DOI: 10.12809/hkmj177037
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
Recommendations on prevention and screening for breast
cancer in Hong Kong
Cancer Expert Working Group on Cancer Prevention
and Screening (August 2016 to July 2018)
TH Lam, MD1; KH Wong, MB, BS, FHKAM
(Medicine)2; Karen KL Chan, MBBChir, FHKAM (Obstetrics and
Gynaecology)3; Miranda CM Chan, MB, BS, FHKAM (Surgery)4;
David VK Chao, FRCGP, FHKAM (Family Medicine)5; Annie NY
Cheung, MD, FHKAM (Pathology)6; Cecilia YM Fan, MB, BS, FHKAM
(Family Medicine)7; Judy Ho, MB, BS, FHKAM (Surgery)8;
EP Hui, MD (CUHK), FHKAM (Medicine)9; KO Lam, MB, BS, FHKAM
(Radiology)10; CK Law, FHKCR, FHKAM (Radiology)11;
WL Law, MS, FHKAM (Surgery)12; Herbert HF Loong, MB, BS, FHKAM
(Medicine)13; Roger KC Ngan, FRCR, FHKAM (Radiology)14;
Thomas HF Tsang, MB, BS, FHKAM (Community Medicine)15; Martin
CS Wong, MD, FHKAM (Family Medicine)16; Rebecca MW Yeung, MD,
FHKAM (Radiology)17; Anthony CH Ying, MB, BS, FHKAM (Radiology)18;
Regina Ching, MB, BS, FHKAM (Community Medicine)19
1 School of Public Health, Li Ka Shing
Faculty of Medicine, The University of Hong Kong, Hong Kong
2 Department of Health, Hong Kong
3 The Hong Kong College of Obstetricians
and Gynaecologists, Hong Kong
4 Hospital Authority (Surgical), Hong
Kong
5 The Hong Kong College of Family
Physicians, Hong Kong
6 The Hong Kong College of Pathologists,
Hong Kong
7 Professional Development and Quality
Assurance, Department of Health, Hong Kong
8 World Cancer Research Fund Hong Kong,
Hong Kong
9 Hong Kong College of Physicians, Hong
Kong
10 Department of Clinical Oncology, The
University of Hong Kong, Hong Kong
11 Hong Kong College of Radiologists,
Hong Kong
12 The College of Surgeons of Hong Kong,
Hong Kong
13 Department of Clinical Oncology, The
Chinese University of Hong Kong, Hong Kong
14 Hong Kong Cancer Registry, Hospital
Authority, Hong Kong
15 Hong Kong College of Community
Medicine, Hong Kong
16 The Jockey Club School of Public
Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
17 Hospital Authority (Non-surgical),
Hong Kong
18 The Hong Kong Anti-Cancer Society,
Hong Kong
19 Centre for Health Protection,
Department of Health, Hong Kong
Corresponding author: Dr Regina Ching (regina_ching@dh.gov.hk)
Abstract
In Hong Kong, breast cancer is the most common
cancer among women and poses a significant health care burden. The
Cancer Expert Working Group on Cancer Prevention and Screening (CEWG)
was set up in 2002 by the Cancer Coordinating Committee to review and
assess local and international scientific evidence, and to formulate
recommendations for cancer prevention and screening. After considering
the local epidemiology, emerging scientific evidence, and local and
overseas screening practices, the CEWG concluded that it was unclear
whether population-based breast cancer screening did more harm than good
in local asymptomatic women at average risk. The CEWG considers that
there is insufficient evidence to recommend for or against
population-based mammography screening for such individuals. Women who
consider breast cancer screening should be adequately informed about the
benefits and harms. The CEWG recommends that all women adopt primary
preventive measures, be breast aware, and seek timely medical attention
for suspicious symptoms. For women at high risk of breast cancer, such
as carriers of confirmed BRCA1/2 deleterious mutations and those
with a family history of breast cancer, the CEWG recommends that they
seek doctor’s advice for annual mammography screening and the age at
which the process should commence. Additional annual screening by
magnetic resonance imaging is recommended for confirmed BRCA1/2
mutation carriers or women who have undergone radiation therapy to the
chest between the age of 10 and 30 years. Women at moderate risk of
breast cancer should discuss with doctors the pros and cons of breast
cancer screening before making an informed decision about mammography
screening every 2 to 3 years.
Introduction
In Hong Kong, the Cancer Coordinating Committee
(CCC) is a high-level committee chaired by the Secretary for Food and
Health to steer the direction of work and advice on local strategies for
cancer prevention and control. Under the auspices of the CCC, the Cancer
Expert Working Group on Cancer Prevention and Screening (CEWG) was set up
in 2002 to review local and international scientific evidence, and to
assess and formulate local recommendations.
This article describes the local breast cancer
burden, preventive measures, as well as the rationale that underlies
screening recommendations made by the CEWG that were reaffirmed in
September 2017.
