DOI: 10.12809/hkmj187348
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Joint recommendations on management of anaemia in
patients with gastrointestinal bleeding in Hong Kong
LY Mak, MB, BS1,2; CW Lau, MB, BS3;
YT Hui, MB, BS2; C Ng, MB, BS2; E Shan, MB, BS2;
Michael KK Li, MB, BS2; James YW Lau, MD4; Philip WY
Chiu, MD4; HT Leong, MB, BS4; J Ho, MD1;
Justin CY Wu, MD1; CK Lee, MB, BS3; WK Leung, MD1,2
1 Hong Kong Society of Gastroenterology
2 Hong Kong IBD Society
3 Hong Kong Red Cross Blood Transfusion
Service
4 Hong Kong Society of Digestive
Endoscopy
Corresponding author: Prof WK Leung (waikleung@hku.hk)
Abstract
The demand for blood products continues to grow
in an unsustainable manner in Hong Kong. While anaemia associated with
gastrointestinal bleeding (GIB) is the leading indication for
transfusion, there is no local recommendation regarding best practices
for transfusion. We aimed to provide evidence-based recommendations
regarding management of anaemia in patients with acute and chronic GIB.
We reviewed all original papers, meta-analyses, systematic reviews, or
guidelines that were available in PubMed. For acute GIB, a restrictive
transfusion strategy, targeting a haemoglobin threshold of 7 to 8 g/dL,
should be adopted because overtransfusion is associated with
significantly higher all-cause mortality and re-bleeding. A liberal
transfusion strategy should only be considered in patients with
co-existing symptomatic coronary artery disease, targeting a haemoglobin
threshold of 9 to 10 g/dL. When acute GIB settles, patients should be
prescribed iron supplements if iron deficiency is present. For chronic
GIB, iron stores should be replenished aggressively via iron
supplementation before consideration of blood transfusion, except in
patients with symptoms of severe anaemia. Oral iron replacement is the
preferred first-line therapy, while intravenous iron is indicated for
patients with inflammatory bowel disease, poor response or poor
tolerability to oral iron, and in whom a rapid correction of iron
deficit is preferred. Intravenous iron is underutilised and the risk of
anaphylactic reaction to current preparations is extremely low. These
recommendations are provided to local clinicians to facilitate judicious
and appropriate use of red cell products and iron replacement therapy in
patients with GIB.
Introduction
Gastrointestinal bleeding (GIB) is a leading
indication for blood transfusion in Hong Kong. In a recent report issued
by The Hong Kong Red Cross Blood Transfusion Service, 242 379 units of red
cells (RBCs) were issued in 2016, an increase of 34% from 2006. More than
90% of blood products were used by patients in public hospitals; more than
70% of RBCs were utilised in medical/geriatric and surgery departments.1 Blood demand is expected to
continue rising because of the ageing population, in whom the highest
amount of blood was used, compared with younger age-groups. The respective
units of blood use per 1000 person-years were: 0 to 14 years (8.0), 15 to
64 years (17.5), 65 to 74 years (58.4), 75 to 84 years (117.7) and ≥85
years (209.3).1 Importantly,
compared with many western countries, Hong Kong is using more RBCs per
population. In 2016, Hong Kong used 33.0 units of RBCs per 1000
population, compared with 20.7 in Singapore, 25.3 in Japan, 19.0 in
Western Australia, 23.5 in New Zealand, 28.5 in England and North Wales,
and 20.8 in Canada (unpublished data from Hong Kong Red Cross Blood
Transfusion Service). Possible explanations for lower usages in other
nations include the adoption of restrictive transfusion practices and more
frequent utilisation of iron replacement therapy. With the continuously
rising demand for blood products in Hong Kong, unmatched by a
corresponding increase in blood donors, there is a pressing need to
institute sustainable transfusion practices, such that blood products can
be used appropriately.
