Hong
Kong Med J 2018 Oct;24(5):444–50 | Epub 28 Sep 2018
DOI: 10.12809/hkmj177111
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Effect of paternal age on semen parameters and live
birth rate of in-vitro fertilisation treatment: a retrospective analysis
SF Lai, MB, BS, FHKAM (Obstetrics and Gynaecology)1,2;
Raymond HW Li, MB, BS, FHKAM (Obstetrics and Gynaecology)1,2;
William SB Yeung, PhD1,2; Ernest HY Ng, MD, FHKAM (Obstetrics
and Gynaecology)1,2
1 Department of Obstetrics and
Gynaecology, The University of Hong Kong, Pokfulam, Hong Kong
2 Department of Obstetrics and
Gynaecology, Kwong Wah Hospital, Yau Ma Tei, Hong Kong
Corresponding author: Dr SF Lai (lsf087@ha.org.hk)
Abstract
Objective: To determine the
effect of paternal age on semen parameters and the live birth rate from
in-vitro fertilisation (IVF) treatment.
Methods: We performed a
retrospective cohort study of couples undergoing a first IVF cycle
between 2004 and 2014 in a tertiary assisted reproduction centre in Hong
Kong.
Results: We analysed 3549 cases.
Paternal age ≥40 years was negatively correlated with semen volume,
progressive motility, total motility and total normal motile count
(P<0.005) and positively correlated with sperm concentration
(P<0.001). There was no correlation with sperm count, normal
morphology, or total motile count. Subgroup analyses in Chinese men only
and in men with normal versus abnormal semen parameters showed the same
correlations. Paternal age was positively associated with maternal age
(P<0.001) and miscarriage (P=0.006), and negatively associated with
ongoing pregnancy and live birth (P<0.001). Logistic regression
showed that maternal age, total number of oocytes retrieved, and number
of embryos transferred were significant factors which independently
predicted the likelihood of live birth from IVF (all P<0.001).
Conclusion: Paternal age was
negatively correlated with some semen parameters, which showed a
significant decline after age 40 years. However, paternal age is not
predictive of the live birth from IVF treatment.
New knowledge added by this study
- Paternal age negatively correlates with some semen parameters.
- Paternal age is not an independent predictor of the likelihood of a live birth from in-vitro fertilisation treatment, after controlling for the maternal age, the number of oocytes retrieved, and the number of embryos transferred.
- Infertile couples can be counselled that although there is a decline in some semen parameters with paternal age ≥40, the live birth rate of in-vitro fertilisation treatment depends primarily on maternal age, number of oocytes retrieved and number of embryos transferred, but not on paternal age.
Introduction
In recent years, marriages and pregnancies are
occurring later and later in life, which was confirmed in a recent
large-scale analysis conducted in the US.1
Local statistics in Hong Kong support this trend, as the median age at
first marriage for both sexes has risen over the past 20 years.2 3 Extensive
data are available on the adverse effects of increasing maternal age on
in-vitro fertilisation (IVF) outcomes4
5 6
but little information was on the adverse effects of increasing paternal
age.
Two systematic reviews have looked at the effect of
paternal age on semen parameters and assisted reproduction outcomes.7 8 Dain et al7 demonstrated that paternal age did
not affect pregnancy, miscarriage, and live birth rates. The authors also
revealed that semen volume decreased with paternal age, but sperm
motility, concentration and morphology did not. Later, in another
systematic review8 of 12 studies on oocyte donor cycles, the same group
showed that advancing paternal age is not associated with adverse
outcomes, including pregnancy and live birth rates. They also showed that,
except for volume and possibly motility, sperm characteristics such as
concentration and morphology did not alter with age. However, most papers
studied did not report the live birth rate, which is the most important
clinical outcome for the patients involved.
The existing information regarding the adverse
effects of increasing paternal age on semen parameters and IVF outcomes is
mostly from Western populations.7 8 9
Therefore, we conducted this study to determine the effect of paternal age
on semen parameters and on the live birth rate of IVF treatment in the
Hong Kong population, which is mainly of Chinese ethnicity.
Methods
Subject inclusion and exclusion
We retrieved all first IVF cycles carried out
between 2004 and 2014 at the Centre of Assisted Reproduction and
Embryology, The University of Hong Kong–Queen Mary Hospital from the
Assisted Reproduction Clinical Database of the Centre. Only the first IVF
cycles using ejaculated semen were included for analysis, to avoid any
potential bias. Cases requiring preimplantation genetic diagnosis or using
donor sperm or surgically retrieved sperm were excluded from the study.
