loose stools should rarely be present without the
use of laxatives, and there should be insufficient criteria for irritable
bowel syndrome. These criteria need to be fulfilled for the last 3 months
with symptom onset at least 6 months prior to diagnosis.
It is important to recognise that there is a
significant overlap between CIC and constipation-predominant irritable
bowel syndrome. Both conditions exist on a continuous spectrum, but the
latter is distinguished by the presence of abdominal pain.5 Patients with CIC may periodically encounter symptoms
of constipation related to irritable bowel syndrome.
The long duration required after symptom onset is a
major limitation of the Rome IV criteria. Instead, cases with recurrent
presentation to the clinic with consistent symptoms should be considered
for diagnosis of CIC. The chronic constipation diagnostic tool proposed by
the Asian Neurogastroenterology and Motility Association, which requires a
duration of only 3 months after symptom onset for diagnosis, has been
demonstrated as a useful alternative to the Rome III criteria in Asian
patients.12
The Bristol Stool Scale is a useful guide for
facilitating communication with patients, although it may not include the
subset of patients who present with difficulty passing stool but do not
have type 1 or 2 stool consistency. Patients should also be encouraged to
keep a stool diary and record their bowel habits to facilitate accurate
diagnosis.4 10
Statement 5: Chronic constipation can be classified as
normal-transit, slow-transit, or defaecatory disorder
This pathophysiological classification categorises
constipation according to colonic transit time and additional functional
or anatomical disruption that leads to obstructed defaecation. It is made
with the aid of advanced tests performed by gastroenterologists but should
not significantly affect first-line management in the primary care
setting. These pathophysiological mechanisms may co-exist and contribute
to treatment refractoriness in some patients. In each of these categories,
there may be underlying secondary causes that need careful evaluation. For
example, slow transit constipation can be a gastrointestinal manifestation
commonly seen in patients with Parkinson’s disease. Slow transit
constipation is strongly associated with irritable bowel syndrome.
Anorectal structural pathology, such as rectocele, may contribute to
defaecatory disorder.
Rectal hyposensitivity has been proposed as a
mechanism associated with constipation, but it is generally believed to be
a consequence of CIC, resulting from chronic rectal distension, rather
than a cause.13
Non-pharmacological management
Statement 6: Dietary and lifestyle adjustments,
including a high-fibre diet, adequate hydration, and physical activity,
should be made before starting pharmacological treatment. Patients with
pelvic floor dysfunction should be referred for physiotherapy
In recent years, the Hong Kong Chinese diet has
become increasingly low in dietary fibre. The dietary fibre intake of the
Hong Kong Chinese population is estimated to be only 10 to 12 g/day—barely
half of that seen in Western societies (about 20 g/day).14 Although some patients may benefit from a fibre-rich
diet, there are currently no data showing that increasing dietary fibre
will help to relieve constipation.8
15 Furthermore, excessive fibre
intake may actually worsen symptoms in severely constipated patients.15 Particularly, elderly patients and those with
inadequate fluid intake or on diuretics may be at risk.8 Hence, a high-fibre diet should be accompanied by
adequate hydration to avoid symptoms of faecal impaction. Consumption of
fruits high in soluble fibre, including papayas and kiwis, can be
recommended to patients.
Dysbiosis has been associated with constipation in
some studies. However, there is insufficient evidence to recommend
probiotics as an effective remedy for CIC.8
10 16
Developing good toilet habits can be beneficial.
Patients should be encouraged to schedule routine bathroom time
(postprandial, when urge may be higher) and use simple manoeuvres, such as
elevating the feet with a footstool.5
Prolonged sitting (>10 minutes) on the toilet is not recommended.
Statement 7: Data on the use of traditional Chinese
medicine in the management of chronic idiopathic constipation are
conflicting
A 16-week randomised double-blind clinical trial
conducted in Hong Kong on 291 patients with CIC showed that the hemp
seed-containing Chinese herbal formula, MaZiRenWan, was significantly more
effective than placebo at increasing the number of complete spontaneous
bowel movements (CSBMs; P<0.005 at week 8 and week 16) but not more
effective than the stimulant laxative senna (P=0.14 at week 8).17 18 19 Patients should be asked about their use of Chinese
medicine, as is commonly used for constipation. It may substantiate the
effectiveness of the treatment or contribute to adverse effects.
