Hong
Kong Med J 2019 Feb;25(1):48–57 | Epub 14 Jan 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Percutaneous mechanical thrombectomy in the treatment
of acute iliofemoral deep vein thrombosis: a systematic review
PC Wong, MB, BS; YC Chan, MB, BS, MD; Y Law, MB, BS;
Stephen WK Cheng, MB, BS, MS
Department of Surgery, Queen Mary Hospital,
Pokfulam, Hong Kong
Corresponding author: Dr YC Chan (ycchan88@hkucc.hku.hk)
Abstract
Background: Conventional
treatment of deep vein thrombosis (DVT) of the lower extremities by
anticoagulation alone has been proven to be insufficient to prevent
recurrence and post-thrombotic syndrome (PTS). Early restoration of
venous patency and preservation of valvular function by endovascular
surgery has been advocated. The aim of this study was to review the
efficacy and safety of percutaneous mechanical thrombectomy (PMT)
against catheter-directed thrombolysis (CDT) in the treatment of acute
iliofemoral DVT.
Methods: Three hundred
sixty-nine articles were identified through screening of the PubMed,
EMBASE, and Cochrane databases from January 2006 to December 2016.
Results: Fifteen retrospective
studies and one prospective registry, totalling 1170 patients, were
recruited for qualitative synthesis. The venous patency rate ranged from
75% to 100% with mean follow-up of 12.3 months. The rates of PTS and
recurrent DVT were less than 17% and 15%, respectively. The overall
mortality rate was 0.26%. Compared with CDT, PMT was shown to reduce PTS
at 1 year (Villalta score: 2.1 ± 3.0 in the PMT group and 5.1 ± 4.1 in
the CDT group, P=0.03) and bleeding complications (packed cells
transfused: 0.2 ± 0.3 units in the pharmacomechanical thrombectomy group
and 1.2 ± 0.7 units in the CDT group, P<0.05).
Conclusion: Percutaneous
mechanical thrombectomy is a safe and effective treatment for acute
iliofemoral DVT in terms of restoration of venous patency, prevention of
DVT recurrence, and PTS. Compared with CDT alone, PMT offers a lower
risk of PTS and bleeding complications.
Introduction
Deep vein thrombosis (DVT) is a major cause of
morbidity and mortality, as it can lead to post-thrombotic syndrome (PTS)
and pulmonary embolism. According to the American College of Chest
Physicians treatment guidelines, DVT has conventionally been treated with
low-molecular-weight heparin, unfractionated heparin, or fondaparinux
followed by vitamin K antagonists for at least 3 months.1 This recommended regimen is adequate for prevention of
thrombus extension, but its effect on clot lysis is minimal. The reported
6-month venous patency rate in patients treated with anticoagulation alone
was only 47.4%. Eventually, up to 55.6% of patients with iliofemoral DVT
developed PTS as a result of valvular dysfunction.2 Up to 5% to 10% of patients had severe PTS in the form
of venous ulceration, which caused significant morbidity and
socio-economic cost.3
In view of the suboptimal treatment outcomes of
anticoagulation, aggressive means have been developed to achieve early
restoration of venous patency and thus preservation of valvular function.
A Cochrane review suggested that early thrombus removal by means of
systemic thrombolysis can prevent venous dysfunction and PTS. However, its
use was limited by its significantly increased risk of bleeding.4
Endovascular modalities including catheter-directed
thrombolysis (CDT) and percutaneous mechanical thrombectomy (PMT) were
developed to achieve accelerated thrombolysis with less bleeding risk.