In addition to the inadequate supply of blood
products, transfusion is not without risks. Approximate risks per unit of
RBC transfusion are 1:60 for febrile reaction, 1:100 for
transfusion-associated circulatory overload, 1:250 for allergic reaction,
and 1:12 000 for transfusion-related acute lung injury. In Hong Kong, the
most recent estimated risks for transmission of hepatitis B virus (1:58
000), hepatitis C virus (1:8 000 000), and human immunodeficiency virus
(1:2 400 000) are not negligible.2
3 4
5 6
7 8
9 10
There are additional risks of overtransfusion. Hence, blood transfusion
should be instituted appropriately with good indications which should
outweigh the potential risks.
Because of these issues and the lack of
standardisation of local clinical practices for blood transfusion, the aim
of this joint recommendation paper by the Hong Kong Society of
Gastroenterology, the Hong Kong IBD Society, the Hong Kong Society of
Digestive Endoscopy, and the Hong Kong Red Cross Blood Transfusion Service
was to provide evidence-based recommendations for the management of
anaemia in patients with acute and chronic GIB; this will facilitate more
judicious and appropriate use of RBC products, as well as other
alternative measures to control anaemia resulting from GIB.
Types of gastrointestinal bleeding
Gastrointestinal bleeding can be classified on the
basis of the speed of blood loss, site of bleeding (upper or lower GIB),
or aetiology of bleeding. For the purpose of this recommendation paper,
only the speed of blood loss (ie, acute or chronic) is considered. Acute
GIB, also known as overt GIB, is defined as frank bleeding from the
gastrointestinal tract, with or without iron deficiency. Clinically
visible bleeding typically presents as haematemesis, coffee-ground
vomiting, melena or haematochezia. Conversely, chronic GIB, also known as
occult bleeding, is defined as guaiac positive stool accompanying iron
deficiency.11 12 13 In this
group of patients, blood is not visible macroscopically; they are
typically managed in an out-patient setting. Iron deficiency is inevitable
in this context of blood loss, because every 1 mL of blood contains 0.5 mg
of elemental iron; a decrease of 1 g/dL haemoglobin results in
approximately 200 mg elemental iron loss.
Some patients present with acute massive
exsanguinating GIB, where life-saving blood transfusion is essential.
There are no universally accepted definitions for massive exsanguinating
GIB. Some trials have defined it as the need for transfusion of at least 4
units of blood during a period of 24 hours in-hospital, or hypotension
with systolic blood pressure <90 mm Hg.14
In the acute care setting, massive bleeding is defined as 50% blood volume
loss within 3 hours, or a rate of 150 mL per minute. In patients with
haemodynamic instability, initial resuscitation is the primary goal and
blood transfusion is often dictated by haemodynamic status, including the
degree of depletion of intravascular volume and clinical signs of organ
hypoperfusion. Thus, these patients are excluded from clinical trials of
transfusion strategies, as discussed in the following sections, and should
be managed accordingly.
Acute gastrointestinal bleeding
Transfusion strategies
The haemoglobin threshold below which RBC
transfusion should be given has been controversial. Older observational
studies and smaller controlled trials suggested that transfusion may be
harmful for patients with hypovolemic anaemia due to GIB.15 16 17 18 19 Recently, increasing evidence from randomised
controlled trials has suggested that a restrictive transfusion strategy is
preferred in patients with acute GIB.2
3 20
In most trials, a restrictive transfusion strategy has referred to a
haemoglobin threshold of 7 to 8 g/dL, whereas a threshold of 9 to 10 g/dL
is used in liberal transfusion strategy. A restrictive transfusion
strategy has been associated with significantly lower short-term
mortality. In a study by Villanueva et al,3
the hazard ratio (HR) for death at 6 weeks was lower in the restrictive
strategy group than in the liberal strategy group (HR=0.55; 95% confidence
interval [CI]=0.33-0.92; P=0.002). Moreover, re-bleeding risk was
significantly lower for the restrictive transfusion group than the liberal