Pharmacological management
Statement 8: Pharmacological management should be
considered if lifestyle and dietary measures do not provide adequate
relief of chronic idiopathic constipation. First-line pharmacological
treatments recommended in primary care include bulking agents, osmotic
laxatives, and stool softeners. Combination therapy with agents across
different classes/mechanisms can be considered before moving to
second-line therapy
Commonly used bulk-forming agents include soluble
fibre, such as psyllium, methylcellulose, and polycarbophil and insoluble
fibre, such as wheat bran. A meta-analysis of three randomised controlled
trials involving 293 patients with CIC showed that soluble fibre
supplementation increases stool frequency (relative risk [RR]=0.25; 95%
confidence interval [CI]=0.16-0.37).16
Long-term use of bulking agents, especially insoluble fibre, is
discouraged because of their propensity to cause bloating and discomfort.16 Caution should be exercised when
giving bulk-forming agents to patients already on a high-fibre diet, as
this may worsen faecal impaction.
Electrolyte-free polyethylene glycol (PEG) and
lactulose are commonly recommended osmotic laxatives in CIC. A
meta-analysis of 10 randomised controlled trials concluded that PEG was
superior to lactulose in outcomes of stool frequency, consistency, and
relief of abdominal pain.19 In
general, PEG is also better tolerated (less likely to cause bloating and
gas) than lactulose, which may result in better compliance by patients.6 Given the favourable efficacy and
safety profile, long-term use of PEG is acceptable as a first-line
treatment for CIC.8 The use of
phosphate solution is strongly discouraged because of the high potential
for serious complications, including phosphate nephropathy and electrolyte
imbalances, especially in elderly people.5
20 21
The panel does not recommend the stool softener docusate as a primary
treatment for CIC given that it was shown to be inferior to psyllium at
improving stool frequency in a randomised controlled trial involving 170
patients with CIC.22
Patients require adequate counselling on regular,
consistent use of medications, regardless of clinical status after dose
titration, to prevent the development of severe refractory symptoms.
Treatment goals should be realistic. Various
outcome measures and definitions of treatment response have been reported
in clinical trials on constipation. In general, achieving one additional
spontaneous bowel movement per week from baseline is recommended as an
indicator of initial positive response. Patients should be counselled that
the normal bowel frequency is a minimum of 3 times per week rather than
once daily. In addition to achieving the desired bowel frequency, a
successful treatment response should also encompass improvement in quality
of life and prevention of complications.
Treatment failure can be considered after 2 months
(alongside non-pharmacological measures) before proceeding to second-line
therapy.6 This period is also
dependent on patient preference.
Statement 9: Linaclotide can be considered as
second-line treatment for chronic idiopathic constipation. Diarrhoea may
be an adverse effect in some patients, and patients should be educated
about this possibility before initiating therapy. Careful vigilance for
severe diarrhoea is recommended before long-term regular use
Second-line pharmacotherapeutic options for CIC
include prokinetic agents (prucalopride) and prosecretory agents
(lubiprostone, linaclotide, plecanatide).6
23 Both linaclotide and
lubiprostone have shown high-quality evidence of efficacy and safety for
treatment of CIC.16 Currently,
linaclotide (290 μg) is the only agent in this category that is registered
for use in Hong Kong. Because the general recommended daily dose of
linaclotide for CIC is 145 μg, once-every-2-days dosing of the 290-μg
capsule is recommended to minimise diarrhoea and improve tolerability.5 6
Four randomised clinical trials involving 651
patients demonstrated that lubiprostone was superior to placebo for
