Hong
Kong Med J 2018 Oct;24(5):460–5 | Epub 28 Sep 2018
DOI: 10.12809/hkmj177181
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Mortality and morbidity of extremely low birth weight
infants in Hong Kong, 2010-2017: a single-centre review
KL Hon, MB, BS, MD1; Sharon Liu, MB,
ChB2; Joey CY Chow, MB, ChB2; Kathy YC Tsang, MPhil1;
Hugh S Lam, MBBChir, MD1; KW So, MB, BS, MRCP3; Yvonne
KY Cheng, MB, ChB, MRCOG4; Alexander KC Leung, MB, BS, FRCPC5;
William Wong, MB, BS1
1 Department of Paediatrics, The Chinese
University of Hong Kong, Shatin, Hong Kong
2 Faculty of Medicine, The Chinese
University of Hong Kong, Shatin, Hong Kong
3 Department of Paediatrics, Prince of
Wales Hospital, Shatin, Hong Kong
4 Department of Obstetrics and
Gynaecology, The Chinese University of Hong Kong, Shatin, Hong Kong
5 Department of Pediatrics, University
of Calgary, Canada
Corresponding author: Prof KL Hon (ehon@hotmail.com)
Abstract
Background: Extremely low
birth weight (ELBW) infants exhibit high rates of mortality and
morbidity. We retrospectively assessed factors associated with mortality
and morbidity among ELBW infants.
Methods: Perinatal demographic
data were reviewed for all ELBW infants born between 2010 and 2017 at a
tertiary neonatal unit.
Results: For non-survivors (21%
of ELBW infants) and survivors, the median gestational ages were 24.1
and 26.2 weeks, respectively, and median birth weights were 650 g and
780 g, respectively (all P<0.001). Regression analyses showed that
non-survival was positively associated with lower gestational age
(adjusted odds ratio [aOR]=6.71 for every 1-week decrease; 95%
confidence interval [CI]=1.73-26.00; P=0.006) and grade 3 or 4
intraventricular haemorrhage (aOR=29.23; 95% CI=1.39-613.84; P=0.030);
non-survival was negatively associated with the presence of
bronchopulmonary dysplasia (aOR=0.01; 95% CI= <0.001-0.23; P=0.005);
length of neonatal intensive care unit stay for survivors was positively
associated with the presence of necrotising enterocolitis
(B-coefficient=89.60; 95% CI=43.86-135.34; P<0.001); and length of
hospital stay for survivors was positively associated with the presence
of necrotising enterocolitis (B-coefficient=2.08; 95% CI=0.43-3.73;
P=0.015) and a low Apgar score at 1 minute (B-coefficient= –0.63; 95%
CI= –1.04 to –0.22; P=0.003).
Conclusion: Extremely low birth
weight infants exhibited significant mortality and morbidity; there was
no survival prior to 23.6 weeks’ gestation or below 550 g birth weight.
The presence of grade 3 or 4 intraventricular haemorrhage was
independently associated with non-survival. Survivors were significantly
more likely to exhibit bronchopulmonary dysplasia; survivors with
necrotising enterocolitis were more likely to require longer stays in
the neonatal intensive care unit and in hospital.
New knowledge added by this study
- Extremely low birth weight infants in Hong Kong showed significant mortality and morbidity: there was no survival prior to 23.6 weeks’ gestation or below 550 g birth weight in this series.
- The presence of grade 3 or 4 intraventricular haemorrhage was independently associated with non-survival.
- Survivors were significantly more likely to exhibit bronchopulmonary dysplasia.
- Survivors with necrotising enterocolitis were significantly more likely to require longer stays in both the neonatal intensive care unit and hospital.
- Parents in Hong Kong with extremely low birth weight infants should be counselled regarding expectations for infant survival and associated complications.
- Hong Kong hospitals can modify their practices to support an increased rate of survival and decreased rate of complications among extremely low birth weight infants.
- These findings may guide future neonatal health policy and funding in Hong Kong.