transfusion group (10% vs 16%, respectively; P=0.01; HR=0.68; 95%
CI=0.47-0.98).3 In a subgroup of
patients with cirrhosis, the survival advantage conferred by a restrictive
transfusion strategy remained for those with Child-Pugh class A or B
disease (HR=0.30; 95% CI=0.11-0.85). Additionally, a restrictive
transfusion strategy is not associated with harm in terms of risks of
myocardial infarction, pulmonary oedema, stroke, pneumonia, or
thromboembolism. In a meta-analysis of four randomised controlled trials
that examined this issue, restrictive transfusion was associated with a
lower risk of all-cause mortality (relative risk [RR]=0.65, 95%
CI=0.44-0.97; P=0.03) and a lower overall re-bleeding rate (RR=0.58, 95%
CI=0.40-0.80; P=0.004).21 It has
become clear that a restrictive transfusion strategy should be adopted for
acute GIB; this is currently recommended in many international guidelines.22 23
24 25
The above recommendation includes exceptions where
a more liberal transfusion strategy should be adopted. This is
particularly true for patients with concurrent symptomatic coronary artery
disease. It is estimated that up to 14% of patients with acute upper GIB
exhibit coexisting coronary artery disease.26
In a prior analysis, these patients showed greater risk of death,
myocardial infarction or unscheduled revascularisation at 30 days if a
restrictive transfusion strategy (haemoglobin threshold of 8 g/dL) was
adopted, compared with a liberal transfusion strategy (haemoglobin
threshold of 10 g/dL) [25.5% vs 10.9%, respectively, risk difference=15%,
95% CI=0.7-29.3%; P=0.054].27
Haemostasis
Ongoing bleeding should be controlled whenever
possible, including endoscopic, radiographic or surgical interventions to
reduce the blood loss and hence, transfusion requirement.22 23
Correction of coagulopathy and use of antifibrinolytic agents should be
considered in appropriate cases. For patients who are using antithrombotic
or anticoagulant therapies, specific reversal agents can be considered.
Ideally, a multidisciplinary team including a haematologist, cardiologist,
neurologist, and gastroenterologist or surgeon should be involved to
ensure the best decision regarding discontinuation of medications or the
use of reversal agents after balancing risk of bleeding versus risk of
thromboembolic events.22
Iron therapy after initial haemostasis
Patients with acute GIB typically exhibit
iron-deficiency anaemia. Although haemoglobin <10 g/dL was associated
with doubling of short-term mortality,28
iron replacement therapy should be considered in stable patients with
borderline low haemoglobin, rather than blood transfusion. In a randomised
controlled trial, oral or intravenous iron supplementation significantly
reduced the proportion of patients with anaemia at 3 months after acute
GIB.29 Unfortunately, this was
often underutilised and only 16% of patients with acute GIB were
prescribed with iron supplements upon discharge.30
Chronic gastrointestinal bleeding
Replenishing the iron store
Iron deficiency should always be corrected by iron
replacement before consideration of blood transfusion in the context of
chronic GIB. Adults typically have approximately 50 mg/kg of total bodily
elemental iron; two thirds is stored in haem and one third is stored in
the form of ferritin or haemosiderin. Approximately 20 mg of iron is
recycled daily in the bone marrow and spleen to maintain haem synthesis,
and approximately 1 to 2 mg/day of additional dietary iron is needed to
balance losses in urine, sweat, and stool. Assuming absorption of 10% of
iron in the medicinal form, the daily elemental iron requirement is
approximately 10 mg; this requirement is higher for menstruating women and
pregnant mothers.31 32 Dietary iron is present in two main forms. Haem iron
is found in meat-based foods and fish. Absorption of haem iron is
independent of body iron status. Non-haem iron is found in plant-based
foods, cereals, or egg yolks. Absorption of non-haem iron, in contrast to
haem iron, is enhanced if the body’s iron store declines. It is absorbed
in its ferrous form in the duodenum and proximal jejunum; therefore, an
acidic environment favours iron absorption. Another important molecular
mechanism of iron absorption involves hepcidin, which regulates