Local epidemiology of female breast cancer
Since the early 1990s, breast cancer has become the
most common cancer among women in Hong Kong. According to the Hong Kong
Cancer Registry,1 there were 3900
newly registered female breast cancer cases in 2015, accounting for 26.1%
of all new cancer cases among women. The median age at diagnosis was 56
years. The age-standardised incidence rate (ASIR) of female breast cancer
was 58.8 per 100 000 standard population. In addition, 575 new cases of
carcinoma in situ of breast cancer (also known as ductal carcinoma in situ
[DCIS]) were reported in 2015, and the highest age-specific incidence rate
was 33.8 per 100 000 female population at age 70 to 74 years. More than
half (66%) of DCIS cases were diagnosed in females aged ≥50 years.
There were 702 registered deaths due to breast
cancer in 2016, representing 12.2% of and the third leading cause of
female cancer deaths.2 The
age-standardised mortality rate (ASMR) of female breast cancer was 10.2
per 100 000 standard population. There has been a rising trend of new
cases and deaths of female breast cancer over the past three decades.
After adjusting for population ageing, the ASIR has maintained an
increasing trend while the ASMR has remained relatively stable. Although
the ASIR of female breast cancer has been increasing in Hong Kong, it
remained lower than the West (eg, UK or Australia) and some Asian
countries (eg, Singapore) in 2012 (Fig3 4 5).
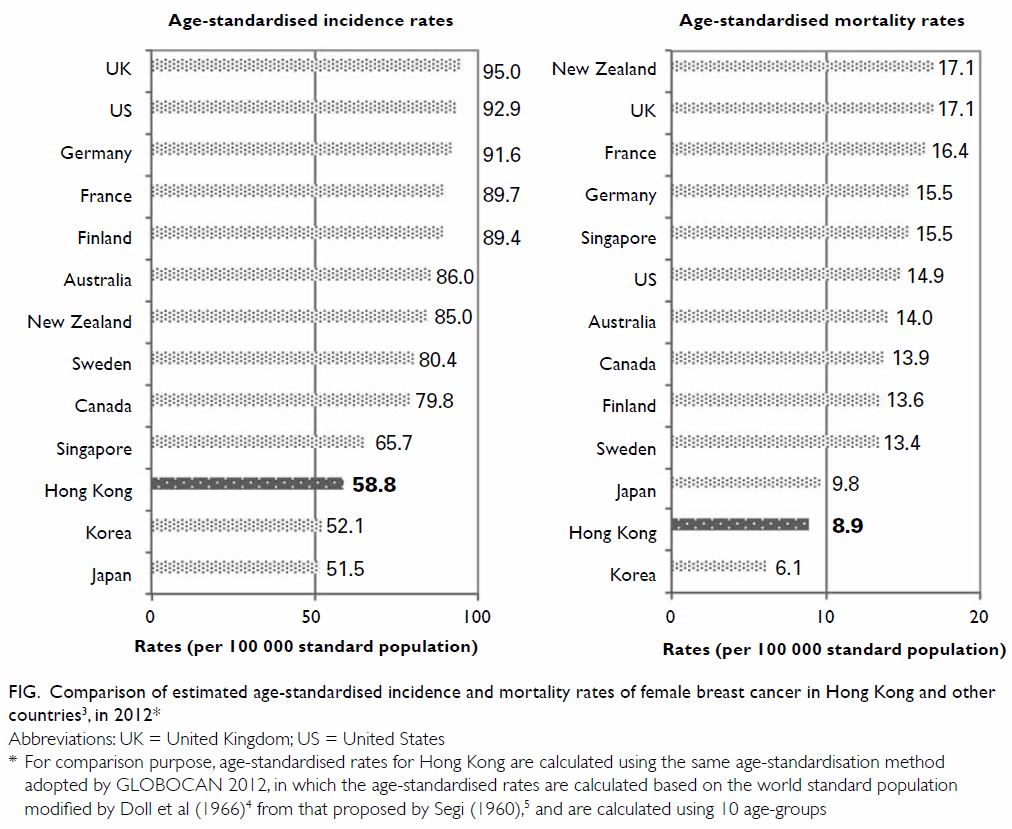
Figure. Comparison of estimated age-standardised incidence and mortality rates of female breast cancer in Hong Kong and other countries3, in 2012
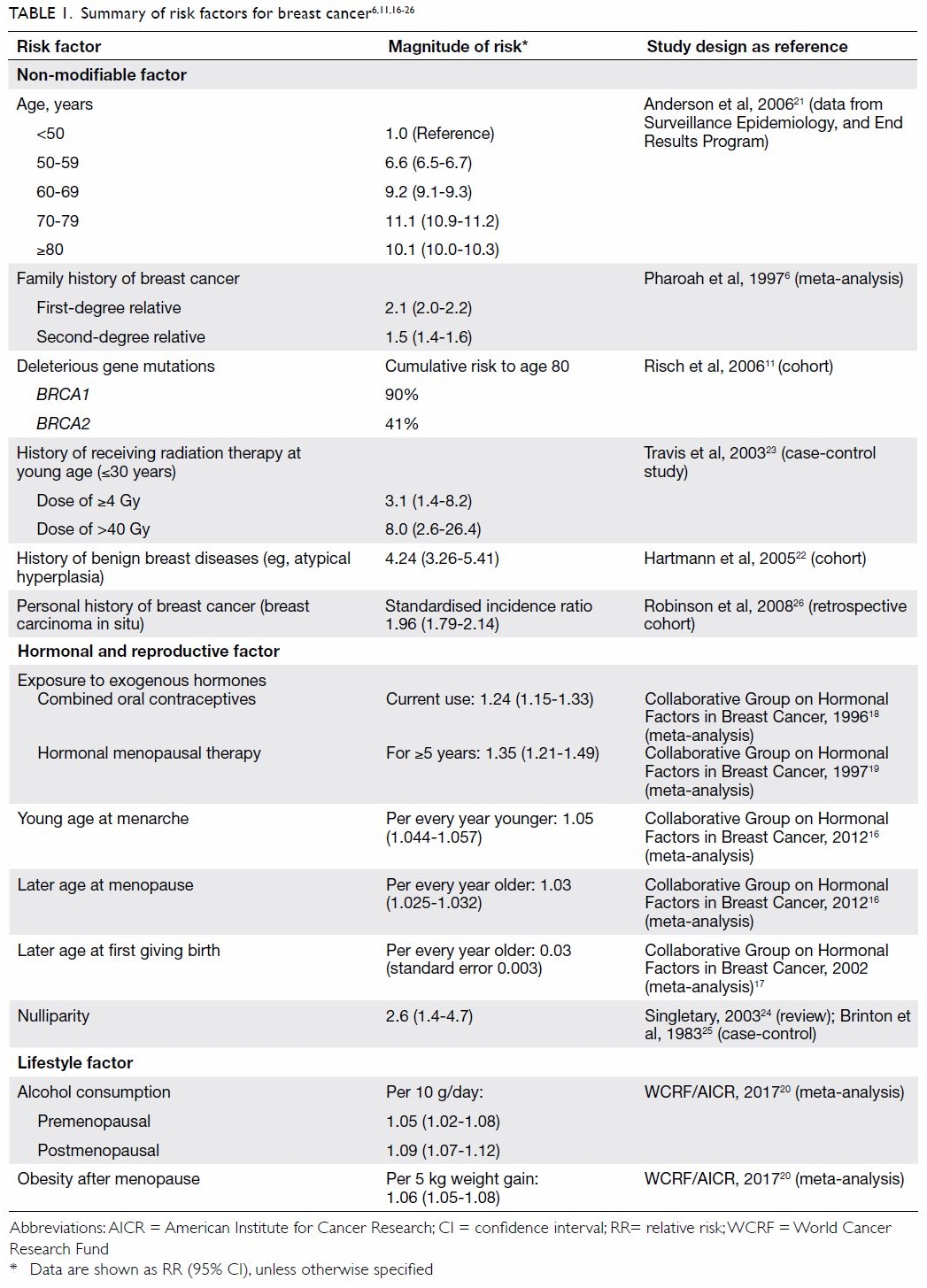
Risk factors for female breast cancer
A range of factors account for woman’s risk of
breast cancer, of which family history being a strong known one. Risk
increases with degree of relatedness of affected relatives, number of
affected relatives, and their age at diagnosis.6
7 8
Having one first-degree relative with breast cancer doubles a woman’s risk
while having an affected second-degree relative increases risk by 50%.6 The risk increases especially when the relative has
been diagnosed before the age of 50.7
Women with certain deleterious gene mutations are
at higher risk of breast cancer. Germline mutations in BRCA1/2
genes are associated with 40% to 90% lifetime risk of breast cancer and
are the most common cause of hereditary breast cancer. Other less common
gene mutations (eg, TP53, PTEN) are also associated with
an increased risk.8 9 10 11 It has been estimated that BRCA1/2 mutations
contribute to 5% to 10% of breast cancer cases in western countries.8 10 There are
limited data on the prevalence of BRCA mutations in the general
population of Hong Kong. Latest findings (as of September 2017) from the
Hong Kong Hereditary Breast Cancer Family Registry of 2549 clinically
high-risk breast or ovarian cancer patients revealed that BRCA mutation
was found in 9.6% of patients, among whom 45.1% were BRCA1 and
54.9% were BRCA2.12 This
is noticeably different from patients in western countries where the
majority of mutations are of BRCA1. In 2011, the Registry started