treatment of CIC (RR=0.67; 95% CI=0.58-0.77).16
Diarrhoea and nausea occurred significantly more frequently with
lubiprostone.16
The efficacy and safety of linaclotide were
evaluated in two 12-week randomised double-blind placebo-controlled
dual-dose (145 μg and 290 μg) trials involving 1276 patients with chronic
constipation. In both trials, a significantly higher proportion of
patients receiving linaclotide achieved the primary endpoint (≥3 CSBMs per
week and an increase of ≥1 CSBMs from baseline during at least 9 of the 12
weeks) compared with those receiving placebo (P<0.01 for all
comparisons).24 In addition,
linaclotide significantly improved stool frequency and consistency,
reduced straining, and reduced abdominal symptoms (bloating and
discomfort). A meta-analysis that combined these two trials and a previous
phase II dose-ranging trial conducted in 310 patients with CIC reported
that 79% of those receiving linaclotide failed to respond to therapy, as
compared with 94.9% of placebo-treated patients (RR=0.84; 95%
CI=0.80-0.87), and diarrhoea was more common with linaclotide treatment
(RR=3.08; 95% CI=1.27-7.48).25 In
a subsequent phase IIIb trial conducted in 483 patients with chronic
constipation and significant abdominal bloating, linaclotide significantly
improved bowel symptoms and bloating compared with placebo.26
Two large randomised placebo-controlled trials
(n=2731) have recently demonstrated the efficacy of plecanatide (3-mg and
6-mg doses) at improving CIC.27 28 Both trials reported a significant
improvement in the proportion of durable CSBM responders and a small
incidence of diarrhoea.27 28
Because prosecretory agents induce active secretion
of electrolytes and fluids into the intestinal lumen, monitoring baseline
renal function is advisable in selected patients who are at risk of
dehydration or renal dysfunction.23
The Food and Drug Administration has classified linaclotide as a pregnancy
category C drug.29
Statement 10: Stimulant laxatives should be regarded as
rescue therapy for chronic idiopathic constipation, not as first-line
agents, and used only on an as-needed basis (less than daily). Regular
chronic use of stimulant laxatives is discouraged. Long-term use of
glycerine suppositories and/or water enemas is acceptable
Stimulant laxatives increase intestinal motility
and intestinal secretion. Commonly used stimulant laxatives include
Agiolax, senna (Senokot), and bisacodyl (Dulcolax). Frequent use of these
agents may lead to long-term dependency or abuse, and therefore, general
practitioners or specialists should counsel patients on limiting their
use.30 The concern that stimulant
laxatives may cause permanent damage to the autonomic nervous system of
the colon has not been proven.15
Statement 11: Surgery should only be used as a last
resort for slow-transit constipation or to treat identified disorders that
require surgical correction. Exclusion of defaecatory disorder and whole
gut slow transit problems is important
Defaecatory disorder commonly coexists with slow-
or normal-transit constipation.31
This is a common cause that contributes to poor laxative treatment
response. These patients must be properly assessed by gastroenterologists
with expertise in management of functional bowel disorder before being
recommended for surgery.
Surgery is generally not effective for management
of refractory CIC and is associated with significant morbidity.10 Rare conditions like megacolon may be an indication
for surgery. The proposed management algorithm for CIC is summarised in
the Figure.
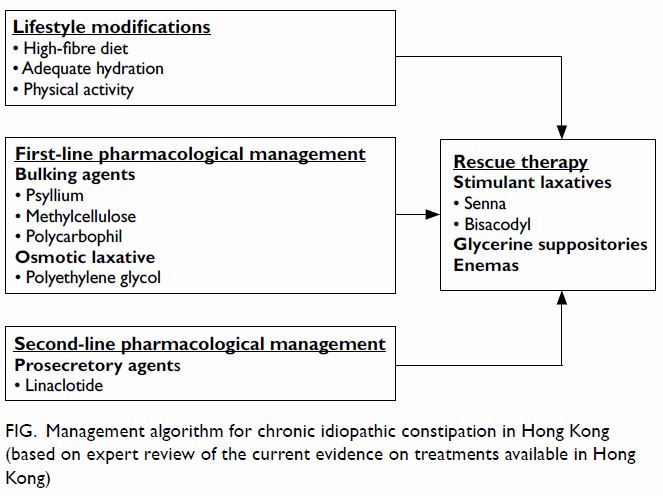
Figure. Management algorithm for chronic idiopathic constipation in Hong Kong (based on expert review of the current evidence on treatments available in Hong Kong)
Conclusions
Chronic constipation is a common gastrointestinal
disorder in patients presenting to primary care providers. These consensus
statements give a general overview of diagnostic and treatment approaches
to CIC appropriate for primary care physicians in Hong Kong.