Introduction
Extremely low birth weight (ELBW) infants (birth
weight <1000 g) have high rates of mortality and morbidity.1 2 The survival
of ELBW infants has improved significantly with the widespread use of
exogenous surfactant agents, maternal steroids, mechanical ventilation,
and advancements in neonatal technologies.1
3 4
5 6
7 The minimum age of viability is
currently regarded as 21 to 22 weeks’ gestation; accordingly, there have
been scattered reports of survivors born at 21 to 22 weeks’ estimated
gestation.2 8 9 10 11
Periviable birth is defined as delivery occurring from 20 weeks to 25
weeks 6 days of gestation.12
Notably, the rate of survival at 3 years for infants born at 22 weeks’
gestation has been reported as approximately 36% in some centres.13 This study aimed to review mortality and morbidity of
ELBW infants at a tertiary neonatal unit in Hong Kong.
Methods
Demographic and clinical data were analysed for
consecutive neonates admitted to the neonatal unit of a
university-affiliated teaching hospital (Prince of Wales Hospital) in Hong
Kong between 1 January 2010 and 30 June 2017. During the study period, the
Prince of Wales Hospital provided regional neonatal intensive care service
for the Eastern New Territories of Hong Kong, with a catchment population
of over 1.1 million (approximately 25% were children aged <12 years). A
standard data form was used for data abstraction by the investigators. All
Clinical Management System records, in-patient records, and computerised
laboratory data were examined. Numerical data were expressed as median
(interquartile range) and compared by using the Mann-Whitney U
test; categorical data were compared by using the Chi squared test, or
Fisher’s exact test for data fields that contained >20% cells with an
expected count <5. Backward binary logistic regression was conducted on
mortality; backward linear regressions were conducted on lengths of
neonatal intensive care unit (NICU) stay and hospital stay for survivors.
Corresponding Kaplan-Meier survival curves were constructed with survival
distribution comparisons by log-rank test using SPSS (Windows version
20.0; IBM Corp, Armonk [NY], US). Two-tailed P values <0.05 were