Ovarian stimulation
Details of the treatment protocol has been
previously described.10 In brief,
women received ovarian stimulation following either the long
gonadotropin-releasing hormone (GnRH) agonist or antagonist protocol. A
baseline ultrasound was performed on day 2 to 3 of the cycle to exclude
pre-existing ovarian cysts. Serum oestradiol (E2) concentration was
measured to confirm the basal level. In the long GnRH agonist protocol,
intranasal buserelin acetate (Suprecur; Sanofi, France) was started on day
21 of the preceding cycle at 150 μg 4 times a day and continued until the
day of ovulation trigger. In the GnRH antagonist protocol, subcutaneous
injection of ganirelix 0.25 mg (Orgalutran; Organon, The Netherlands) or
cetrorelix 0.25 mg (Cetrotide; Merck Serono, Germany) was started on day 6
after gonadotropin injection until the day of ovulation trigger. Human
menopausal gonadotropin (hMG, Menogon; Ferring, Switzerland) or
recombinant follicle-stimulating hormone (Gonal-f [Merck Serono, Germany]
or Puregon [Organon, The Netherlands]) injection was started at a dosage
as determined by the antral follicle count. Ovarian response was monitored
by transvaginal ultrasound. Human chorionic gonadotropin (hCG; Profasi [10
000 units; MSD, US] or Ovidrel [250 μg; Merck Serono, Switzerland]) was
given to trigger the final oocyte maturation when there were at least
three follicles ≥16 mm in diameter, of which one follicle was ≥18 mm.
Triptorelin 0.2 mg (Decapeptyl; Ferring, Switzerland) was used to replace
hCG if serum E2 concentration was >25 000 pmol/L, if more than 15
follicles were ≥14 mm in diameter on the day of hCG administration, or
when the patient had evidence of ovarian hyperstimulation syndrome.
Transvaginal ultrasound-guided oocyte retrieval was performed 34 to 36
hours after the ovulatory dose of hCG or triptorelin injection.
Instruction on abstinence of sex for 2 to 7 days was given prior to
submission of any semen sample. Fresh ejaculated semen samples were
evaluated according to World Health Organization guidelines11 12 and the
same protocol (including the strict criteria for assessing sperm
morphology) was adopted throughout the period covered in this study.
Intracytoplasmic sperm injection (ICSI) was
performed when the normal morphology of a recent semen sample was <3%
or the total motile sperm count after sperm preparation was <0.2
million. The same criteria for ICSI versus conventional insemination was
adopted throughout the study period. Fertilisation was assessed 16 to 20
hours after insemination. Embryo transfer was performed under ultrasound
guidance 2 days after retrieval. Up to three embryos were transferred
before 2006 and a maximum of two embryos were transferred after 2006.
Luteal phase was supported with vaginal progesterone pessaries (Cyclogest
400 mg twice a day [Cox Pharmaceuticals, Barnstaple, United Kingdom] or
Endometrin 100 mg twice a day [Ferring, Switzerland]) or intramuscular
injection of hCG 1500 units every 6 days for two doses. Urine pregnancy
test was performed 16 days after embryo transfer. Where the pregnancy test
was positive, ultrasound scans were performed at 6 and 8 weeks of
gestation to confirm fetal viability and number.
Total motile count (TMC) was defined as total sperm
count with progressive motility (total count × % with progressive
motility). Total normal motile count (TNMC) was defined as sperm count
with progressive motility and normal morphology (total count × % with
progressive motility × % with normal morphology).
Pregnancy was defined by a positive urine or serum
hCG test. Miscarriage was defined as a pregnancy which became non-viable
before 24 weeks of gestation; this included biochemical pregnancies and
miscarriages before 24 weeks. An ongoing pregnancy was defined as presence
of intrauterine sac(s) with positive fetal heart pulsation at 8 weeks of
gestation. A live birth was defined as the complete expulsion or
extraction from a woman of a conceptus after 24 completed weeks of
gestational age which, after such separation, showed evidence of life.
Statistical analyses
The key outcomes of this study were live birth and
semen parameters including volume, concentration, count, progressive
motility, total motility, normal morphology, TMC, and TNMC.
Statistical analysis was performed using SPSS
Windows version 24.0 (IBM Corp, Armonk [NY], US) and MedCalc (Version 12,
Belgium). Paternal age of our cohort was not normally distributed as shown
by Kolmogorov-Smirnov test (P<0.001). Therefore, non-parametric tests
were used for analysis. The Mann-Whitney U or Kruskal-Wallis H
tests were used to compare continuous variables among groups. Spearman’s
correlation was used to determine correlations between continuous
variables. Chi squared test was used for analysis of categorical
variables. Logistic regression analysis was used to examine factors
predicting live birth, first by univariate analysis of individual
variables, and those factors showing significance were subsequently
entered into multivariate analysis. A two-tailed value of P<0.05 was
considered statistically significant.