ferroportin-mediated release of iron from enterocytes and macrophages. In
a chronic inflammatory state, hepcidin is increased and negatively
regulates iron homeostasis.33
In patients with iron-deficiency anaemia, the daily
recommended iron requirement substantially increases to 150 to 200 mg
elemental iron per day to replenish the deficit; approximately 4 weeks are
needed to fully correct the iron deficit.32
Iron replacement therapy is indicated in these patients, because dietary
iron intake alone is unlikely to replace this deficit. The Ganzoni
equation is used in some studies to estimate the iron deficit as follows:
iron deficit (mg) = body weight (kg) × [target haemoglobin (g/dL) − actual
haemoglobin (g/dL)] × 2.4 + 500 mg.34
However, many clinicians view this formula as inconvenient and may
underestimate iron deficit35;
therefore, it is not widely used in clinical practice. A simplified
fixed-dose regimen, as used for treatment of patients with inflammatory
bowel disease (IBD), may be considered for iron replacement (Table
1).36
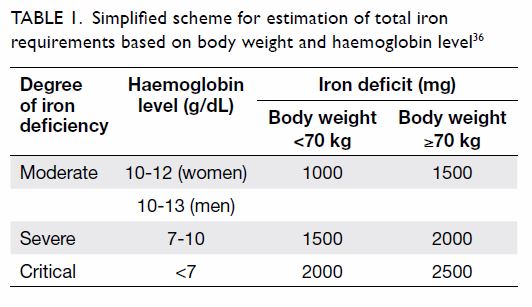
Table 1. Simplified scheme for estimation of total iron requirements based on body weight and haemoglobin level36
Route and dosing of iron replacement therapy
Iron replacement can be administered in either oral
or intravenous form. In most cases, the oral route remains first-line
treatment because of its convenience, low cost and avoidance of
hospitalisation, as well as the potential risks of anaphylactic reaction
with intravenous iron. However, gastrointestinal upset, such as nausea and
constipation, is very common with oral iron replacement, which decreases
patient compliance. Additionally, this method requires a few weeks or
months to replenish depleted iron stores in the body. To further
complicate treatment, oral iron therapy is ineffective in a few clinical
situations. First, in patients with chronic inflammation, hepcidin is
upregulated and exerts a negative effect on intestinal iron absorption.
Second, in patients with achlorhydria (eg, those undergoing long-term
treatment with proton pump inhibitors), or a history of vagotomy or
gastric bypass, the acidic gastric environment that maintains the ferrous
state of iron is lost; thus, absorption is largely impaired. Other causes
of poor response to oral iron replacement include small bowel
malabsorption (eg, IBD, prior small bowel resection, or celiac disease)
and co-administration of iron with coffee or tea. In particular, IBD
patients with iron-deficiency irondeficiency anaemia are recommended to
receive intravenous iron as first-line therapy, because of its greater
effectiveness than oral iron.37
The “Day-14 haemoglobin”, ie, increase in haemoglobin by ≥1 g/dL on day 14
after oral iron therapy, is a useful tool to determine whether and when to
transit from oral to intravenous iron.
Because of the above caveats related to oral iron
replacement, intravenous iron replacement can be considered as an
alternative. Intravenous iron may also be considered in accordance with
patient preference, as some patients cannot tolerate the adverse effects
of oral iron or prefer rapid correction of iron deficiency. An older
preparation of intravenous iron, in the form of high-molecular-weight iron
dextran, was underutilised in the past because of potential anaphylactic
reactions. However, this preparation has been removed from the US and
Europe. In recent years, the safety of intravenous iron has been vastly
improved by newer well-tolerated preparations, such as iron sucrose and
iron isomaltoside. According to the US Food and Drug Administration, the
cumulative rate of serious adverse reactions is <1:200 000 with
different intravenous compounds (iron sucrose, ferric gluconate and
low-molecular-weight iron dextran).38
39 Intravenous iron is now
generally considered safe and more effective than oral preparations.40 41 42
Table 2 shows the recommended dosing of commonly
used preparations of oral and intravenous iron replacement available in
Hong Kong.43 A 300-mg iron
sulphate tablet contains 20% to 30% elemental iron. Typical dosing of an
oral iron sulphate tablet would be 300 mg administered twice daily, which
would supply approximately 120 to 180 mg of elemental iron to the patient.