Catheter-directed thrombolysis was shown to be superior to anticoagulation
alone in terms of higher thrombolysis rate and lower rates of recurrence
and PTS.2 It features loco-regional
delivery of thrombolytic agent over the DVT site via a transluminal
catheter. The dosage of the thrombolytic agent can be reduced compared
with that of systemic thrombolysis, and thus, a reduction in bleeding
complications can be achieved. Its benefits have been validated by a
number of randomised controlled trials and meta-analyses, but its
application rate remains low because of its substantial bleeding risk and
cost.5
Percutaneous mechanical thrombectomy is another
form of endovascular treatment, in which thrombectomy devices are passed
to the site of DVT and blood clots are removed by different mechanical
means. It can also be used as an adjunctive device to CDT or
pharmacomechanical thrombectomy. When these two devices are used in
combination, the dosage of thrombolytic agents can be lowered further and
the duration of procedure can be shortened.6
According to the American College of Chest Physicians guidelines, PMT
provides the greatest benefits for young and functionally active patients
with acute presentation (<14 days, or presence of phlegmasia cerulea
dolens) of extensive proximal DVT.1
Percutaneous mechanical thrombectomy has provided promising results in
various studies, while high-level evidence to guide its implementation is
still lacking. Against this background, this article aimed to review the
evidence about PMT regarding its procedural outcomes and safety profile in
the treatment of DVT.
Methods
Literature search
A systematic review was conducted in accordance
with the PRISMA (Preferred Reporting Items for Systematic reviews and
Meta-Analysis) statement (http://www.prisma-statement.org/). An electronic
search was performed using the PubMed, EMBASE, and Cochrane Databases from
January 2006 to December 2016. The medical subject heading (MeSH) terms
used were “mechanical thrombectomy” and “venous thrombosis” or “deep vein
thrombosis”.
Study selection
The inclusion criteria were as follows: DVT of the
lower extremities; human study; study population aged ≥18 years; and
articles published in English. Reviews and case reports were excluded. All
studies of interest were obtained as full-text articles and assessed by
two authors independently. Final decisions on inclusion in the study were
made by the entire research team.
Data extraction and outcome measurement
Relevant data were extracted with the following
items recorded: author, title, year of publication, number and age of
patients, co-morbidities, duration of follow-up, onset of symptoms,
location of DVT, type of thrombectomy device, and adjunctive modalities.
Efficacy was measured in terms of rates of venous patency, recurrence, and
PTS. Complications including bleeding, pulmonary embolism, and mortality
were recorded. Secondary outcomes included dosage of thrombolytic agents,
cost, and duration of procedure.
Data analysis
Statistical meta-analysis was not performed because
of the heterogeneity of the original data. Therefore, descriptive data
were summarised and presented in tables to provide a comprehensive
overview of different clinical aspects of the studies.
Results
Our initial search yielded a total of 369 articles,
including 260 articles from PubMed, 98 articles from EMBASE, and 11
studies from the Cochrane Library. Thirty-one duplicated records and 283
irrelevant studies were excluded upon screening of titles and abstracts,
leaving 55 potentially eligible studies. A further 39 articles were
excluded after full-text articles were assessed: 28 review articles; one
study on the patients with inferior vena cava (IVC) filter; one study on
the effect of clot age; seven studies without full text; and two
non-English studies. Fifteen retrospective studies and one prospective
registry were included into our analysis, in which seven articles reported
comparative evidence of PMT versus CDT. There were no published randomised
trials available.
Baseline patient demographics and characteristics
of the studies are summarised in Table 1.6 7 8
9 10
11 12
13 14
15 16
17 18
19 20
21 A total of 1170 patients were
included (range, 16-329 patients) with a mean age of 53.5 years (range, 16
to 88 years). The mean follow-up time was 12.3 months (range, 1-82
months).
Four different categories of thrombectomy devices
were used among the included studies: rheolytic devices,6 7 8 9 10 11
aspiration devices,12 13 14 15 16
rotational devices,16 17 18 19 20 21 and ultrasound-enhanced thrombolysis devices.14 They aimed to achieve transcatheter removal of
thrombi via different mechanical means.
The AngioJet system (Possis Medical, Minneapolis
[MN], US) is a rheolytic device that generates high-velocity saline jets
at the side of catheter, which create a localised low-pressure zone and
thus result in maceration and aspiration of the thrombus.
During aspiration thrombectomy, the thrombus was
aspirated out through the percutaneous catheter as the catheter was
gradually pulled out. The process was repeated until complete removal of
the thrombus or at least 90% disappearance of thrombi. Two of the
aspiration systems were Aspirex (Straub Medical, Wangs, Switzerland) and
the Trellis infusion system (Covidien, Mansfield [MA], US), which is a
sophisticated system that contains an oscillation drive unit that mixes
the thrombus with thrombolytic agents between two occlusion balloons.