Diagnosis of CIC is made using the revised Rome IV
criteria, along with simple laboratory tests and physical examinations,
which are important for excluding secondary causes, such as medications,
electrolyte imbalances, structural abnormalities, and metabolic or
pathological disorders.5 8 Patients presenting with alarming features should be
referred to gastroenterologists or surgeons for appropriate further
assessments.
Dietary and lifestyle adjustments should be
attempted first before initiating pharmacological treatments for CIC.
Soluble fibre supplementation may improve stool frequency, but excessive
use of bulking agents (especially insoluble fibre) may lead to bloating
and faecal impaction. Among osmotic agents, PEG is more effective and
better tolerated than lactulose.6 19
Linaclotide may be considered as second-line
therapy for patients who have failed fibre and osmotic laxatives.6 It is the only prosecretory agent currently registered
for use in Hong Kong. Linaclotide has been demonstrated to generate
significant improvements of constipation symptoms in CIC compared with
placebo, but diarrhoea is a significant concern, especially with the
higher dose that is normally used (290 μg). Lubiprostone, linaclotide, and
plecanatide are all superior to placebo for treatment of CIC, but no
head-to-head trials comparing these medications have been conducted thus
far.
Author contributions
All authors contributed to the concept, acquisition
of data, interpretation of data, drafting of the manuscript, and critical
revision for important intellectual content. All authors had full access
to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
English language editing and writing support,
funded by an unrestricted educational grant from AstraZeneca Hong Kong
Limited, was provided by Cassandra Thomson and Shirani Kanaganayagam of
MIMS (Hong Kong) Limited.
References
1. Cheng C, Chan AO, Hui WM, Lam SK. Coping
strategies, illness perception, anxiety and depression of patients with
idiopathic constipation: a population-based study. Aliment Pharmacol Ther
2003;18:319-26. Crossref
2. Heaton KW, Radvan J, Cripps H, Mountford
RA, Braddon FE, Hughes AO. Defecation frequency and timing, and stool form
in the general population: a prospective study. Gut 1992;33:818-24. Crossref
3. Johanson JF, Kralstein J. Chronic
constipation: a survey of the patient perspective. Aliment Pharmacol Ther
2007;25:599-608. Crossref
4. Rao SS, Meduri K. What is necessary to
diagnose constipation. Best Pract Res Clin Gastroenterol 2011;25:127-40. Crossref
5. Lacy BE, Mearin F, Chang L, et al. Bowel
disorders. Gastroenterology 2016;150:1393-407. Crossref
6. Tse Y, Armstrong D, Andrews CN, et al.
Treatment algorithm for chronic idiopathic constipation and
constipation-predominant irritable bowel syndrome derived from a Canadian
national survey and needs assessment on choices of therapeutic agents. Can
J Gastroenterol Hepatol 2017;2017:8612189. Crossref
7. Nehra V, Bruce BK, Rath-Harvey DM,
Pemberton JH, Camilleri M. Psychological disorders in patients with
evacuation disorders and constipation in a tertiary practice. Am J
Gastroenterol 2000;95:1755-8.
8. Shin JE, Jung HK, Lee TH, et al.
Guidelines for the diagnosis and treatment of chronic functional
constipation in Korea, 2015 revised edition. J Neurogastroenterol Motil
2016;22:383-411. Crossref
9. Lembo A, Camilleri M. Chronic
constipation. N Engl J Med 2003;349:1360-8. Crossref
10. Sobrado CW, Neto IJ, Pinto RA, Sobrado
LF, Nahas SC, Cecconello I. Diagnosis and treatment of constipation: a
clinical update based on the Rome IV criteria. J Coloproctol
2018;38:137-44. Crossref
11. Power AM, Talley NJ, Ford AC.
Association between constipation and colorectal cancer: systematic review
and meta-analysis of observational studies. Am J Gastroenterol
2013;108:894-903.