However, the recommended dosing for patients with IBD might be lower.
According to the European Crohn’s and Colitis Organisation Consensus, no
more than 100 mg elemental iron per day should be administered to patients
with IBD, as a result of a few preclinical or early reports of the adverse
effects of oral iron on exacerbation of disease activity, carcinogenesis,
and alteration of intestinal microbiota.37
The benefits of lower iron dosing are not limited to IBD patients. A
recent randomised unblinded trial showed that alternate daily dosing of
iron is superior to daily dosing of iron in terms of efficacy
(specifically related to hepcidin regulation) and tolerability.44 Most oral preparations of iron replacement therapy
are equally effective, as long as compliance is ensured. Liquid
preparations may minimise gastrointestinal upset and avoid the risk of
iron tablet–induced gastric erosion.45
46 Co-administration of oral iron
with ascorbic acid is advocated by some experts because of the theoretical
enhancement of iron absorption by reduction of ferric iron to the ferrous
form.47 Indeed, oral ascorbic acid
administration was associated with a dose-dependent increase of oral iron
absorption in healthy volunteers.48
Although large-scale studies of patients with iron-deficiency anaemia are
lacking, oral ascorbic acid is well tolerated and may be considered for
concomitant administration with oral iron.
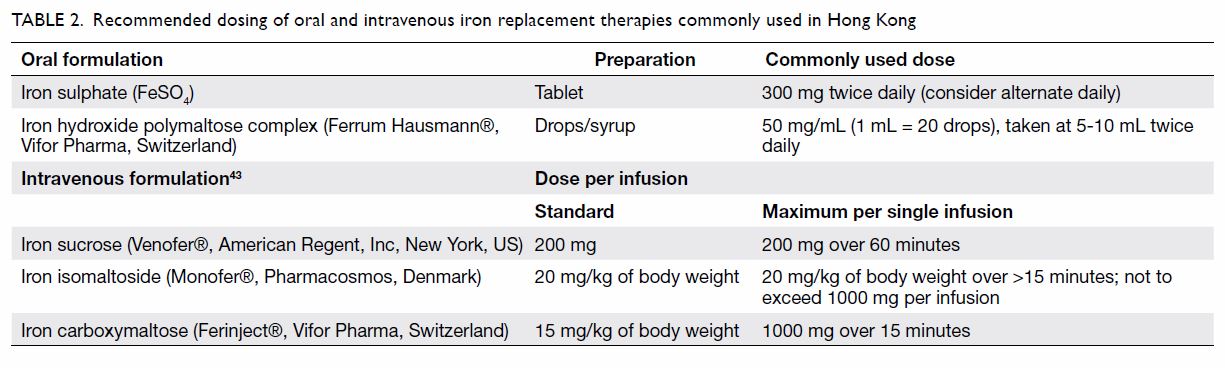
Table 2. Recommended dosing of oral and intravenous iron replacement therapies commonly used in Hong Kong
Intravenous iron can be considered as first choice
in patients with a high probability of non-compliance, small bowel
malabsorption, severe anaemia, or multiple co-morbidities that affect
hepcidin-regulated iron absorption. There are two preparations of
intravenous iron commonly available in Hong Kong: iron sucrose (Venofer®,
American Regent, Inc, New York, US) and iron isomaltoside (Monofer®,
Pharmacosmos, Denmark). Monofer® can be administered at a
maximum of 20 mg/kg or 1000 mg per single dose weekly. Premedication to
prevent anaphylaxis is not routinely needed, but patients should be
monitored for at least 30 minutes after drug administration.
Blood transfusions should not be routinely used in
chronic GIB and are reserved for patients with severe anaemic symptoms,
where blood transfusion would provide rapid relief of the symptoms.
Typically, 1 unit of RBC provides approximately 200 mg elemental iron,
which would increase haemoglobin by 1 g/dL.