Rotational devices feature high-frequency
revolution of a helix that is controlled by a foot pedal. At least four
different types of rotational devices were included, including the Amplatz
thrombectomy device (Microvena, White Bear Lake [MN], US), Rotarex (Straub
Medical, Wangs, Switzerland), Trerotola (Arrow International, Redding
[PA], US), and Cleaner (Rex Medical, Fort Worth [TX], US and Argon Medical
Devices, Inc, Plano [TX], US). They all consisted of a motor-driven
fragmentation helix or basket that was rotated in the thrombosed vein. The
thrombus was then aspirated out via the catheter.
An ultrasound-enhanced thrombolysis device (EKOS
Corporation, Bothell [WA], US) was selectively used in one study14 for patients with inadequate thrombus removal despite
the use of a rotational device. A high-frequency ultrasound wave was
emitted through transducers inside the catheter to achieve maceration of
the thrombus and mix it with thrombolytic agents.
Efficacy
Nine non-comparative and seven comparative studies
were included in our analysis. Efficacy in terms of rates of venous
patency, PTS, and recurrent thrombosis is shown in Table 2.6 7 8
9 10
11 12
13 14
15 16
17 18
19 20
21 Venous patency was measured
most frequently by Duplex ultrasound (n=9) followed by computed
tomographic (CT) venography (n=4) and contrast venography (n=2). Imaging
modalities were not mentioned in three studies. Venous patency was further
quantified according to a 3-tier system in five studies: Grade I (<50%
clot lysis), Grade II (50%-99% clot lysis), and Grade III (100% clot
lysis).22 Venous patency was