considered statistically significant.
Results
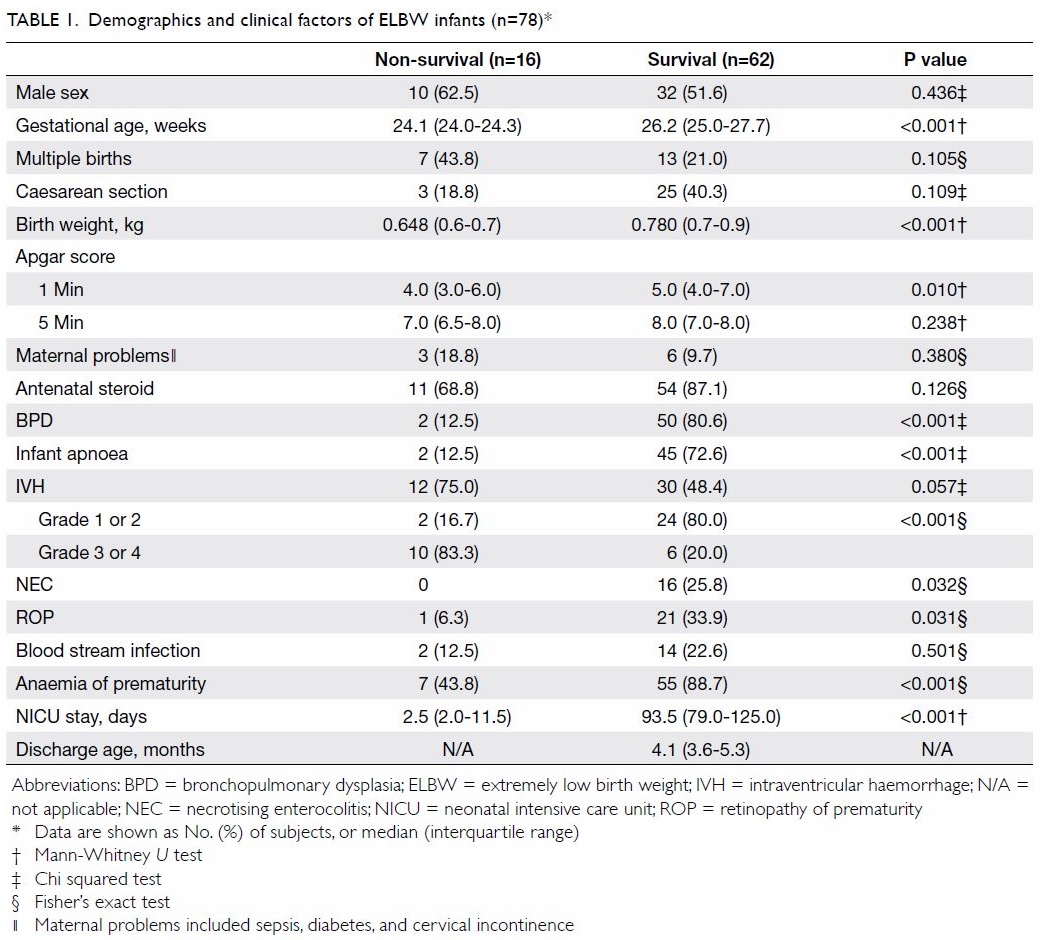
A total of 78 ELBW neonates were admitted to the
NICU between 1 January 2010 and 30 June 2017; 16 of these neonates died
(mortality 21%) [Table 1]. For non-survivors and survivors, the
median gestational ages were 24.1 and 26.2 weeks, respectively, and birth
weights were 650 and 780 g, respectively (P<0.001). The median
(interquartile range) of NICU stay before death was 2.5 (2.0-11.5) days.
The median durations of NICU stay and total hospital stay among survivors
before discharge were 93.5 days and 4.1 months, respectively. Backward
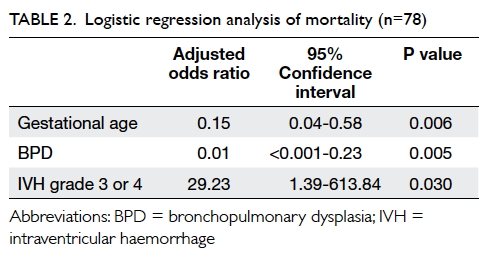
binary logistic regression analysis showed that non-survival was
associated with lower gestational age (adjusted odds ratio [aOR]=6.71 for
every 1-week decrease; 95% confidence interval [95% CI]=1.73-26.00;
P=0.006) and grade 3 or 4 intraventricular haemorrhage [IVH] (aOR=29.23;
95% CI=1.39-613.84; P=0.030); non-survival was negatively associated with
the presence of bronchopulmonary dysplasia (BPD) [aOR=0.01; 95%
CI=<0.001-0.23; P=0.005), after adjustment for multiple births,
caesarean section, birth weight, Apgar score at 1 minute, antenatal
steroid administration, infant apnoea, necrotising enterocolitis (NEC),
retinopathy of prematurity (ROP), and anaemia of prematurity in the first
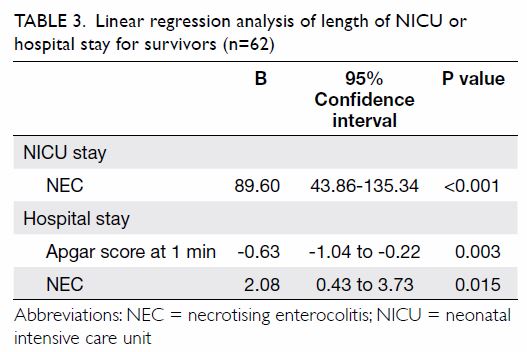
step of the regression analysis (Table 2). Backward linear regression revealed that
the length of NICU stay for survivors was positively associated with the
presence of NEC (B-coefficient=89.60; 95% CI=43.86-135.34; P<0.001),
after including gestational age, caesarean section, birth weight, Apgar
score at 1 minute, and blood stream infection in the first step of the
regression analysis (Table 3). The length of hospital stay was positively
associated with presence of NEC (B-coefficient=2.08; 95% CI=0.43-3.73;
P=0.015), but negatively associated with Apgar score at 1 minute
(B-coefficient= –0.63; 95% CI= –1.04 to –0.22; P=0.003), after including
gestational age, birth weight, Apgar score at 1 minute, and Apgar score at
5 minute in the first step of the regression analysis (Table
3).