to employ a four-gene panel including TP53 and PTEN.10 13 Since
then, 15 (0.6%) and two (0.08%) patients carrying TP53 and PTEN
mutations have been identified, respectively.12
Additional established risk factors for female
breast cancer include a history of receiving radiation therapy at a young
age, history of breast cancer, ovarian cancer or endometrial cancer,
history of benign breast disease (eg, atypical hyperplasia), exposure to
exogenous hormones (eg, combined oral contraceptives or hormone
replacement therapy), reproductive factors (eg, early menarche or late
menopause, nulliparity, late first live birth), alcohol consumption,
obesity after menopause, and increasing age.6
8 14
15 16
17 18
19 20
21 22
23 24
25 26
A summary of these risk factors for breast cancer and the magnitude of
risk is presented in Table 1.6 11 16
17 18
19 20
21 22
23 24
25 26
Primary prevention and breast awareness
Certain breast cancer risk factors are related to
personal lifestyle and behaviour. Women can lower their risk by adopting
primary preventive measures such as undertaking moderate-intensity or
equivalent aerobic physical activity for at least 150 minutes per week,
avoidance of alcohol, maintaining a healthy body weight with body mass
index between 18.5 and 22.9 and waist circumference less than 80 cm,
bearing children at an earlier age and breastfeeding for a longer
duration.8 14 15 17 20 Alcohol
is a Group I carcinogen as classified by the International Agency for
Research on Cancer (IARC), World Health Organization. There is strong
evidence that alcohol can cause, inter alia, female breast cancer. With
respect to cancer risk, there is no safe level of alcohol consumption. For
women, drinking 10 grams of alcohol per day (eg, 250 mL of beer with 5%
alcohol content, a small glass (~100 mL) of red or white wine with 12%
alcohol content increases the risk of premenopausal breast cancer by 5%
and postmenopausal breast cancer by 9%.20
The higher the intake, the higher the risk, not only of breast cancer but
at least six or seven other cancers.14
Symptoms of early breast cancer may not be easily
noticed. The CEWG recommends all women to be breast aware, that is, be
familiar with the normal look and feel of their breasts and visit the
doctor promptly if suspicious symptoms appear, such as presence of a
breast or axillary lump, change in skin texture of the breast or nipple,
or nipple rash, discharge, or retraction.
Screening for the general female population at average
risk
Breast self-examination, clinical breast
examination, and mammography are widely used breast cancer screening
modalities. The CEWG considers there is insufficient evidence to recommend
regular breast self-examination as a screening tool due to its low
sensitivity in detecting breast cancer, no proven benefit in reducing
breast cancer mortality, and greater harm due to the increased detection
of benign lesions and biopsies performed.27
The CEWG is also of the view that there is insufficient evidence to
recommend clinical breast examination since its effectiveness in reducing
breast cancer mortality cannot be concluded from the limited studies