measured at 6 months in four studies and at 1 year in the other 12
studies. Venous patency rates ranged from 75% to 100%.
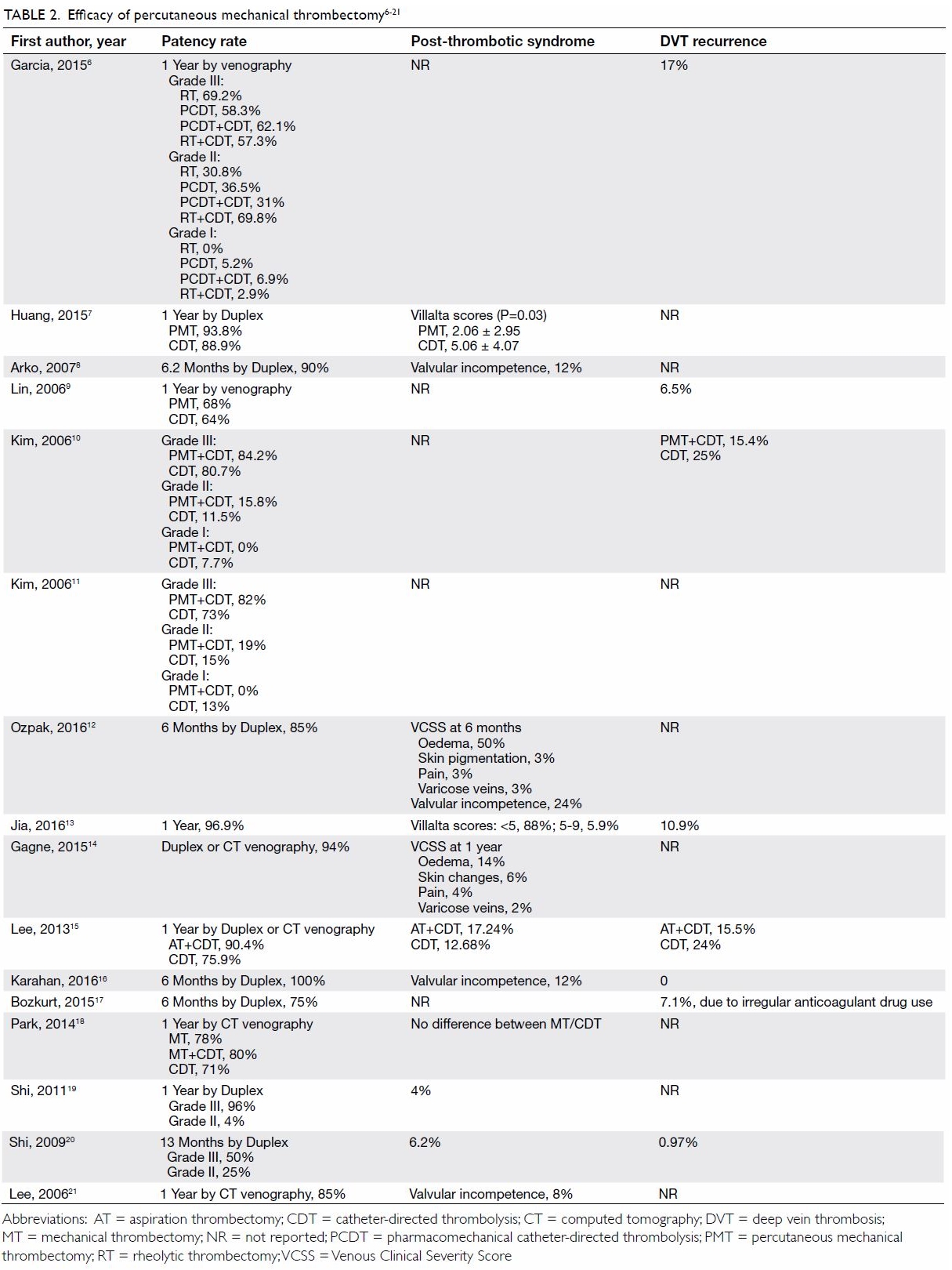
Table 2. Efficacy of percutaneous mechanical thrombectomy6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
Rates of PTS were reported in terms of Villalta
score (n=2)23 or Venous Clinical
Severity Score (VCSS) [n=2].14
Four studies reported the rates of valvular incompetence from 8% to 24%.
The rates of DVT recurrence, reported in eight studies, ranged from 0% to
17%.
Complications
The major complications of thrombectomy are shown
in Table 3.6 7 8
9 10
11 12
13 14
15 16
17 18
19 20
21 Six studies reported rates of
pulmonary embolism ranging from 0.3% to 17%, but none of them was
clinically significant. No patient had pulmonary embolism in the remaining
11 studies. Garcia et al6 reported
major bleeding complications in 3.6% of patients, including intracranial
bleeding, gastrointestinal bleeding secondary to gastritis or gastric
cancer, retroperitoneal bleeding, and haemolytic anaemia requiring
transfusion. Minor bleeding complications were reported at frequencies of
up to 28%, most of which were access site bleeding. Blood transfusion and
surgical intervention were seldom required. No operative mortality was
reported in 12 studies, while only two cases of fatal intracranial
haemorrhage were noted in two separate studies. Another mortality was
reported by Garcia et al,6 with an
unknown cause of death. The overall mortality in this series was 0.26%.
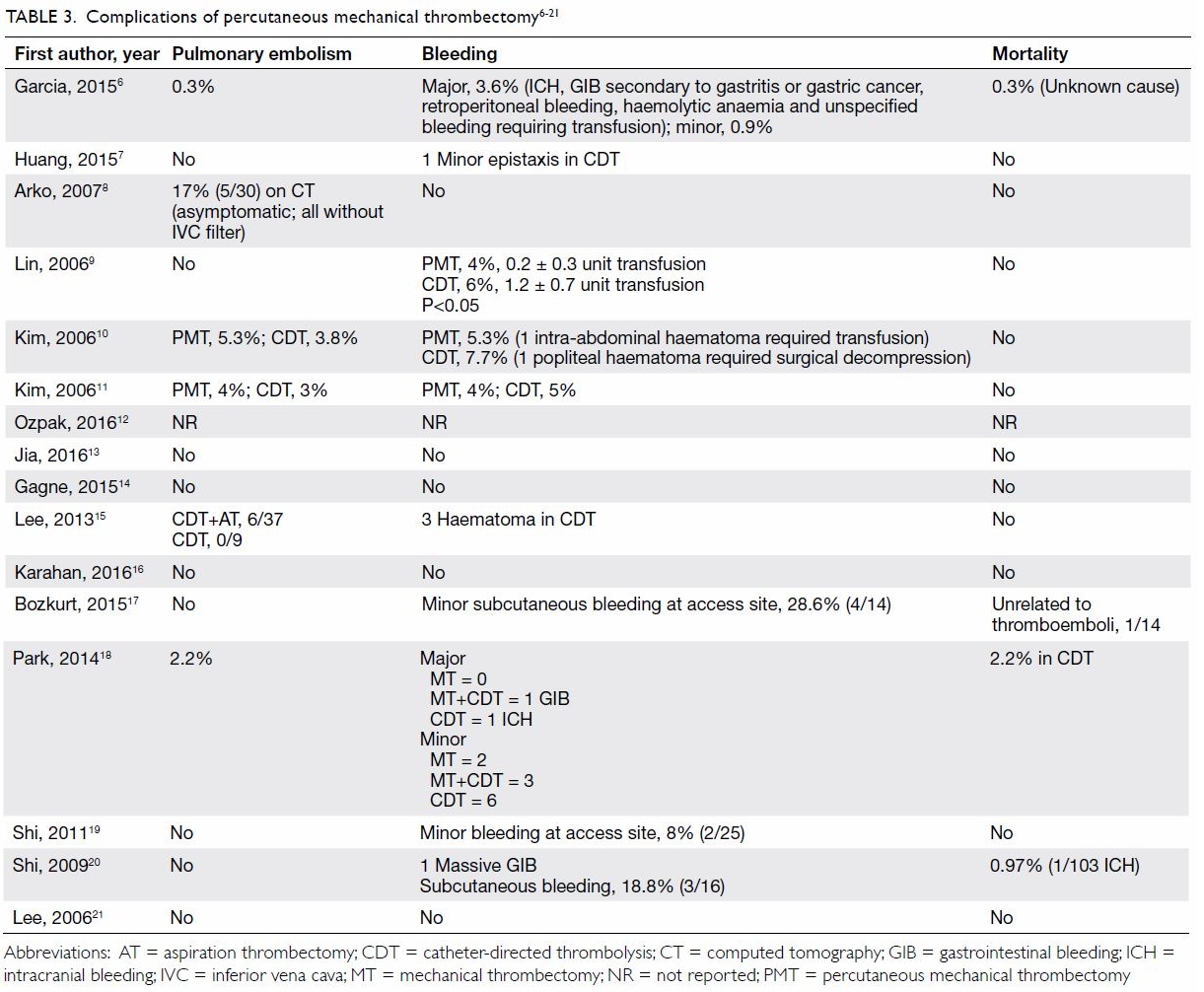
Table 3. Complications of percutaneous mechanical thrombectomy6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