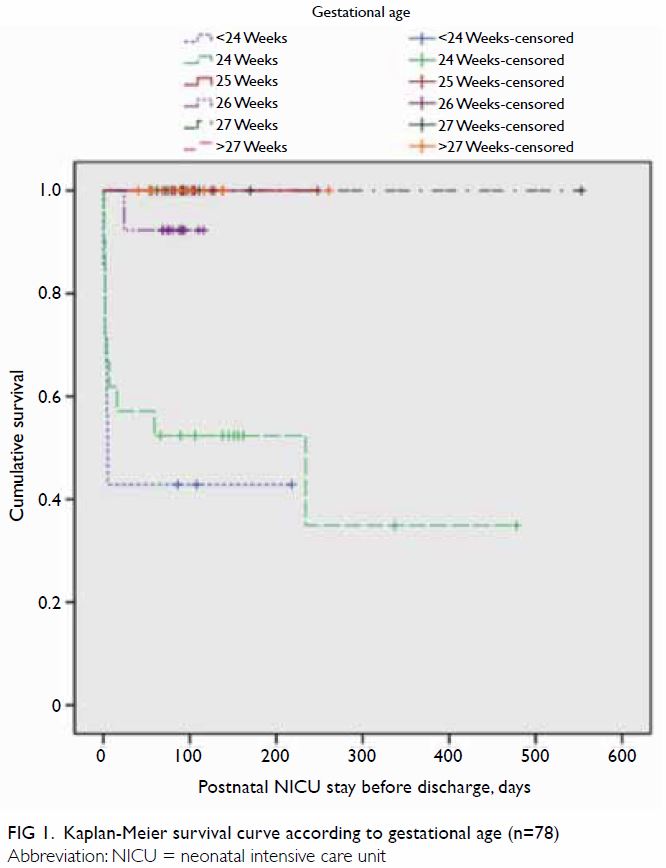
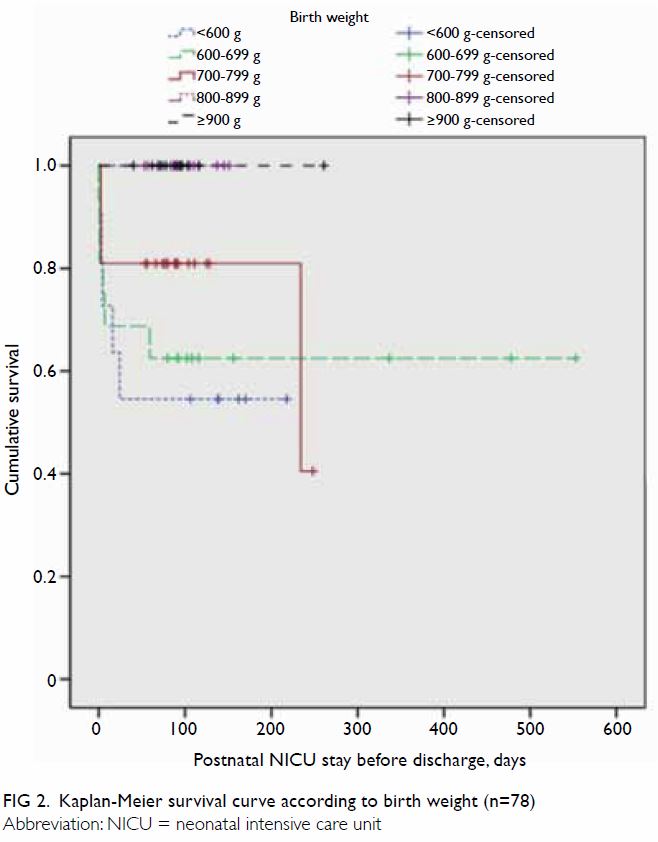
Survival curves were constructed for gestational
age (Fig 1) and birth weight (Fig 2). Median Kaplan-Meier survival estimates for
gestational ages <24, 24, and 26 weeks were 43%, 52%, and 92%,
respectively (no death records were available for other groups), during
the period of postnatal NICU stay before discharge; importantly, their
survival distributions were significantly different (χ2=31.1;
P<0.001). Median Kaplan-Meier survival estimates for birth weights
<600 g, 600 to 699 g, and 700 to 799 g were 55%, 63%, and 81%,
respectively (no death records were available for other groups); their
survival distributions were significantly different (χ2=13.9;
P=0.008) [Fig 2].