Results
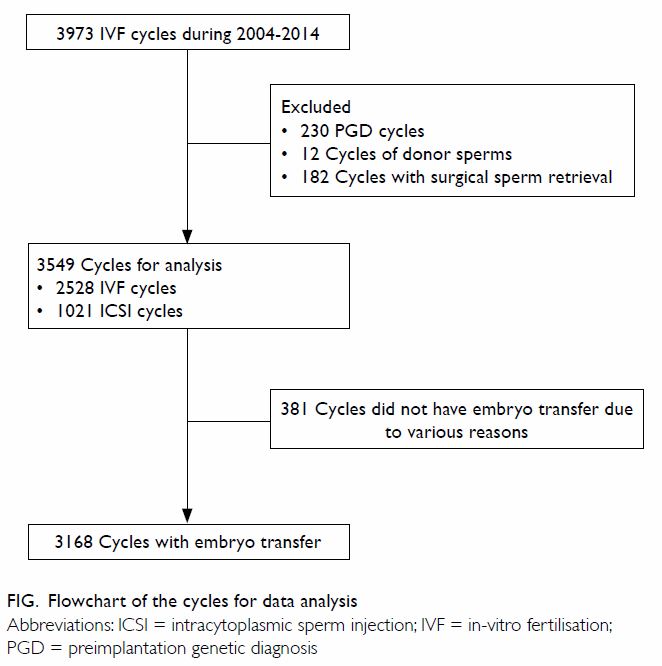
A total of 3973 first IVF cycles were performed
during the study period (Fig). Of these, 424 cases were not selected (230
with a preimplantation genetic diagnosis, 12 using donor sperm, and 182
using surgically retrieved sperm). In ICSI cases, if the sperm
concentration was <3 million/mL, morphology would not be evaluated
(n=207). Sperm concentration, count and/or normal morphology could not be
evaluated in the fresh semen samples of 84 men with severe male factors.
Hence, TNMC was only available in 3258 cases.
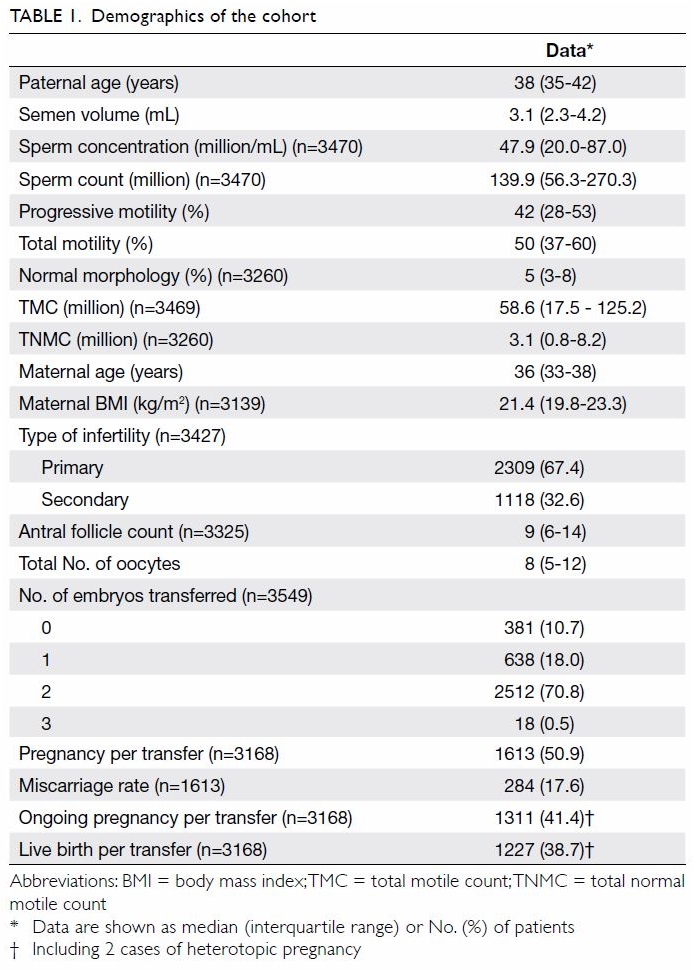
Cohort demographics are shown in Table
1. The causes of infertility were as follows: tuboperitoneal factor
(n=620; 17.5%), endometriosis (n=294; 8.3%), male factor (n=1483; 41.8%),
unexplained (n=603; 17.0%), and mixed factors (n=549; 15.5%). Among those
analysed, conventional insemination was performed in 2528 (71.2%) cycles
and ICSI was required in 1021 (28.8%) cycles. There were 381 (10.7%) cases
which did not have fresh embryo transfer for various reasons, leaving 3168
cases with fresh embryo transfer. There were 1613 pregnancies, giving a
pregnancy rate of 50.9% per transfer. Of these pregnancies, 241 (14.9%)
miscarried before 8 weeks of gestation. An additional 43 (2.7%) cases
miscarried after 8 weeks of gestation. Four (0.2%) women terminated their
pregnancy due to congenital abnormalities (n=3) or for social reasons
(n=1). Five (0.3%) pregnancies ended in intrauterine death.