Secondary outcomes
Seven studies reported comparative evidence about
PMT versus CDT (Table 46 9 10
11 15
18). Huang et al7 showed that PMT significantly reduced PTS at 1 year,
with lower Villalta scores in the PMT group (2.1±3.0) than in the CDT
group (5.1±4.1; Wilcoxon rank-sum test, P=0.03). However, no statistical
difference was shown in Villalta scores in another retrospective study
conducted by Park et al.18
Lin et al9
compared bleeding complications between the two groups in terms of the
number of units of packed red blood cells transfused. There was a
significant reduction of blood transfusion from 1.2±0.7 units in the CDT
group to 0.2±0.3 units in the PMT group (Pearson Chi squared, P≤0.05).9
The dosage of thrombolytic infusion and average
procedural time were significantly reduced in the CDT with adjunctive PMT
group compared with the CDT alone group, as reported in at least four
different retrospective studies.10
11 15
18
From the economic perspective, two retrospective
studies performed cost analysis, and PMT was found to be associated with
44% to 49% reduction in total hospital costs.9
10 It was also consistent with
shorter hospital and intensive care unit (ICU) stays in the PMT group
(4.6±1.3 days of hospital stay and 0.6±0.3 days of ICU stay in the PMT
group vs 8.4±2.3 days of hospital stay and 2.4±1.2 days of ICU stay in the
CDT group; Pearson Chi squared, P<0.02 to 0.04).
No statistically significant differences in venous
patency or symptom improvement between the two groups were reported in
this series of comparative studies.