Discussion
Extremely low birth weight infants, especially
those born at periviable gestations of 22 to 23 weeks, exhibit
significantly higher mortality. In this cohort of ELBW infants, we
confirmed two independent factors associated with ELBW non-survival:
gestation age below 24 weeks and the presence of grade 3 or 4 IVH.
Although not specifically analysed in this series, congenital anomalies
did not appear to be a pertinent risk factor influencing survival among
ELBW infants. Among infants in the non-survival group, the median time
until death was 2.5 days after birth; in our series, no survival was
observed for ELBW infants whose birth occurred prior to 23.6 weeks or who
exhibited birth weight of <550 g. These local data are expected to be
useful in counselling pregnant women who are at risk for the delivery of
ELBW infants. Non-survival was associated with an increased aOR of 6.7 for
every 1-week decrease in gestation.
In addition to perinatal mortality, long-term
survival was also low. A previous report stated that first-year survival
was 15.5% for infants whose birth weight was <500 g.14 Infants with ELBW are more susceptible to all
complications of prematurity, both during the immediate neonatal period
and after discharge from the nursery. A study by the Eunice Kennedy
Shriver National Institute of Child Health and Human Development Neonatal
Research Network,15 undertaken to
relate other known risk factors with the likelihood of survival and
impairment, reported that 83% of infants born at 22 to 25 weeks’ gestation
received intensive care involving mechanical ventilation. Of the infants
whose outcomes were known at 18 to 22 months, 49% died, 61% died or had
profound impairment, and 73% died or had impairment.
Additional reports have suggested that other
factors should be considered, in combination with gestational age, when
determining the likelihood of favourable outcomes with intensive care.15 16 17 18 19 According to the data from a 2011 cohort study,
infants born at 23 to 25 weeks’ gestation who received antenatal exposure
to corticosteroids exhibited a lower rate of mortality and complications,
compared with infants who did not have such exposure.16 Recently, chorioamnionitis was linked to preterm
birth and neonatal infection. In a longitudinal observational study that
included 2390 extremely preterm infants (gestational age <27 weeks),
Pappas et al20 reported that
antenatal exposure to chorioamnionitis appeared to increase the odds of
cognitive impairment, as well as death/neurodevelopmental impairment.
Survival occurred in 79% of ELBW infants at our
centre. Extremely low birth weight survivors exhibited significant
morbidity. Our series showed that BPD, severe IVH, NEC, and ROP were
present in 81%, 20%, 26%, and 34% of survivors, respectively. Notably, the
survivors experienced long stays in the NICU and hospital prior to
discharge. Necrotising enterocolitis is particularly associated with long
stays in the NICU and hospital. The sequelae of low birth weight have been
well-studied, but less information is available regarding sequelae of
ELBW.21 Low birth weight is
generally closely associated with fetal and perinatal mortality and
morbidity, inhibited growth and cognitive development, and a risk of
chronic diseases later in life. At the population level, the proportion of
infants with low birth weight is an indicator of a multifaceted public
health problem that includes long-term maternal malnutrition, poor health,
hard work, and poor health care during pregnancy. On an individual basis,
low birth weight is an important predictor of newborn health and survival,
and is associated with a higher risk of infant and childhood mortality.21 In this regard, ELBW represents
the most severe subset of low birth weight outcomes.