available.28 29 30
Ultrasonography, used as an adjunct to mammography
in women with radiologically dense breasts, has the potential to depict
small breast cancers not visible on mammography.31
However, both the Cochrane review in 201332
and the IARC review in 20158 33 concluded that there is insufficient evidence that
ultrasonography as an adjunct to mammography screening can decrease breast
cancer mortality.
Evidence from some western countries suggests that
organised breast screening programmes using mammography are effective in
the detection of tumours at an earlier stage and reduction of breast
cancer mortality in their populations. Nevertheless disadvantages such as
false-positive or false-negative results, overdiagnosis (the diagnosis of
breast cancer, in particular of DCIS, as a result of screening that would
not have been diagnosed or never have caused harm in a patient’s lifetime
if screening had not taken place), overtreatment, and potential
complications arising from subsequent invasive investigations or treatment
may outweigh the benefits.1 34 35
A Cochrane review in 2013 estimated that
mammography screening resulted in a 15% reduction in breast cancer
mortality and a 30% increase in overdiagnosis and overtreatment. For every
2000 women invited for mammography screening over a 10-year period, one
woman would be prevented from dying of breast cancer; 10 healthy women
would be treated unnecessarily; and more than 200 women would be falsely
alarmed and experience significant psychological distress because of
false-positive findings.36
In UK, the Independent Breast Review in 2013 showed
that mammography screening led to a relative risk reduction in breast
cancer mortality of 20% and an estimated 11% overdiagnosis rate.37
The Swiss Medical Board reported in 2013 that for
every 1000 women who underwent regular mammography screening, one to two
women’s lives could be saved, but around 100 women would undergo
unnecessary investigations and treatment. The cost-effectiveness ratio was
very unfavourable. The Board concluded that introduction of a mammography
screening programme was not recommended and a time limit should be set on
existing programmes. The Board further recommended that thorough medical
assessment and comprehensive information about the benefits and harms of
screening should be provided to women considering mammography screening.38
The 25-year follow-up of the Canadian National
Breast Screening Study in 2014 revealed that women aged 40 to 59 years who
underwent annual mammography screening received no benefit in terms of
breast cancer mortality but resulted in 22% overdiagnosis, prompting the
need of policy-makers to reassess the rationale of screening.34
In 2015, the IARC evaluated the cancer-preventive
and adverse effects of different breast cancer screening methods. It was
estimated that women aged 50 to 69 years invited for mammography screening
had a 24% reduced risk of mortality from breast cancer. Notwithstanding
this, the evaluation concluded sufficient evidence that mammography
screening led to overdiagnosis at an average rate of 6.5% (range, 1-10%).