Comparison between types of thrombectomy devices
Three studies compared outcomes of different
thrombectomy devices. Garcia et al6
created the first prospective multi-centre (PEARL) registry to document
the use of the AngioJet rheolytic device. A total of 329 patients were
stratified into four treatment subgroups: (1) rheolytic thrombectomy (RT)
alone; (2) RT plus CDT; (3) pharmacomechanical CDT (PCDT), and (4) PCDT
combined with CDT. Each of these subgroups differed in terms of the
presence, timing, and delivery means of thrombolytic agents. Rheolytic
thrombectomy was given before or after CDT in subgroup 2, and PCDT was
defined as delivery of lytic agent through an AngioJet catheter. This
registry demonstrated no statistical difference in venous patency rate
between the subgroups, while a significant reduction in procedural time in
non-CDT group was observed (Table 4). The investigators concluded that RT was
effective and safe, and therefore, the needs for concomitant CDT and
intensive care could potentially be reduced.
Shi et al20
and Arko et al8 compared the
outcomes of Amplatz versus Rotarex and Trellis versus AngioJet devices,
which again showed no significant differences in clinical outcomes between
the two groups.
Use of adjunctive treatments
Inferior vena cava filter placement, angioplasty,
and stenting were the most commonly performed adjunctive treatments in
addition to thrombectomy. Inferior vena cava filters were used in 46% to
100% of patients among 11 studies. The majority of the filters were
removed shortly after the procedure without major complications. Lee et al15 reported that 6 of 37 patients
had thrombus entrapment in prophylactic IVC filters in the thrombectomy
group compared with 0 of 9 patients in the CDT alone group. Arko et al8 reported that 17% of patients showed asymptomatic
pulmonary embolism on CT after thrombectomy, in which all patients did not
receive IVC filters. This showed that prophylactic IVC filtration could be
a useful measure for prevention of pulmonary embolism, especially in
patients who undergo aggressive thrombectomy.
Angioplasty with or without stenting was performed
in 15 studies, ranging from 14% to 80% of patients. The two main
indications were iliac vein compression syndrome (May-Thurner syndrome)
and residual thrombus after thrombectomy. One study reported a
significantly improved iliac vein patency rate in the group with stents
(28.95%) than without stents (11.29%; log rank test, P=0.026).15
Discussion
Catheter-directed thrombolysis and PMT are both
emerging techniques for treatment of acute DVT of the lower extremities
that have the advantage of early restoration of venous patency and thus
reduction of post-thrombotic complications. A 2015 meta-analysis compared
the efficacy of CDT plus anticoagulation versus that of anticoagulation
alone in the treatment of proximal DVT. It showed that additional CDT was
associated with significantly improved 6-month venous patency and PTS
rates. However, there was a two-fold increase in bleeding complications in
the CDT group, and concomitant close monitoring under intensive care
setting has had a substantial economic burden.5
These two main reasons have precluded the incorporation of CDT into the
standard treatment recommendation despite encouraging procedural outcomes.
As compared with CDT, PMT is another endovascular
option that has provided promising clinical outcomes with better
controlled bleeding risk. This review has served as a comprehensive
overview of clinical and safety outcomes across different categories of
thrombectomy devices. It demonstrated well that the procedural outcomes of
both PMT alone and that with pharmacomechanical devices were non-inferior
to that of CDT in treatment of acute DVT in the lower extremities. The
rates of PTS, bleeding complications, and hospital costs of PMT were all
shown to be favourable to those of CDT alone. In addition, the mortality
risk of PMT was minimal and comparable to that of patients treated with
anticoagulation alone: 0.4% recurrent fatal venous thromboembolism and
0.2% fatal major bleeding events.24
As illustrated in this review, the balanced risks and benefits of PMT
provide a basis for the future initiation of randomised controlled trials
on its use.
In addition, PMT is potentially superior to CDT
especially in patients in whom thrombolysis therapy is contra-indicated.
According to the Society of Interventional Radiology recommendations, CDT
is absolutely contra-indicated in patients with recent cerebrovascular
events, neurosurgery or intracranial trauma, active internal bleeding, and
those with absolute contra-indications to anticoagulation. Other strong
relative contra-indications are listed in the Standard of Practice25: recent major surgery, obstetrical delivery or major
trauma within 10 days, etc. These patients are prone to the development of
DVT, and they have been conventionally treated with anticoagulation or IVC
filters. Percutaneous mechanical thrombectomy is another option in this
clinically challenging situation. Further studies on this particular group
of high-risk patients are necessary to investigate the efficacy and safety
of this novel technique.