12. Gwee KA, Bergmans P, Kim J, et al.
Assessment of the Asian Neurogastroenterology and Motility Association
chronic constipation criteria: An Asian multicenter cross-sectional study.
J Neurogastroenterol Motil 2017;23:262-72. Crossref
13. Whitehead WE, Bharucha AE. Diagnosis
and treatment of pelvic floor disorders: what’s new and what to do.
Gastroenterology 2010;138:1231-5. Crossref
14. Zhang R, Wang Z, Fei Y, et al. The
difference in nutrient intakes between Chinese and Mediterranean, Japanese
and American diets. Nutrients 2015;7:4661-88. Crossref
15. Müller-Lissner SA, Kamm MA,
Scarpignato C, Wald A. Myths and misconceptions about chronic
constipation. Am J Gastroenterol 2005;100;232-42.
16. Ford AC, Moayyedi P, Lacy BE, et al.
American College of Gastroenterology monograph on the management of
irritable bowel syndrome and chronic idiopathic constipation. Am J
Gastroenterol 2014;109 Suppl 1:S2-26. Crossref
17. Wang X, Yin J. Complementary and
alternative therapies for chronic constipation. Evid Based Complement
Alternat Med 2015;2015:396396. Crossref
18. Zhong LL, Cheng CW, Kun W, et al.
Efficacy of MaZiRenWan, a Chinese herbal medicine, in patients with
functional constipation in a randomized controlled trial. Clin
Gastroenterol Hepatol 2018 Apr 12. Epub ahead of print. Crossref
19. Cheng CW, Bian ZX, Zhu LX, Wu JC, Sung
JJ. Efficacy of a Chinese herbal proprietary medicine (Hemp Seed Pill) for
functional constipation. Am J Gastroenterol 2011;106:120-9. Crossref
20. Lee-Robichaud H, Thomas K, Morgan J,
Nelson RL. Cochrane review: lactulose versus polyethylene glycol for
chronic constipation. Evidence-Based Child Health 2011;6:824-64. Crossref
21. Zheng S, Yao J. Expert consensus on
the assessment and treatment of chronic constipation in the elderly. Aging
Med 2018;1:8-17. Crossref
22. Abcar A, Hever A, Momi JS, Sim JJ.
Acute phosphate nephropathy. Perm J 2009;13:48-50. Crossref
23. Lembo AJ, Schneier HA, Shiff SJ, et
al. Two randomized trials of linaclotide for chronic constipation. N Engl
J Med 2011;365:527-36. Crossref
24. McRorie JW, Daggy BP, Morel JG,
Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium
for treatment of chronic constipation. Aliment Pharmacol Ther
1998;12:491-7. Crossref
25. Jiang C, Xu Q, Wen X, Sun H. Current
developments in pharmacological therapeutics for chronic constipation.
Acta Pharm Sin B 2015;5:300-9. Crossref
26. Ford AC, Suares NC. Effect of
laxatives and pharmacological therapies in chronic idiopathic
constipation: systematic review and meta-analysis. Gut 2011;60:209-18. Crossref
27. Lacy BE, Schey R, Shiff SJ, et al.
Linaclotide in chronic idiopathic constipation patients with moderate to
severe abdominal bloating: a randomized, controlled trial. PLoS One
2015;10:e0134349. Crossref
28. Miner PB Jr, Koltun WD, Wiener GJ, et
al. A randomized phase III clinical trial of plecanatide, a uroguanylin
analog, in patients with chronic idiopathic constipation. Am J
Gastroenterol 2017;112:613-21. Crossref
29. Linzess (linaclotide capsules):
highlights of prescribing information. Available from:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202811s000lbl.pdf.
Accessed 19 Mar 2019.
30. Thomas RH, Allmond K. Linaclotide
(Linzess) for irritable bowel syndrome with constipation and for chronic
idiopathic constipation. P T 2013;38:154-60.
31. Roerig JL, Steffen KJ, Mitchell JE,
Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs
2010;70:1487-503. Crossref