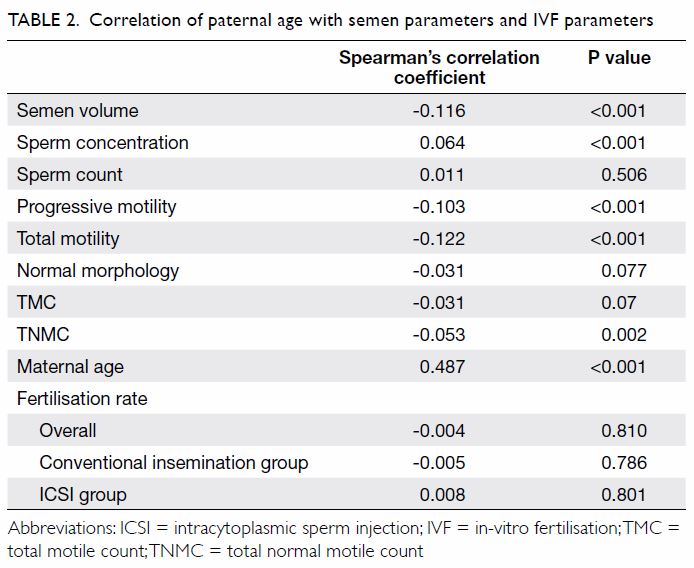
Paternal age was negatively correlated with the
semen volume, progressive motility, total motility, and TNMC (P<0.005),
as shown in Table 2. Paternal age was positively correlated with
sperm concentration (P<0.001). Paternal age was not correlated with
sperm count, normal morphology, or TMC.
We divided paternal age into two groups (<40
years and ≥40 years) and analysed the difference in semen parameters using
the Mann-Whitney U test. Results showed a significant decline in
semen volume, progressive motility, total motility and TNMC (P<0.005),
but a significant increase in sperm concentration for age ≥40 years
(P<0.001). There was no significant difference in sperm count, normal
morphology, TMC or fertilisation rate between the age-groups (P>0.05).
These findings support the correlations shown in Table 2.
The analyses were repeated in the subset including
Chinese men only (n=3394, 95.6%), and showed similar correlations between
age and semen parameters and IVF outcomes.
When men were grouped into those with normal and
abnormal semen parameters, the negative correlation of paternal age with
semen volume, progressive motility, total motility and TNMC and the
positive correlation with sperm concentration remained the same as that
for the whole cohort.
Paternal age was not significantly associated with
the fertilisation rate in the conventional IVF group (P=0.786), in the
ICSI group (P=0.801), or overall (P=0.810) as shown in Table
2. Paternal age was positively correlated with maternal age
(Spearman’s correlation coefficient: 0.487; P<0.001). Paternal age was
significantly lower in those who attained pregnancy, ongoing pregnancy and
live birth compared with those who did not (P<0.001; Mann-Whitney U
test). In contrast, paternal age was significantly higher in cases that
ended in miscarriage than in those that achieved live birth (P=0.006;
Mann-Whitney U test).
The clinical and demographic characteristics of
patients with or without a live birth in the first IVF cycles are compared
in Table 3. Women who had a live birth were
significantly younger (median 35.0 vs 36.0 years), had a younger partner
(median 37.0 vs 38.0 years), higher antral follicle count (median 11 vs
8), more oocytes retrieved (median 9 vs 7), and had double embryo transfer
(86.5% vs 74.6%). There was no statistically significant difference in the
maternal BMI nor in TNMC between these two groups. The analysis showed
similar findings between pregnant versus non-pregnant groups and between
those with ongoing pregnancy and those without.