Nevertheless, this review has several limitations.
The studies were heterogeneous in terms of outcome measurements and the
use of thrombectomy devices. Post-thrombotic syndrome was measured in
terms of Villalta score, VCSS, or valvular incompetence rate. Although
these systems were well-defined objective scales for monitoring and
documentation of PTS, it was difficult to compare efficacy across studies.
Similarly, the important index of venous patency rate was variously
measured by Duplex ultrasound, CT venogram, or venography. Inaccuracies
during direct comparison between studies were unavoidable.
Other adjunctive modalities in addition to the
principal thrombectomy devices including iliac vein angioplasty, stenting,
and prophylactic IVC filter were used in a major proportion of the
studies. No standardised criteria were outlined for the usage of these
devices, and they created a confounding factor during data analysis. With
inadequate information on sub-categorisation of the study populations,
analysis specific to each type of adjunctive devices was not feasible.
Most of the studies were retrospective, and no randomised trials were
available for quantitative analysis.
Conclusion
Percutaneous mechanical thrombectomy is a safe and
effective treatment for acute iliofemoral DVT in terms of restoration of
venous patency, prevention of DVT recurrence, PTS, and pulmonary embolism.
The overall clinical outcomes of PMT are superior to those with
anticoagulation alone. Compared with CDT alone, adjunctive PMT has a lower
risk of PTS and bleeding complications. Randomised studies to demonstrate
the efficacy of PMT versus anticoagulation and CDT and compare the
efficacy of different types of PMT devices would be most beneficial to
guide future strategies for treatment of acute proximal DVT.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: PC Wong, YC Chan, Y Law.
Drafting of the manuscript: PC Wong, YC Chan, Y Law.
Critical revision: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: PC Wong, YC Chan, Y Law.
Drafting of the manuscript: PC Wong, YC Chan, Y Law.
Critical revision: All authors.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration
The paper was presented as an abstract in the 21st
Asian Congress of Surgery by the Asian Surgical Association, 22-23
November 2017, Tokyo, Japan.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
References
1. Kearon C, Kahn SR, Agnelli G, Goldhaber
S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous
thromboembolic disease: American College of Chest Physicians
Evidence-Based Clinical Practice Guidelines (8th edition). Chest
2008;133(6 Suppl):454S-545S. Crossref
2. Enden T, Haig Y, Kløw NE, et al.
Long-term outcome after additional catheter-directed thrombolysis versus
standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT
study): a randomised controlled trial. Lancet 2012;379:31-8. Crossref
3. Baldwin MJ, Moore HM, Rudarakanchana N,
Gohel M, Davies AH. Post-thrombotic syndrome: a clinical review. J Thromb
Haemost 2013;11:795-805. Crossref
4. Watson LI, Armon MP. Thrombolysis for
acute deep vein thrombosis. Cochrane Database Syst Rev 2004;(4):CD002783.
Crossref
5. Du GC, Zhang MC, Zhao JC.
Catheter-directed thrombolysis plus anticoagulation versus anticoagulation
alone in the treatment of proximal deep vein thrombosis—a meta-analysis.
Vasa 2015;44:195-202. Crossref
6. Garcia MJ, Lookstein R, Malhotra R, et
al. Endovascular management of deep vein thrombosis with rheolytic
thrombectomy: final report of the prospective multicenter PEARL
(Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of
Catheter Lengths) registry. J Vasc Interv Radiol 2015;26:777-85. Crossref
7. Huang CY, Hsu HL, Kuo TT, Lee CY, Hsu
CP. Percutaneous pharmacomechanical thrombectomy offers lower risk of
post-thrombotic syndrome than catheter-directed thrombolysis in patients
with acute deep vein thrombosis of the lower limb. Ann Vasc Surg
2015;29:995-1002. Crossref
8. Arko FR, Davis CM 3rd, Murphy EH, et al.
Aggressive percutaneous mechanical thrombectomy of deep venous thrombosis:
early clinical results. Arch Surg 2007;142:513-9. Crossref
9. Lin PH, Zhou W, Dardik A, et al.
Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for
treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg
2006;192:782-8. Crossref
10. Kim HS, Patra A, Paxton BE, Khan J,
Streiff MB. Catheter-directed thrombolysis with percutaneous rheolytic
thrombectomy versus thrombolysis alone in upper and lower extremity deep
vein thrombosis. Cardiovasc Intervent Radiol 2006;29:1003-7. Crossref
11. Kim HS, Patra A, Paxton BE, Khan J,
Streiff MB. Adjunctive percutaneous mechanical thrombectomy for
lower-extremity deep vein thrombosis: clinical and economic outcomes. J
Vasc Interv Radiol 2006;17:1099-104. Crossref
12. Ozpak B, Ilhan G, Ozcem B, Kara H. Our
short-term results with percutaneous mechanical thrombectomy for treatment
of acute deep vein thrombosis. Thorac Cardiovasc Surg 2016;64:316-22. Crossref
13. Jia Z, Tu J, Zhao J, et al. Aspiration
thrombectomy using a large-size catheter for acute lower extremity deep
vein thrombosis. J Vasc Surg Venous Lymphat Disord 2016;4:167-71. Crossref
14. Gagne P, Khoury T, Zadeh BJ,
Rajasinghe HA. A multicenter, retrospective study of the effectiveness of
the trellis-8 system in the treatment of proximal lower-extremity deep
vein thrombosis. Ann Vasc Surg 2015;29:1633-41. Crossref
15. Lee JH, Kwun WH, Suh BY. The results
of aspiration thrombectomy in the endovascular treatment for iliofemoral
deep vein thrombosis. J Korean Surg Soc 2013;84:292-7. Crossref
16. Karahan O, Kutas HB, Gurbuz O, et al.
Pharmacomechanical thrombolysis with a rotator thrombolysis device in
iliofemoral deep venous thrombosis. Vascular 2016;24:481-6. Crossref
17. Bozkurt A, Kırbaş İ, Kösehan D,
Demirçelik B, Nazlı Y. Pharmacomechanical thrombectomy in the management
of deep vein thrombosis using the cleaner device: an initial single-center
experience. Ann Vasc Surg 2015;29:670-4. Crossref
18. Park KM, Moon IS, Kim JI, et al.
Mechanical thrombectomy with Trerotola compared with catheter-directed
thrombolysis for treatment of acute iliofemoral deep vein thrombosis. Ann
Vasc Surg 2014;28:1853-61. Crossref
19. Shi HJ, Huang YH, Shen T, Xu Q.
Percutaneous mechanical thrombectomy for acute massive lower extremity
deep venous thrombosis. Surg Laparosc Endosc Percutan Tech 2011;21:50-3. Crossref
20. Shi HJ, Huang YH, Shen T, Xu Q.
Percutaneous mechanical thrombectomy combined with catheter-directed
thrombolysis in the treatment of symptomatic lower extremity deep venous
thrombosis. Eur J Radiol 2009;71:350-5. Crossref
21. Lee KH, Han H, Lee KJ, et al.
Mechanical thrombectomy of acute iliofemoral deep vein thrombosis with use
of an Arrow-Trerotola percutaneous thrombectomy device. J Vasc Interv
Radio 2006;17:487-95. Crossref
22. Porter JM, Moneta GL. Reporting
standards in venous disease: an update. International Consensus Committee
on Chronic Venous Disease. J Vasc Surg 1995;21:635-45. Crossref
23. Kahn SR, Partsch H, Vedantham S.
Definition of post-thrombotic syndrome of the leg for use in clinical
investigations: a recommendation for standardization. J Thromb Haemost
2009;7:879-83. Crossref
24. Carrier M, Le Gal G, Wells PS, Rodger
MA. Systematic review: case-fatality rates of recurrent venous
thromboembolism and major bleeding events among patients treated for
venous thromboembolism. Ann Intern Med 2010;152:578-89. Crossref
25. Vedantham S, Thorpe PE, Cardella JF,
et al. Quality improvement guidelines for the treatment of lower extremity
deep vein thrombosis with use of endovascular thrombus removal. J Vasc
Interv Radiol 2009;20(7 Suppl):S227-39. Crossref