© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Ciliated muconodular papillary tumour of the lung
mimicking mucinous adenocarcinoma: a case report and literature review
Florence MF Cheung, MB, BS, FHKAM (Pathology)1;
J Guan, MD, PhD1; QG Luo, MD2; Alan DL Sihoe,
MBBChir, FHKAM (Surgery)2; XP Shen, MD3
1 Department of Pathology,
University of Hong Kong—Shenzhen Hospital, Shenzhen, Guangdong, China
2 Department of Thoracic Surgery,
University of Hong Kong—Shenzhen Hospital, Shenzhen, Guangdong, China
3 Department of Radiology,
University of Hong Kong—Shenzhen Hospital, Shenzhen, Guangdong, China
Corresponding author: Dr Florence MF Cheung (fmfcheung@gmail.com)
Case report
Solitary lung nodules of <3 cm in diameter
within the lung parenchyma and with no other abnormalities are often
picked up incidentally during routine radiographic imaging. The incidence
of cancer for such nodules has been estimated to be 10% to 70%. Management
strategy depends on the clinical probability of cancer; nodule size,
features, and growth rate ascertained by radiology; and the surgical risk
to the patient. We report a case of such a nodule revealed by radiology
and suspicious for malignancy. Subsequent excision and pathological
examination revealed unexpected findings.
The index patient was a 61-year-old Chinese man
from Northern China with history of laryngeal cancer treated successfully
by local surgery and radiotherapy 6 years prior to present admission.
Routine chest computed tomography (CT) scan revealed a peripheral lung
nodule 9 mm in diameter in the right lower lobe. Follow-up CT scan 1 year
later revealed minimal increase in size to 10 mm and the patient was
referred to the Department of Thoracic Surgery, University of Hong
Kong–Shenzhen Hospital in November 2015 for further treatment. The patient
was a former chronic smoker for more than 10 years (10 cigarettes/day) but
had stopped smoking upon diagnosis of laryngeal cancer.
Physical examination of the patient was
unremarkable. High-resolution CT scan confirmed the presence of a
peripheral lung nodule in his right lower lobe lateral-basal segment that
was suspicious for malignancy. It measured 12 mm in diameter and had a
spiculated border with a central cavity (Fig a). Another high-density 2-mm nodule was present
in the right upper lobe subpleural region associated with apical fibrosis.
There was pleural thickening and mildly increased peripheral lung markings
in bilateral lower lobes. The peribronchiolar and hilar lymph nodes were
not enlarged. After further examination and assessment of the surgical
risk, video-assisted thoracoscopy was decided, with patient consent.
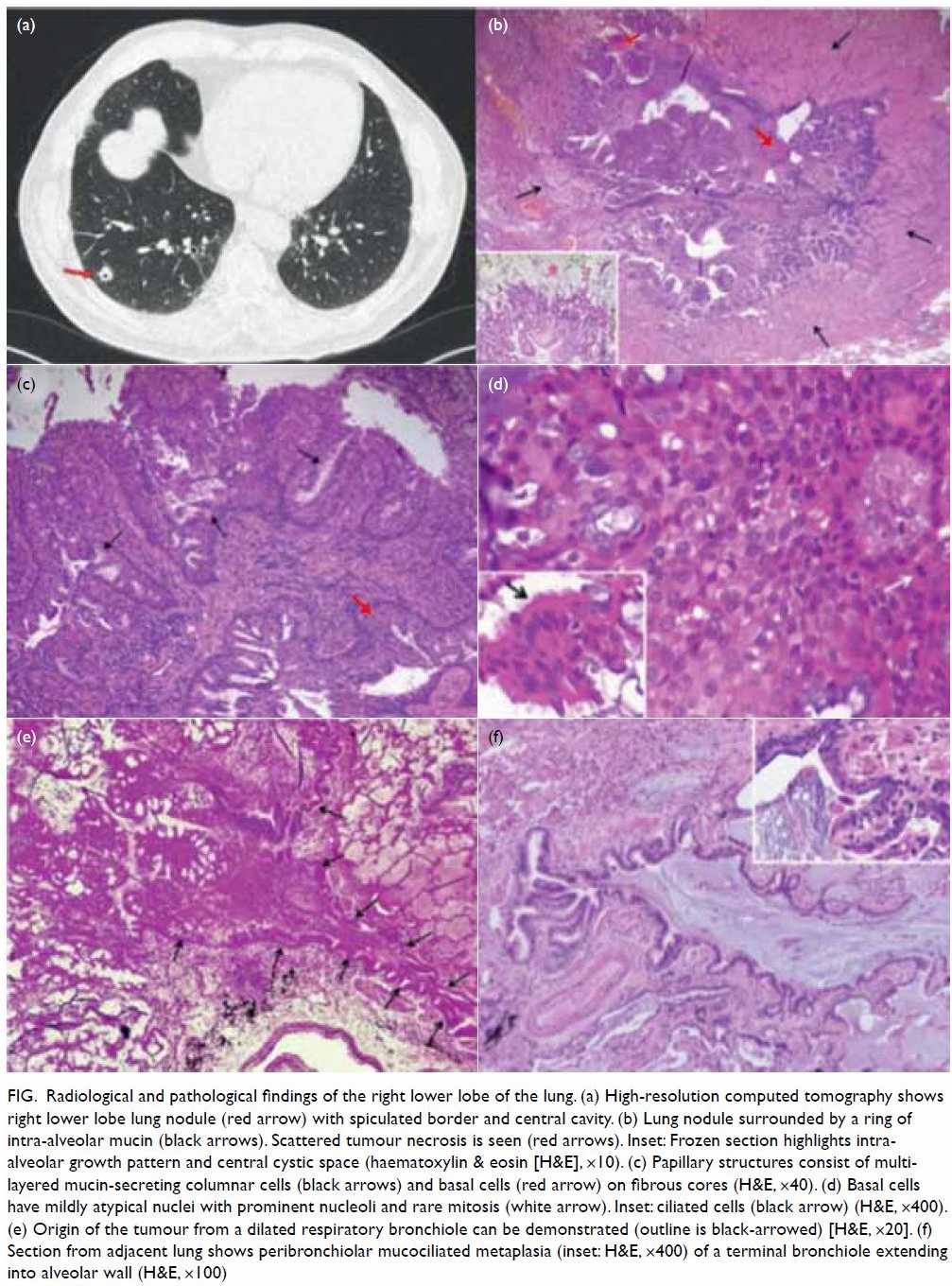