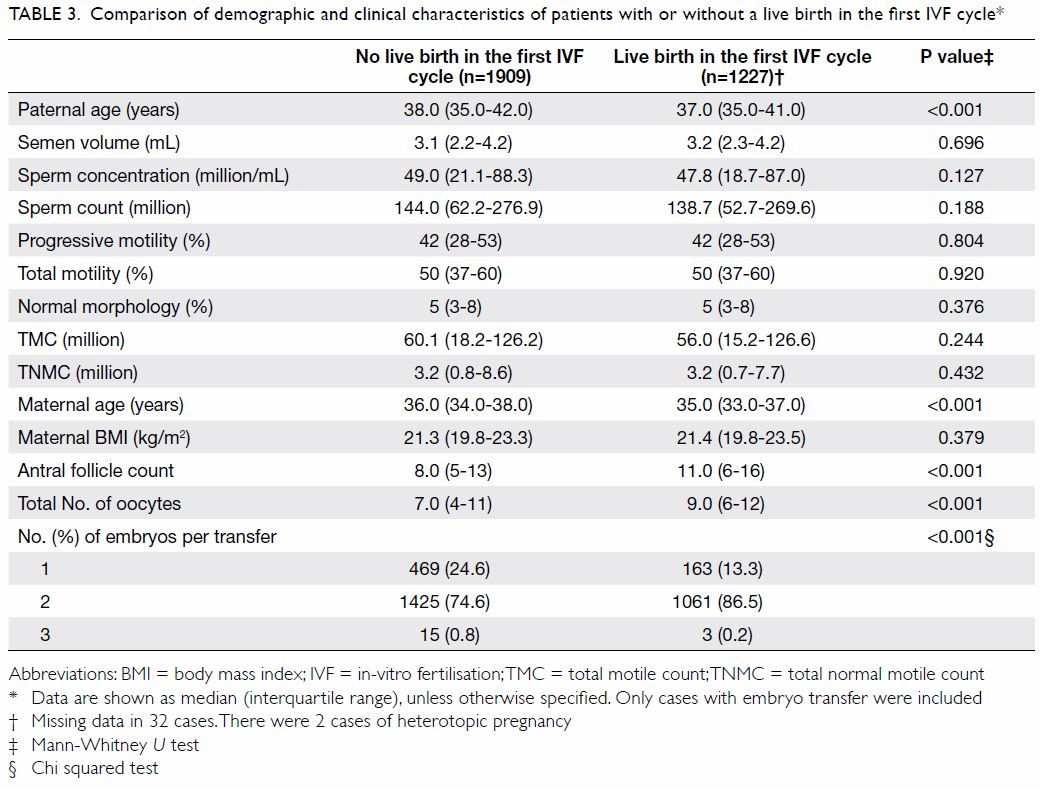
Table 3. Comparison of demographic and clinical characteristics of patients with or without a live birth in the first IVF cycle
Logistic regression on individual variables by
univariate analysis was used to analyse the prediction on the live birth
in the first IVF cycle. Only paternal age, maternal age, total number of
oocytes retrieved, and number of embryos transferred were found to be
significant predictors. On combining these variables in a multivariate
analysis, maternal age, total number of oocytes retrieved and number of
embryos transferred, but not paternal age, were the significant factors
which independently predicted the likelihood of live birth in the first
IVF cycles after controlling for the others (P<0.001), as shown in Table 4.
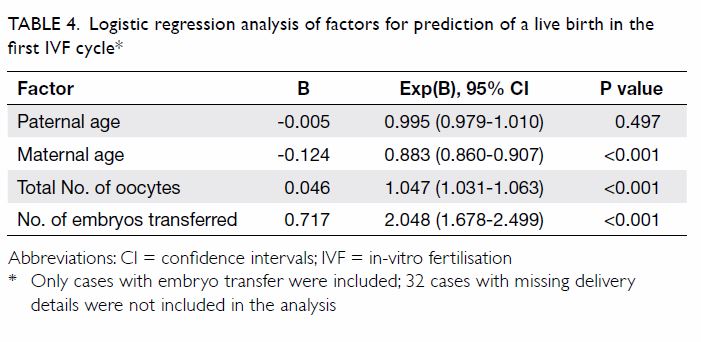
Table 4. Logistic regression analysis of factors for prediction of a live birth in the first IVF cycle
Discussion
This is the first large-scale study on the effect
of paternal age on semen parameters and IVF outcomes in our region, with
the majority of patients being Chinese. Nearly 4000 first IVF cycles were
analysed. Most previous studies have reported on oocyte donation models.7 8
Our cohort is the largest sample size in the literature based on
autologous oocytes and fresh semen samples, with live birth as one of the
key outcomes. Logistic regression analysis was employed to differentiate
the factors affecting live birth in the first IVF cycles.