Bronchopulmonary dysplasia, grade 3 or 4 IVH, and
ROP occurred in a significant proportion of the survivors. Our morbidity
data are comparable to those reported in multiple large studies.8 22 23 In recent years, ELBW infants have constituted more
than 97% of cases of BPD.24
Importantly, NEC is the most common intestinal emergency in preterm
infants.25 26 The routine use of antenatal steroids and surfactant
therapy has resulted in increased survival of infants with ELBW, thereby
increasing the survival rate in the group at the greatest risk.27
The incidence and severity of IVH are inversely
related to gestational age. Infants with ELBW are at particular risk for
IVH; however, the use of antenatal steroids decreases the incidence of
IVH. Prognosis is correlated with the grade of IVH28; up to 40% of infants with grade 3 IVH exhibit
significant cognitive impairment, and up to 90% of infants with grade 4
IVH exhibit major neurologic sequelae, requiring lifetime care. Notably, a
study of 1064 infants born at ≤28 weeks’ gestation found that, unless it
was accompanied or followed by a white matter lesion, low-grade IVH was
associated with a modest to nonexistent risk of adverse developmental
outcomes during infancy.29
The outcomes of ELBW infants are evolving as
therapy and supportive care continue to change.1
Clinical focus should be placed on the prevention of premature births, as
well as equipping NICU staff and facilities with the necessary skills and
resources, respectively, to implement evidence-based interventions that
improve the survival of ELBW infants. Efforts to minimise injury, preserve
growth, and identify interventions focused on antioxidant and
anti-inflammatory pathways are currently being evaluated. Thus, treatment
and prevention of long-term deficits must be developed in the context of
an evolving target. Ensuring health in cases of extreme prematurity (≤23
weeks’ gestation) is extremely difficult.2
Most centres do not have minimum birth weight criteria for resuscitation;
often, a “trial of life” may be discussed with the parents before the
birth of the infant so that the infant can be resuscitated and evaluated
for viability after birth. Viability is the term frequently used to
indicate the potential for a fetus to be liveborn and capable of surviving
to a specified endpoint (eg, a designated time, attainment of a certain
age or landmark event, admission to the NICU, or discharge from the
hospital). Many institutions have generated centre-specific data regarding
the probability of survival to aid in discussions with families prior to
delivery. Discussions regarding the withdrawal of treatment or support are
often necessary when the family and medical team agree that the
continuation of medical treatment is not in the infant’s best interests.
Naturally, these circumstances involve numerous ethical, moral, and legal
issues; they may generate more questions than answers. Therefore, each
centre caring for ELBW infants must carefully follow and analyse their
particular survival statistics, in order to better inform and guide
parents concerning the outcomes and prognoses of these periviable infants.
Author contributions
Concept or design: KL Hon, S Liu, JCY Chow.
Acquisition of data: KL Hon, S Liu, JCY Chow.
Analysis and interpretation of data: KL Hon, KYC Tsang.
Drafting of the article: KL Hon, Y Cheng, AKC Leung.
Critical revision for important intellectual content: All authors.
Acquisition of data: KL Hon, S Liu, JCY Chow.
Analysis and interpretation of data: KL Hon, KYC Tsang.
Drafting of the article: KL Hon, Y Cheng, AKC Leung.
Critical revision for important intellectual content: All authors.
Declaration
As an editor of the journal, KL Hon was not
involved in the peer review of the article. All authors have disclosed no
conflicts of interest. All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethics approval for this study was obtained from
the Clinical Research Ethics Committee of The Chinese University of Hong
Kong.
References
1. Glass HC, Costarino AT, Stayer SA, Brett
CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth
Analg 2015;120:1337-51. Crossref
2. Blencowe H, Cousens S, Chou D, et al.
Born too soon: the global epidemiology of 15 million preterm births.
Reprod Health 2013;(10 Suppl 1):S2. Crossref
3. Anderson JG, Baer RJ, Partridge JC, et
al. Survival and major morbidity of extremely preterm infants: a
population-based study. Pediatrics 2016;138:e20154434. Crossref
4. García-Muñoz Rodrigo F, García-Alix
Pérez A, García Hernández JA, Figueras Aloy J; Grupo SEN1500. Morbidity
and mortality in newborns at the limit of viability in Spain: a
population-based study. [in Spanish]. An Pediatr (Barc) 2014;80:348-56. Crossref
5. Crane JM, Magee LA, Lee T, et al.
Maternal and perinatal outcomes of pregnancies delivered at 23 weeks’
gestation. J Obstet Gynaecol Can 2015;37:214-24. Crossref
6. Jarjour IT. Neurodevelopmental outcome
after extreme prematurity: a review of the literature. Pediatr Neurol
2015;52:143-52. Crossref
7. Partridge JC, Robertson KR, Rogers EE,
Landman GO, Allen AJ, Caughey AB. Resuscitation of neonates at 23 weeks’
gestational age: a cost-effectiveness analysis. J Matern Fetal Neonatal
Med 2015;28:121-30. Crossref
8. Tyson JE, Younes N, Verter J, Wright LL.
Viability, morbidity, and resource use among newborns of 501- to 800-g
birth weight. National Institute of Child Health and Human Development
Neonatal Research Network. JAMA 1996;276:1645-51. Crossref
9. Berger TM, Bernet V, El Aama S, et al.
Perinatal care at the limit of viability between 22 and 26 completed weeks
of gestation in Switzerland. 2011 revision of the Swiss recommendations.