The estimated cumulative risk of false-positive results was about 20% for
a woman who had 10 screens from age 50 to 70 years, leading to short-term
negative psychological consequences.8
33
In some regions of Asia where organised mammography
screening programmes (eg, Singapore, Korea, Taiwan) are implemented, there
is a lack of published peer-reviewed articles in the public domain
documenting systematic programme evaluation or modelling studies that
estimate or report on the extent of overdiagnosis and the number of lives
saved. At the same time, there is evidence of a generally low acceptance
of mammography screening in Asian regions. Data kept by the International
Cancer Screening Network39 showed
that the participation rate of a breast cancer screening programme in 2010
was 19% in Japan and 39.3% in Korea. The Singapore National Health Survey
of 2010 showed that 39.6% women aged 50 to 69 years reported a history of
mammography according to the recommended screening interval in Singapore,
which was within the 2 years preceding the survey.40 In Taiwan, the coverage of mammography screening
among women aged 45 to 69 years was 36% in 2012/2013.41
Furthermore, some international and local evidence
suggests a reduction in breast cancer mortality could be attributable to
improved survival due to treatment advances and improved health service
delivery rather than screening per se.35
42 43
44
In Hong Kong, the ASIR of breast cancer is
relatively low when compared with that in western countries. Therefore,
the positive predictive value of mammography will be lower, generating
more false-positive results and ensuing unnecessary follow-up
investigations, potential complications and psychological distress.45 Furthermore, local modelling studies have shown that
population-based mammography screening is not a cost-effective public
health intervention in Hong Kong as compared with other strategies to
prevent and control breast cancer.46
47
In conclusion, the CEWG considers that there is so
far insufficient evidence to make a definitive recommendation for or
against population-based mammography screening for asymptomatic women at
average risk in Hong Kong. Individuals considering breast cancer screening
should be adequately informed by doctors about the associated benefits and
harms.
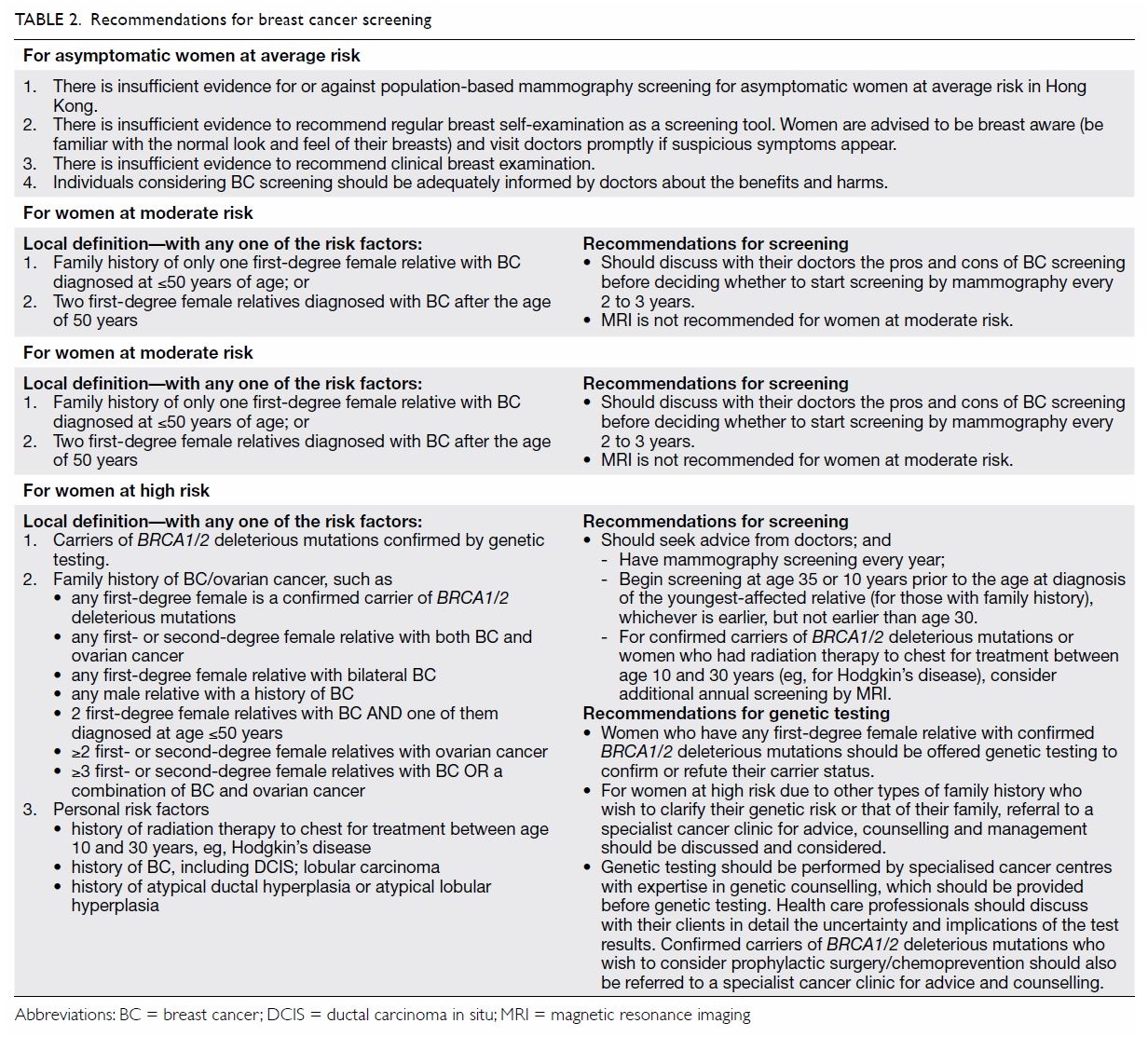
Screening for women at increased risk
Locally, there is lack of consensus on how to
identify women at increased risk of breast cancer. The CEWG has based its
conclusions on international studies and overseas practices to derive a
local definition of increased risk by adopting a set of qualitative risk
stratification criteria, which include BRCA1/2 deleterious
mutation carrier status, characteristics of family history and personal
risk factors. Women at increased risk are categorised as being at ‘high
risk’ or ‘moderate risk’ (Table 2).
Enhanced surveillance for early detection of breast
cancer has been suggested as a secondary preventive measure for women at
increased risk. Although there has been no randomised controlled trial of
mammography screening specifically in women at increased risk, previous
observational studies concluded that mammography screening of high-risk
population could be effective despite differences in study populations,