Our results show that paternal age was negatively
correlated with semen volume, progressive motility, total motility, TNMC
but not sperm count, normal morphology nor TMC. The positive correlation
between paternal age and sperm concentration might be explained by the
decrease in semen volume with age, resulting in an apparent raised sperm
concentration. An increase in sperm concentration was also found in a
recent study conducted on a similar scale.13
Other studies have mostly reported either no significant change14 15 or a
decrease in sperm concentration.16
Although increasing paternal age was not associated with any significant
change in normal morphology, the associated significant reduction in
progressive motility contributed to an overall negative impact on TNMC.
These composite parameters represent the population of sperm relevant to
natural fertility. The decline in overall sperm quality with paternal age
is likely due to the decline in testicular function.17 The number of Leydig cells,18
Sertoli cells19 and germ cells
decreases with paternal age.20
Despite the overall decrease in various semen parameters with age, we
found that the fertilisation rate was not significantly reduced with
increasing age; this is compatible with most previous studies.7 8
Various age cut-offs have been suggested in the
literature for defining advanced paternal age but the most frequently used
cut-off is age 40 years at the time of conception.21 Both the American Society for Reproductive Medicine
and the British Andrology Society recommend that the sperm donor should be
age <40 years.22 23 Our results show that there is an inverse
relationship in semen parameters with paternal age using the cut-off of
age 40 years. Multiple studies have shown that advanced paternal age is
associated with a significant increase in DNA fragmentation24 which in turn is associated with higher rate of IVF
failure.25 As men age, the sperm
chromatin integrity weakens and sperm DNA fragmentation increases.26 Fecundability has been shown to decrease with
increased abnormal sperm chromatin percentage.27
The molecular ageing process has been shown to induce changes in
reproductive hormone profiles, decreasing in sperm quality parameters, and
contributing to male infertility.28
On univariate analysis, paternal age was associated
inversely with live birth. However, paternal age was also positively
correlated with maternal age, meaning that younger men usually have
younger partners and vice versa. Logistic regression analysis indicated
that maternal age but not paternal age was an independent predictor of the
likelihood of live birth, in contrast to some previous studies.29 30 Paternal
age is likely a surrogate marker of maternal age and does not have a
direct effect on IVF outcomes.
The analyses were repeated in the subset including
Chinese men only, revealing similar findings on semen parameters and IVF
outcomes. However, we are cautious of the interpretation of this finding
as the majority of our cohort was Chinese.
Although different ovarian stimulation protocols
and different medications were used throughout the study period,
meta-analyses have shown that there are no differences in the live birth
rates between the long GnRH agonist protocol and the GnRH antagonist
protocol, between the use of hMG and recombinant follicle-stimulating
hormone, nor among the different types of progestogens used for luteal
phase support in terms of pregnancy outcomes.31
32 33
34
Our study has some limitations. We followed the
delivery details of most patients; only 32 patients were lost to
follow-up. However, we do not have the information on any congenital
abnormalities of the live-born or long-term data of the babies born via
IVF/ICSI. The sample size of the study is also too small to evaluate these
outcomes. Hence, we cannot evaluate the long-term effects of advanced
paternal age on their children, if any. However, multiple studies have
shown that advanced paternal age is associated with increased incidence of
schizophrenia,35 36 autism,37 38 and genetic conditions such as
achondroplasia and Apert’s syndrome, despite young maternal age in their
children.39 40 Although the semen parameters analysed were based on
the one-off semen sample provided for insemination, semen parameters are
known to vary with time. We do not have data on paternal body weight,
smoking and drinking habits, medical condition, hormone levels or exact
time of abstinence. The range of paternal age was also relatively narrow
in this cohort.
The present study examined patients who had
undergone IVF treatment. This study represents only one subset of
infertile patients. Patients undergoing other forms of fertility treatment
such as intrauterine insemination would be a valuable subject for further
studies.
Conclusion
Paternal age is negatively correlated with some
semen parameters, which show a significant decline after 40 years.
Maternal age, the number of oocytes retrieved, and the number of embryos
transferred, but not paternal age are predictive of live birth from IVF
treatment.
Author contributions
Concept or design: SF Lai, RHW Li, EHY Ng.
Acquisition of data: SF Lai, RHW Li.
Analysis or interpretation of data: SF Lai, RHW Li.
Drafting of the article: SF Lai.
Critical revision for important intellectual content: RHW Li, WSB Yeung, EHY Ng.