Figure. Radiological and pathological findings of the right lower lobe of the lung. (a) High-resolution computed tomography shows right lower lobe lung nodule (red arrow) with spiculated border and central cavity. (b) Lung nodule surrounded by a ring of intra-alveolar mucin (black arrows). Scattered tumour necrosis is seen (red arrows). Inset: Frozen section highlights intra-alveolar growth pattern and central cystic space (haematoxylin & eosin [H&E], ×10). (c) Papillary structures consist of multi-layered mucin-secreting columnar cells (black arrows) and basal cells (red arrow) on fibrous cores (H&E, ×40). (d) Basal cells have mildly atypical nuclei with prominent nucleoli and rare mitosis (white arrow). Inset: ciliated cells (black arrow) (H&E, ×400). (e) Origin of the tumour from a dilated respiratory bronchiole can be demonstrated (outline is black-arrowed) [H&E, ×20]. (f) Section from adjacent lung shows peribronchiolar mucociliated metaplasia (inset: H&E, ×400) of a terminal bronchiole extending into alveolar wall (H&E, ×100)
During video-assisted thoracoscopy, wedge excision
of the nodule was done. Intra-operative frozen section consultation
revealed a 10-mm papillary glandular tumour 8 mm away from the pleura.
There was profuse mucin production and intra-alveolar extension suspicious
for mucinous adenocarcinoma. Right lower lobectomy was subsequently
performed, and the patient had an uneventful recovery. Microscopic
examination of formalin-fixed paraffin-embedded sections from the nodule
showed an arborising papillary tumour (Fig b) surrounded by intra-alveolar mucin. There was
extension along the alveolar lining at its periphery (Fig b, inset)
simulating ‘lepidic spread’ of adenocarcinoma. The papillary structures (Fig c and d) consisted of fibrous cores covered by
single to multiple layers of mucin-secreting and ciliated (Fig
d, inset) columnar cells and basal cells. There was focal tumour
necrosis, rare mitoses, and mild nuclear atypia. Mucin and inflammatory
cells filled the central cystic space. Origin from a dilated terminal
bronchiole could be traced (Fig e). A batch of immunohistochemical studies
showed CK7+/CK20- tumour cells mostly negative for thyroid transcription
factor-1 except at the periphery, suggestive of residual alveolar lining
cells. Monoclonal carcinoembryonic antigen highlighted the mucinous cells
and p63 stained the basal cells. Proliferative index by Ki67 was low (5%).
The overall picture was consistent with ciliated muconodular papillary
tumour (CMNPT) of the lung. Examination of the lobectomy specimen showed
focal peribronchiolar fibrosis compatible with the effect of smoking.1 These foci often contained peribronchiolar metaplasia
featuring ciliated and mucinous columnar cells (Fig f) occasionally forming small papillae (Fig
f, inset). The 2-mm lesion in the right upper lobe was a fibrotic
nodule. The patient was well 1 year after surgery.