criteria for risk stratification, screening protocols, and measures of
effectiveness.48 49 50 51 Having said that, mammography generally has lower
sensitivity in younger women and those with a genetic predisposition to
breast cancer due to increased mammographic density obscuring the
radiological features of early breast cancer in premenopausal women, and a
higher likelihood of benign mammographic images for BRCA-related
breast cancer.52
Magnetic resonance imaging has been recommended as
an adjunct to routine mammography for surveillance of women at high risk.
Magnetic resonance imaging is more sensitive than mammography for
detection of breast cancer among BRCA1/2 mutation carriers.53 54 The IARC
review found improved sensitivity (95% vs 40%) but lower specificity (80%
vs 95%) of MRI plus mammography compared with mammography alone.8
In this regard, several studies have reported that
breast cancer screening with MRI in women at increased risk has
significantly shifted the stage at diagnosis from advanced stage to
earlier and pre-invasive stage, when compared with other common screening
modalities such as clinical breast examination, mammography, and
ultrasonography.55 56 57 A
modelling study of three large BRCA1/2 screening projects in UK,
Canada, and the Netherlands demonstrated that screening with mammography
and MRI (combined screening) detected relatively more DCIS and smaller
invasive cancers in BRCA2 mutation carriers than BRCA1
mutation carriers, resulting in larger reductions in breast cancer
mortality that ranged from 41.9% (for mammography alone) to 50.1%
(combined screening) for BRCA1 and from 46.8% (for mammography
alone) to 61.6% (combined screening) for BRCA2.58
One survival analysis among 959 UK women with
high-risk genetic mutations reported that 10-year survival was
significantly higher in the MRI-screened carriers of BRCA1/2
mutations (95.3%) compared with unscreened mutation carriers (73.7%).
However, the analysis did not show any significant difference in 10-year
survival between the combined mammography plus MRI and mammography-only
groups.59 The IARC review also
found variable all-cause survival results among the reviewed cohort
studies in women with BRCA1/2 mutation.8
Notwithstanding the above, studies showed that MRI
was superior to mammography in detecting hereditary breast cancer. The
radiation risk and false-positive rate of different screening strategies
should be considered when making individual screening decisions.60 Regarding the effectiveness of screening Chinese
women at higher breast cancer risk, there is currently a lack of local
studies on the role and effectiveness of MRI and/or mammography.
Based on the emerging scientific evidence and
international screening practices, the CEWG recommends that women at high
risk of breast cancer see a doctor and undergo mammography screening every
year, starting at age 35 or 10 years prior to the age at diagnosis of the
youngest affected relative (for those with a family history), whichever is
earlier, but not earlier than age 30. For confirmed carriers of BRCA1/2
deleterious mutations or women who have had radiation therapy to the chest
between age 10 and 30 years (eg, for Hodgkin’s disease), the CEWG
recommends that they consider additional annual screening by MRI.