Acquisition of data: SF Lai, RHW Li.
Analysis or interpretation of data: SF Lai, RHW Li.
Drafting of the article: SF Lai.
Critical revision for important intellectual content: RHW Li, WSB Yeung, EHY Ng.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Declaration
All authors have disclosed no conflicts of
interest. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity.
Ethical approval
Ethics approval was obtained from the Institutional
Review Board of the University of Hong Kong/Hospital Authority Hong Kong
West Cluster. Because this retrospective study was carried out using
existing patient data in an anonymous manner, the requirement for written
informed consent from individual patients was waived.
References
1. Khandwala YS, Zhang CA, Lu Y, Eisenberg
ML. The age of fathers in the USA is rising: an analysis of 168 867 480
births from 1972 to 2015. Hum Reprod 2017;32:2110-16. Crossref
2. Census and Statistics Department, Hong
Kong SAR Government. Hong Kong monthly digest of statistics (January
2015). 15 Jan 2015. Available from:
https://www.censtatd.gov.hk/fd.jsp?file=B10100022015MM01B0100.pdf&product_id=B1010002&lang=1.
Accessed 25 Oct 2017.
3. Census and Statistics Department, Hong
Kong SAR Government. Hong Kong monthly digest of statistics (December
2015). 15 Dec 2015. Available from:
https://www.censtatd.gov.hk/fd.jsp?file=B10100022015MM12B0100.pdf&product_id=B1010002&lang=1.
Accessed 25 Oct 2017.
4. McLernon DJ, Steyerberg EW, Te Velde ER,
Lee AJ, Bhattacharya S. Predicting the chances of a live birth after one
or more complete cycles of in vitro fertilisation: population based study
of linked cycle data from 113 873 women. BMJ 2016;355:i5735. Crossref
5. Toner JP, Coddington CC, Doody K, et al.
Society for Assisted Reproductive Technology and assisted reproductive
technology in the United States: a 2016 update. Fertil Steril
2016;106:541-6. Crossref
6. Bhattacharya S, Maheshwari A, Mollison
J. Factors associated with failed treatment: an analysis of 121 744 women
embarking on their first IVF cycles. PLoS One 2013;8:e82249. Crossref
7. Dain L, Auslander R, Dirnfeld M. The
effect of paternal age on assisted reproduction outcome. Fertil Steril
2011;95:1-8. Crossref
8. Sagi-Dain L, Sagi S, Dirnfeld M. Effect
of paternal age on reproductive outcomes in oocyte donation model: a
systematic review. Fertil Steril 2015;104:857-65.e1. Crossref
9. Sagi-Dain L, Sagi S, Dirnfeld M. The
effect of paternal age on oocyte donation outcomes. Obstet Gynecol Surv
2016;71:301-6. Crossref
10. Li HW, Lee VC, Lau EY, Yeung WS, Ho
PC, Ng EH. Role of baseline antral follicle count and anti-Mullerian
hormone in prediction of cumulative live birth in the first in vitro
fertilisation cycle: a retrospective cohort analysis. PLoS One
2013;8:e61095. Crossref
11. World Health Organization. WHO
Laboratory Manual for the Examination of Human Semen and Sperm-cervical
Mucus Interaction. 4th ed. Cambridge: Cambridge University Press; 1999.
12. World Health Organization. WHO
Laboratory Manual for the Examination and Processing of Human Semen. 5th
ed. Geneva: World Health Organization; 2010.
13. Begueria R, Garcia D, Obradors A,
Poisot F, Vassena R, Vernaeve V. Paternal age and assisted reproductive
outcomes in ICSI donor oocytes: is there an effect of older fathers? Hum
Reprod 2014;29:2114-22. Crossref
14. Duran EH, Dowling-Lacey D, Bocca S,
Stadtmauer L, Oehninger S. Impact of male age on the outcome of assisted
reproductive technology cycles using donor oocytes. Reprod Biomed Online
2010;20:848-56. Crossref
15. Whitcomb BW, Turzanski-Fortner R,
Richter KS, et al. Contribution of male age to outcomes in assisted
reproductive technologies. Fertil Steril 2011;95:147-51. Crossref
16. Luna M, Finkler E, Barritt J, et al.
Paternal age and assisted reproductive technology outcome in ovum
recipients. Fertil Steril 2009;92:1772-5. Crossref
17. Kovac JR, Addai J, Smith RP, Coward
RM, Lamb DJ, Lipshultz Li. The effects of advanced paternal age on
fertility. Asian J Androl 2013;15:723-8. Crossref
18. Neaves WB, Johnson L, Petty CS.
Age-related change in numbers of other interstitial cells in testes of
adult men: evidence bearing on the fate of Leydig cells lost with
increasing age. Biol Reprod 1985;33:259-69.