Swiss Med Wkly 2011;141:w13280. Crossref
10. Obstetric care consensus No. 4:
periviable birth [editorial]. Obstet Gynecol 2016;127:e157-69. Crossref
11. Obstetric care consensus No. 4
summary: periviable birth [editorial]. Obstet Gynecol 2016;127:1184-6. Crossref
12. American College of Obstetricians and
Gynecologists, Society for Maternal-Fetal Medicine. Obstetric care
consensus No. 6: periviable birth. Obstet Gynecol 2017;130:e187-99. Crossref
13. Ishii N, Kono Y, Yonemoto N, et al.
Outcomes of infants born at 22 and 23 weeks’ gestation. Pediatrics
2013;132:62-71. Crossref
14. MacDorman MF, Hoyert DL, Mathews TJ.
Recent declines in infant mortality in the United States, 2005-2011. NCHS
Data Brief 2013;120:1-8.
15. Tyson JE, Parikh NA, Langer J, Green
C, Higgins RD, National Institute of Child Health and Human Development
Neonatal Research Network. Intensive care for extreme prematurity—moving
beyond gestational age. N Engl J Med 2008;358:1672-81. Crossref
16. Carlo WA, McDonald SA, Fanaroff AA, et
al. Association of antenatal corticosteroids with mortality and
neurodevelopmental outcomes among infants born at 22 to 25 weeks’
gestation. JAMA 2011;306:2348-58. Crossref
17. Lee HC, Green C, Hintz SR, et al.
Prediction of death for extremely premature infants in a population-based
cohort. Pediatrics 2010;126:e644-50. Crossref
18. Younge N, Goldstein RF, Bann CM, et
al. Survival and neurodevelopmental outcomes among periviable infants. N
Engl J Med 2017;376:617-28. Crossref
19. Patel RM, Kandefer S, Walsh MC, et al.
Causes and timing of death in extremely premature infants from 2000
through 2011. N Engl J Med 2015;372:331-40. Crossref
20. Pappas A, Kendrick DE, Shankaran S, et
al. Chorioamnionitis and early childhood outcomes among extremely
low-gestational-age neonates. JAMA Pediatr 2014;168:137-47. Crossref
21. Stevens-Simon C, Orleans M.
Low-birthweight prevention programs: the enigma of failure. Birth
1999;26:184-91. Crossref
22. EXPRESS Group, Fellman V,
Hellström-Westas L, et al. One-year survival of extremely preterm infants
after active perinatal care in Sweden. JAMA 2009;301:2225-33. Crossref
23. Jobe AH. The new bronchopulmonary
dysplasia. Curr Opin Pediatr 2011;23:167-72. Crossref
24. Bhandari A, McGrath-Morrow S.
Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia.
Semin Perinatol 2013;37:132-7. Crossref
25. Gordon PV, Swanson JR. Necrotizing
enterocolitis is one disease with many origins and potential means of
prevention. Pathophysiology 2014;21:13-9. Crossref
26. Torrazza RM, Li N, Neu J. Decoding the
enigma of necrotizing enterocolitis in premature infants. Pathophysiology
2014;21:21-7. Crossref
27. Berman L, Moss RL. Necrotizing
enterocolitis: an update. Semin Fetal Neonatal Med 2011;16:145-50. Crossref
28. Merhar SL, Tabangin ME, Meinzen-Derr
J, Schibler KR. Grade and laterality of intraventricular haemorrhage to
predict 18-22 month neurodevelopmental outcomes in extremely low
birthweight infants. Acta Paediatr 2012;101:414-8. Crossref
29. O’Shea TM, Allred EN, Kuban KC, et al.
Intraventricular hemorrhage and developmental outcomes at 24 months of age
in extremely preterm infants. J Child Neurol 2012;27:22-9. Crossref