Further management
Whenever possible, the source of bleeding should be
identified, with haemostasis secured to prevent continuous blood loss. It
may remain difficult in some cases of obscure bleeding, or with multiple
sites of bleeding (eg, multiple small bowel angiodysplasia). Clinicians
should always maintain awareness of other potential causes of anaemia in
these patients, including malabsorption, chronic inflammation,
erythropoietin deficiency and other concurrent nutritional deficiencies,
such as vitamin B12 and folate; these must be corrected to
optimise the haemoglobin level.
Recommendations
1. In massive exsanguinating GIB, blood transfusion
for life-saving purposes should be administered on the basis of
haemodynamic status and response to fluid resuscitation.
2. In acute GIB:
A restrictive transfusion strategy should be adopted, which involves a
haemoglobin threshold of 7 to 8 g/dL; below this threshold, RBC
transfusion should be administered.
Overtransfusion is associated with higher all-cause mortality and
re-bleeding.
A less restrictive transfusion strategy, targeting a haemoglobin level
of 9 to 10 g/dL, is only preferred in patients with coexisting
symptomatic coronary artery disease.
After acute GIB settles, patients should be prescribed iron
supplements. The duration of this supplementation is not yet defined,
but should be titrated in accordance with haemoglobin and iron status.
3. In chronic GIB:
Iron stores should be replenished aggressively via iron
supplementation.
Blood transfusion should not be routinely used and is reserved for
patients with severe anaemic symptoms.
Oral iron replacement is the first-line therapy, whereas intravenous
iron is indicated in patients with IBD, poor response or poor
tolerability to oral iron, and in whom a rapid correction of iron
deficit is preferred.
Oral iron can be given at alternate daily dosing to improve
effectiveness and tolerability.
Co-administration of ascorbic acid with oral iron may be considered.
Intravenous iron is underutilised. The risk of anaphylactic reaction
to current preparations of intravenous iron is extremely low.
Author contributions
This is a Joint Position Statement issued by the
three professional gastrointestinal societies (Hong Kong Society of
Gastroenterology, Hong Kong Society of Digestive Endoscopy, Hong Kong IBD
Society) and the Hong Kong Red Cross Blood Transfusion Service. All
authors were involved in the preparation, drafting, and critical review of
this article.
Funding/support
This article received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Declaration
All authors have disclosed no conflicts of
interest. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity.
References
1. Fact sheet on blood collection and use.
Hong Kong Red Cross Blood Transfusion Service. Apr 2017.
2. Carson JL, Guyatt G, Heddle NM, et al.
Clinical practice guidelines from the AABB: red blood cell transfusion
thresholds and storage. JAMA 2016;316:2025-35. Crossref
3. Villanueva C, Colomo A, Bosch A, et al.
Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J
Med 2013;368:11-21. Crossref
4. The Sanguis Study Group. Use of blood
products for elective surgery in 43 European hospitals. Transfus Med
1994;4:251-68. Crossref
5. Clifford L, Jia Q, Yadav H, et al.
Characterizing the epidemiology of perioperative transfusion-associated
circulatory overload. Anesthesiology 2015;122:21-8. Crossref
6. DeBaun MR, Gordon M, McKinstry RC, et
al. Controlled trial of transfusions for silent cerebral infarcts in
sickle cell anemia. N Engl J Med 2014;371:699-710. Crossref
7. Federowicz I, Barrett BB, Andersen JW,
Urashima M, Popovsky MA, Anderson KC. Characterization of reactions after
transfusion of cellular blood components that are white cell reduced
before storage. Transfusion 1996;36:21-8. Crossref
8. Popovsky MA, Audet AM, Andrzejewski C
Jr. Transfusion-associated circulatory overload in orthopedic surgery
patients: a multi-institutional study. Immunohematology 1996;12:87-9.