19. Johnson L, Zane RS, Petty CS, Neaves
WB. Quantification of the human Sertoli cell population: its distribution,
relation to germ cell numbers, and age related decline. Biol Reprod
1984;31:785-95. Crossref
20. Kuhnert B, Nieschlag E. Reproductive
functions of the ageing male. Hum Reprod Update 2004;10;327-39. Crossref
21. Toriello HV, Meck JM, Professional
Practice and Guidelines Committee. Statement on guidance for genetic
counseling in advanced paternal age. Genet Med 2008;10:457-60. Crossref
22. American Society for Reproductive
Medicine. Practice Committee guidelines. Sperm donation. Available from:
https://www.asrm.org/Guidelines/. Accessed 25 Oct 2017.
23. British Andrology Society. Policy and
guidelines. Sperm donation. Available from: http://www.britishandrology.
org.uk/resources/policy-guidelines/. Accessed 25 Oct 2017.
24. Moskovtsev SI, Willis J, Mullen JB.
Age-related decline in sperm deoxyribonucleic acid integrity in patients
evaluated for male infertility. Fertil Steril 2006;85:496-9. Crossref
25. López G, Lafuente R, Checa MA,
Carreras R, Brassesco M. Diagnostic value of sperm DNA fragmentation and
sperm high-magnification for predicting outcome of assisted reproduction
treatment. Asian J Androl 2013;15:790-4. Crossref
26. Singh NP, Muller CH, Berger RE.
Effects of age on DNA double-strand breaks and apoptosis in human sperm.
Fertil Steril 2003;80:1420-30. Crossref
27. Spanò M, Bonde JP, Hjøllund HI,
Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human
fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril
2000;73:43-50. Crossref
28. Sharma R, Agarwai A, Rohra VK, Assidi
M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm
quality, reproductive outcome and associated epigenetic risks to
offspring. Reprod Biol Endocrinol 2015;13:35. Crossref
29. Frattarelli JL, Miller KA, Miller BT,
Elkind-Hirsch K, Scott RT Jr. Male age negatively impacts embryo
development and reproductive outcome in donor oocyte assisted reproductive
technology cycles. Fertil Steril 2008;90:97-103. Crossref
30. Robertshaw I, Khoury J, Abdallah ME,
Warikoo P, Hofmann GE. The effect of paternal age on outcome in assisted
reproductive technology using the ovum donation model. Reprod Sci
2014;21:590-3. Crossref
31. Lambalk CB, Banga FR, Huirne JA, et
al. GnRH antagonist versus long agonist protocols in IVF: a systematic
review and meta-analysis accounting for patient type. Hum Reprod Update
2017;23:560-79. Crossref
32. Al-Inany HG, Youssef MA, Ayeleke RO,
Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists
for assisted reproductive technology. Cochrane Database Syst Rev
2016;(4):CD001750. Crossref
33. van Wely M, Kwan I, Burt AL, et al.
Recombinant versus urinary gonadotrophin for ovarian stimulation in
assisted reproductive technology cycles. Cochrane Database Syst Rev
2011;(2):CD005354. Crossref
34. Polyzos NP, Messini CI, Papanikolaou
EG, et al. Vaginal progesterone gel for luteal phase support in IVF/ICSI
cycles: a meta-analysis. Fertil Steril 2010;94:2083-7. Crossref
35. Malaspina D, Harlap S, Fennig S, et
al. Advancing paternal age and the risk of schizophrenia. Arch Gen
Psychiatry 2001;58:361-7. Crossref
36. Dalman C, Allebeck P. Paternal age and
schizophrenia: further support for an association. Am J Psychiatry
2002;159:1591-2. Crossref
37. Reichenberg A, Gross R, Weiser M, et
al. Advancing paternal age and autism. Arch Gen Psychiatry
2006;63:1026-32. Crossref
38. Durkin MS, Maenner MJ, Newschaffer CJ,
et al. Advanced parental age and the risk of autism spectrum disorder. Am
J Epidemiol 2008;168:1268-76. Crossref
39. Belloc S, Hazout A, Zini A, et al. How
to overcome male infertility after 40: Influence of paternal age on
fertility. Maturitas 2014;78:22-9. Crossref
40. Jennings MO, Owen RC, Keefe D, Kim ED.
Management and counseling of the male with advanced paternal age. Fertil
Steril 2017;107:324-8. Crossref