Discussion
The term CMNPT of the lung was first used by
Ishikawa in 2002 to describe a 1.5-cm peripheral lung nodule consisting of
ciliated columnar cells, mucous cells and basal cells with papillary
architecture. It was considered benign in view of indolent behaviour and
bland-looking cells. Further reports by Ishikawa2
and others3 4 5 6 7 of similar
tumours under various names (eg, solitary peripheral ciliated glandular
papillomas, peripheral pulmonary papillary/glandular neoplasms with
ciliated cells) supported this group of tumours as a specific entity that
has not been included in the 2015 World Health Organization Classification
of Lung Tumours.8 We searched the
literature and reviewed 12 reports of 33 such tumours (online
supplementary Appendix). Controversy exists whether CMNPT should be
considered a benign tumour, a well-differentiated adenocarcinoma (in view
of frequent intra-alveolar extension), or a spectrum of entities with
possible progression. The consistent small size, slow growth rate, and
lack of recurrence or metastasis after surgery support the benign nature
of this tumour. Differentiation from mucinous adenocarcinoma is difficult
for pathologists, especially during intra-operative frozen section, owing
to the profuse mucin production and lepidic growth pattern. High-power
examination revealing tripartite cell differentiation and lack of
significant atypia in a clinically slow-growing lung nodule should raise
suspicion of CMNPT. Wedge excision with clear margin is the treatment of
choice. Our findings concur with a previous report3 of tumour origin from the terminal bronchiole. The
finding of co-existing peribronchiolar metaplasia with similar cell
components as CMNPT in the rest of the lung is unique. This suggests
progression of disease from smoking-induced metaplasia to neoplasia during
the pathogenesis. Although chronic smoking was noted in most male patients
with CMNPT (14 out of 16 with smoking history specified in the online
supplementary Appendix), co-existing peribronchiolar metaplasia was
only briefly mentioned in one report,4
probably owing to limited sampling in wedge excision for most tumours.
Molecular analysis for BRAF or EGFR mutations was not done
in our case, because there was no therapeutic indication. Studies of CMNPT
by Chuang et al5 and Lau et al6 yielded no KRAS or EGFR mutation. In contrast, Kamata
et al7 reported mutations involving
EGFR, BRAF, PTEN11, CTNNB1, IDH1,
and TP53 in Asian patients and Liu et al4
reported mutations involving BRAF and AKT1 in one
non-Asian patient. Because CMNPT is commonly reported in patients from
East Asia, more reports are expected when awareness of this entity is
raised among pathologists in this region. The pathogenesis, molecular
characteristics, and natural behaviour of CMNPT can be better defined when
more data are available.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: FMF Cheung.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: FMF Cheung.
Critical revision for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: FMF Cheung.
Critical revision for important intellectual content: All authors.
Acknowledgement
The authors would like to thank Dr Siu-wah Pang for
contributing to the diagnosis of this tumour.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Ethics Committee of
the University of Hong Kong–Shenzhen Hospital as original work with no
infringement of personal privacy. The requirement for patient consent was
waived by the Ethics Committee.
References
1. Katzenstein AL, Mukhopadhyay S, Zanardi
C, Dexter E. Clinically occult interstitial fibrosis in smokers:
classification and significance of a surprisingly common finding in
lobectomy specimens. Hum Pathol 2010;41:316-25. Crossref
2. Ishikawa M, Sumitomo S, Imamura N, et
al. Ciliated muconodular papillary tumor of the lung: report of five
cases. J Surg Case Rep 2016;2016.pii:rjw144. Crossref
3. Aida S, Ohara I, Shimazaki H, et al.
Solitary peripheral ciliated glandular papillomas of the lung: a report of
3 cases. Am J Surg Pathol 2008;32:1489-94. Crossref
4. Liu L, Aesif SW, Kipp BR, et al.
Ciliated muconodular papillary tumors of the lung can occur in Western
patients and show mutations in BRAF and AKT1. Am J Surg
Pathol 2016;40:1631-6. Crossref
5. Chuang HW, Liao JB, Chang HC, Wang JS,
Lin SL, Hsieh PP. Ciliated muconodular papillary tumor of the lung: a
newly defined peripheral pulmonary tumor with conspicuous mucin pool
mimicking colloid adenocarcinoma: a case report and review of literature.
Pathol Int 2014;64:352-7. Crossref
6. Lau KW, Aubry MC, Tan GS, Lim CH, Takano
AM. Ciliated muconodular papillary tumor: a solitary peripheral lung
nodule in a teenage girl. Hum Pathol 2016;49:22-6. Crossref
7. Kamata T, Sunami K, Yoshida A, et al.
Frequent BRAF or EGFR mutations in ciliated muconodular
papillary tumors of the lung. J Thorac Oncol 2016;11:261-5. Crossref
8. Travis WD, Brambilla E, Burke AP, et al.
WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon,
France: International Agency for Research on Cancer; 2015.