9. Stramer SL, Notari EP, Krysztof DE, Dodd
RY. Hepatitis B virus testing by minipool nucleic acid testing: does it
improve blood safety? Transfusion 2013;53:2449-58. Crossref
10. Zou S, Dorsey KA, Notari EP, et al.
Prevalence, incidence, and residual risk of human immunodeficiency virus
and hepatitis C virus infections among United States blood donors since
the introduction of nucleic acid testing. Transfusion 2010;50:1495-504. Crossref
11. Raju GS, Gerson L, Das A, Lewis B,
American Gastroenterological Association. American Gastroenterological
Association (AGA) Institute technical review on obscure gastrointestinal
bleeding. Gastroenterology 2007;133:1697-717. Crossref
12. Raju GS, Gerson L, Das A, Lewis B,
American Gastroenterological Association. American Gastroenterological
Association (AGA) Institute medical position statement on obscure
gastrointestinal bleeding. Gastroenterology 2007;133:1694-6. Crossref
13. Gerson LB, Fidler JL, Cave DR,
Leighton JA. ACG clinical guideline: diagnosis and management of small
bowel bleeding. Am J Gastroenterol 2015;110:1265-87. Crossref
14. Yoon W, Jeong YY, Shin SS, et al.
Acute massive gastrointestinal bleeding: detection and localization with
arterial phase multi-detector row helical CT. Radiology 2006;239:160-7. Crossref
15. Blair SD, Janvrin SB, McCollum CN,
Greenhalgh RM. Effect of early blood transfusion on gastrointestinal
haemorrhage. Br J Surg 1986;73:783-5. Crossref
16. Halland M, Young M, Fitzgerald MN,
Inder K, Duggan JM, Duggan A. Characteristics and outcomes of upper
gastrointestinal hemorrhage in a tertiary referral hospital. Dig Dis Sci
2010;55:3430-5. Crossref
17. Hearnshaw SA, Logan RF, Palmer KR,
Card TR, Travis SP, Murphy MF. Outcomes following early red blood cell
transfusion in acute upper gastrointestinal bleeding. Aliment Pharmacol
Ther 2010;32:215-24. Crossref
18. Kravetz D, Sikuler E, Groszmann RJ.
Splanchnic and systemic hemodynamics in portal hypertensive rats during
hemorrhage and blood volume restitution. Gastroenterology 1986;90:1232-40.
Crossref
19. Villarejo F, Rizzolo M, Lópéz E,
Domeniconi G, Arto G, Apezteguia C. Acute anemia in high digestive
hemorrhage. Margins of security for their handling without transfusion of
red globules [in Spanish]. Acta Gastroenterol Latinoam 1999;29:261-70.
20. Jairath V, Kahan BC, Gray A, et al.
Restrictive versus liberal blood transfusion for acute upper
gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster
randomised feasibility trial. Lancet 2015;386:137-44. Crossref
21. Odutayo A, Desborough MJ, Trivella M,
et al. Restrictive versus liberal blood transfusion for gastrointestinal
bleeding: a systematic review and meta-analysis of randomised controlled
trials. Lancet Gastroenterol Hepatol 2017;2:354-360. Crossref
22. Strate LL, Gralnek IM. ACG clinical
guideline: management of patients with acute lower gastrointestinal
bleeding. Am J Gastroenterol 2016;111:459-74. Crossref
23. Gralnek IM, Dumonceau JM, Kuipers EJ,
et al. Diagnosis and management of nonvariceal upper gastrointestinal
hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE)
Guideline. Endoscopy 2015;47:a1-46. Crossref
24. Laine L, Jensen DM. Management of
patients with ulcer bleeding. Am J Gastroenterol 2012;107:345-60. Crossref
25. Jalan R, Hayes PC. UK guidelines on
the management of variceal haemorrhage in cirrhotic patients. Br Soc
Gastroenterol Gut 2000;46:III1-15. Crossref
26. Crooks CJ, West J, Card TR.
Comorbidities affect risk of nonvariceal upper gastrointestinal bleeding.