Women who have any first-degree female relative
with confirmed BRCA1/2 deleterious mutations should be offered
genetic testing to confirm or refute their carrier status. Apart from
this, for women at high risk due to other types of family history of
breast/ovarian cancer (Table 2) who wish to clarify their genetic risk or
that of their family, referral to a specialist cancer clinic for advice,
counselling and management should be discussed and considered. Genetic
testing should be performed by specialised cancer centres with expertise
in genetic counselling that should be provided before genetic testing.
Health care professionals should discuss with their clients in detail the
limitations, uncertainties, and implications of test results.
There exists a group of women whose risk of
developing breast cancer may not be as high as those with a genetic
mutation or strong family history, but who are at moderate risk due to a
family history of breast cancer. The CEWG recommends that women at
moderate risk discuss with their doctor the pros and cons of breast cancer
screening before deciding whether to start screening by mammography every
2 to 3 years. Magnetic resonance imaging is not recommended for women at
moderate risk.
Table 2 summarises the current CEWG recommendations
for breast cancer screening in women at average and increased risk. A set
of leaflets and a booklet on breast cancer prevention and screening are
available (http://www.chp.gov.hk/en/content/9/25/31932.html) to the public
to empower informed decision-making.
Conclusion
After taking into consideration the local
epidemiology, emerging scientific evidence, and local and overseas
screening practices, the CEWG concludes that it is unclear whether breast
cancer screening does more harm than good for the asymptomatic woman at
average risk, and has reaffirmed that there is insufficient evidence so
far to recommend population-based mammography screening for these women.
Individuals considering breast cancer screening should discuss the matter
with their doctors and be adequately informed about the benefits and
harms. Primary prevention, breast awareness, and timely medical attention
for suspicious symptoms are recommended for women of any age. The CEWG
recommends that women at high risk seek medical advice and counselling
about breast cancer screening.
The CEWG will continue to review emerging evidence
for or against breast cancer screening and prevention, including the
outcome of research commissioned by the Research Office of the Food and
Health Bureau at a local institution to develop a validated risk
prediction tool for the local population. The findings will facilitate
formulation by the CEWG of evidence-based recommendations of criteria for
breast cancer screening, especially for those at higher risk.
Declaration
As editors of this journal, DVK Chao, HHF Loong,
and MCS Wong were not involved in the peer review process of this article.
All other authors have no conflicts of interest to disclose. All authors
had full access to the data, contributed to the study, approved the final
version for publication, and take responsibility for its accuracy and
integrity. An earlier version of this article was published online in the
Centre for Health Protection website, September 2017.
References
1. Hong Kong Cancer Registry, Hospital
Authority. Female Breast Cancer in 2015. Available from:
http://www3.ha.org.hk/cancereg/pdf/factsheet/2015/breast_2015.pdf.
Accessed 21 Dec 2017.
2. Department of Health, Census and
Statistics Department, Hong Kong SAR Government. Mortality statistics in
2016. Available from:
https://www.chp.gov.hk/en/healthtopics/content/25/53.html. Accessed 21 Dec
2017.
3. Ervik M, Lam F, Ferlay J, Mery L,
Soerjomataram I, Bray F. Cancer today. Lyon, France: International Agency
for Research on Cancer. Cancer Today. Available from:
http://gco.iarc.fr/today. Accessed 19 Sep 2017.
4. Doll R, Payne P, Waterhouse J. Cancer
Incidence in Five Continents: A Technical Report. Berlin: Springer Verlag;
1966.
5. Segi M. Cancer Mortality for Selected
Sites in 24 Countries (1950-57). Sendai: Tohoku University School of
Public Health; 1960.
6. Pharoah PD, Day NE, Duffy S, Easton DF,
Ponder BA. Family history and the risk of breast cancer: a systematic
review and meta-analysis. Int J Cancer 1997;71:800-9. Crossref
7. Kharazmi E, Chen T, Narod S, Sundquist
K, Hemminki K. Effect of multiplicity, laterality, and age at onset of
breast cancer on familial risk of breast cancer: a nationwide prospective
cohort study. Breast Cancer Res Treat 2014;144:185-92. Crossref
8. International Agency for Research on
Cancer. IARC Handbooks of Cancer Prevention. Volume 15: Breast Cancer
Screening. France: World Health Organization; 2016.
9. Shiovitz S, Korde LA. Genetics of breast
cancer: a topic in evolution. Ann Oncol 2015;26:1291-9. Crossref
10. Kwong A, Chen JW, Shin VY. A new
paradigm of genetic testing for hereditary breast/ovarian cancer. Hong
Kong Med J 2016;22:171-7. Crossref
11. Risch HA, McLaughlin JR, Cole DE, et
al. Population BRCA1 and BRCA2 mutation frequencies and
cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer
Inst 2006;98:1694-706. Crossref
12. Hong Kong Hereditary Breast Cancer
Registry. Our Statistics: Analysis of participants recruited into research
study till September 2017. Available from:
http://www.asiabreastregistry.com/en/hereditary-cancers/our-statistics.Accessed
21 Dec 2017.
13. Kwong A, Shin VY, Au CH, et al.
Detection of germline mutation in hereditary breast and/or ovarian cancers
by next-generation sequencing on a four-gene panel. J Mol Diagn
2016;18:580-94. Crossref
14. World Cancer Research Fund. American
Institute for Cancer Research. Breast Cancer 2010 Report: Food, nutrition,
physical activity, and the prevention of breast cancer. 2010. Available
from:
http://www.wcrf.org/sites/default/files/Breast-Cancer-2010-Report.pdf.