Gastroenterology 2013;144:1384-93.e2. Crossref
27. Carson JL, Brooks MM, Abbott JD, et
al. Liberal versus restrictive transfusion thresholds for patients with
symptomatic coronary artery disease. Am Heart J 2013;165:964-71.e1. Crossref
28. Rockall TA, Logan RF, Devlin HB,
Northfield TC. Risk assessment after acute upper gastrointestinal
haemorrhage. Gut 1996;38:316-21. Crossref
29. Bager P, Dahlerup JF. Randomised
clinical trial: oral vs. intravenous iron after upper gastrointestinal
haemorrhage—a placebo-controlled study. Aliment Pharmacol Ther
2014;39:176-87. Crossref
30. Bager P, Dahlerup JF. Lack of
follow-up of anaemia after discharge from an upper gastrointestinal
bleeding centre. Dan Med J 2013;60:A4583.
31. Office of Dietary Supplements.
National Institutes of Health. US Government. Iron: dietary supplement
fact sheet. Last updated Mar 2018. Available from:
https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/. Accessed Apr
2018.
32. Alleyne M, Horne MK, Miller JL.
Individualized treatment for iron-deficiency anemia in adults. Am J Med
2008;121:943-8. Crossref
33. Olsson KS, Norrby A. Comment to:
Hepcidin: from discovery to differential diagnosis. Haematologica
2008;93:90-7. Haematologica 2008;93:e51. Crossref
34. Ganzoni AM. Intravenous iron-dextran:
therapeutic and experimental possibilities [in German]. Schweiz Med
Wochenschr 1970;100:301-3.
35. Koch TA, Myers J, Goodnough LT.
Intravenous iron therapy in patients with iron deficiency anemia: dosing
considerations. Anemia 2015;2015:763576. Crossref
36. Stein J, Hartmann F, Dignass AU.
Diagnosis and management of iron deficiency anemia in patients with IBD.
Nat Rev Gastroenterol Hepatol 2010;7:599-610. Crossref
37. Dignass AU, Gasche C, Bettenworth D,
et al. European consensus on the diagnosis and management of iron
deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis
2015;9:211-22. Crossref
38. Chertow GM, Mason PD, Vaage-Nilsen O,
Ahlmén J. Update on adverse drug events associated with parenteral iron.
Nephrol Dial Transplant 2006;21:378-82. Crossref
39. Yessayan L, Sandhu A, Besarab A, et
al. Intravenous iron dextran as a component of anemia management in
chronic kidney disease: a report of safety and efficacy. Int J Nephrol
2013;2013:703038. Crossref
40. Kulnigg S, Stoinov S, Simanenkov V, et
al. A novel intravenous iron formulation for treatment of anemia in
inflammatory bowel disease: the ferric carboxymaltose (FERINJECT)
randomized controlled trial. Am J Gastroenterol 2008;103:1182-92. Crossref
41. Johnson-Wimbley TD, Graham DY.
Diagnosis and management of iron deficiency anemia in the 21st century.
Therap Adv Gastroenterol 2011;4:177-84. Crossref
42. Koduru P, Abraham BP. The role of
ferric carboxymaltose in the treatment of iron deficiency anemia in
patients with gastrointestinal disease. Therap Adv Gastroenterol
2016;9:76-85. Crossref
43. Camaschella C. Iron-deficiency anemia.
N Engl J Med 2015;372:1832-43. Crossref
44. Stoffel NU, Cercamondi CI, Brittenham
G, et al. Iron absorption from oral iron supplements given on consecutive
versus alternate days and as single morning doses versus twice-daily split
dosing in iron-depleted women: two open-label, randomised controlled
trials. Lancet Haematol 2017;4:e524-33. Crossref
45. Meliţ LE, Mărginean CO, Mocanu S,
Mărginean MO. A rare case of iron-pill induced gastritis in a female
teenager: A case report and a review of the literature. Medicine
(Baltimore) 2017;96:e7550. Crossref
46. Hashash JG, Proksell S, Kuan SF,
Behari J. Iron pill-induced gastritis. ACG Case Rep J 2013;1:13-5. Crossref
47. Atanassova BD, Tzatchev KN. Ascorbic
acid—important for iron metabolism. Folia Med (Plovdiv) 2008;50:11-6.
48. Brise H, Hallberg L. Effect of
ascorbic acid on iron absorption. Acta Med Scand Suppl 1962;376:51-8. Crossref