Accessed 19 Sep 2017.
15. International Agency for Research on
Cancer, World Health Organization. List of classifications by cancer sites
with sufficient or limited evidence in humans. Vol 1-117. Available from:
http://monographs.iarc.fr/ENG/Classification/Table4.pdf. Accessed 19 Sep
2017.
16. Collaborative Group on Hormonal
Factors in Breast Cancer. Menarche, menopause, and breast cancer risk:
individual participant meta-analysis, including 118 964 women with breast
cancer from 117 epidemiological studies. Lancet Oncol 2012;13:1141-51. Crossref
17. Collaborative Group on Hormonal
Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative
reanalysis of individual data from 47 epidemiological studies in 30
countries, including 50 302 women with breast cancer and 96 973 women
without the disease. Lancet 2002;360:187-95. Crossref
18. Collaborative Group on Hormonal
Factors in Breast Cancer. Breast cancer and hormonal contraceptives:
collaborative reanalysis of individual data on 53 297 women with breast
cancer and 100 239 women without breast cancer from 54 epidemiological
studies. Lancet 1996;347:1713-27. Crossref
19. Collaborative Group on Hormonal
Factors in Breast Cancer. Breast cancer and hormone replacement therapy:
collaborative reanalysis of data from 51 epidemiological studies of 52,705
women with breast cancer and 108,411 women without breast cancer. Lancet
1997;350:1047-59. Crossref
20. World Cancer Research Fund. American
Institute for Cancer Research. Continuous update project. Analysing
research on cancer prevention and survival. Diet, nutrition, physical
activity and breast cancer. 2017. Available from:
https://wcrf.org/sites/default/files/Breast-Cancer-2017-Report.pdf.
Accessed 19 Sep 2017.
21. Anderson WF, Pfeiffer RM, Dores GM,
Sherman ME. Comparison of age distribution patterns for different
histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers
Prev 2006;15:1899-905. Crossref
22. Hartmann LC, Sellers TA, Frost MH, et
al. Benign breast disease and the risk of breast cancer. N Engl J Med
2005;353:229-37. Crossref
23. Travis LB, Hill DA, Dores GM, et al.
Breast cancer following radiotherapy and chemotherapy among young women
with Hodgkin disease. JAMA 2003;290:465-75. Crossref
24. Singletary SE. Rating the risk factors
for breast cancer. Ann Surg 2003;237:474-82. Crossref
25. Brinton LA, Hoover R, Fraumeni JF, Jr.
Reproductive factors in the aetiology of breast cancer. Br J Cancer
1983;47:757-62.
26. Robinson D, Holmberg L, Møller H. The
occurrence of invasive cancers following a diagnosis of breast carcinoma
in situ. Br J Cancer 2008;99:611-5. Crossref
27. Kösters JP, Gøtzsche PC. Regular
self-examination or clinical examination for early detection of breast
cancer. Cochrane Database Syst Rev 2003;(2):CD003373. Crossref
28. Mittra I, Mishra GA, Singh S, et al. A
cluster randomized, controlled trial of breast and cervix cancer screening
in Mumbai, India: methodology and interim results after three rounds of
screening. Int J Cancer 2010;126:976-84. Crossref
29. Pisani P, Parkin DM, Ngelangel C, et
al. Outcome of screening by clinical examination of the breast in a trial
in the Philippines. Int J Cancer 2006;118:149-54. Crossref
30. Sankaranarayanan R, Ramadas K, Thara
S, et al. Clinical breast examination: preliminary results from a cluster
randomized controlled trial in India. J Natl Cancer Inst 2011;103:1476-80.
Crossref
31. Ohuchi N, Suzuki A, Sobue T, et al.
Sensitivity and specificity of mammography and adjunctive ultrasonography
to screen for breast cancer in the Japan Strategic Anti-cancer Randomized
Trial (J-START): a randomised controlled trial. Lancet 2016;387:341-8. Crossref
32. Gartlehner G, Thaler K, Chapman A, et
al. Mammography in combination with breast ultrasonography versus
mammography for breast cancer screening in women at average risk. Cochrane
Database Syst Rev 2013;(4):CD009632. Crossref
33. Lauby-Secretan B, Scoccianti C, Loomis
D, et al. Breast-cancer screening—viewpoint of the IARC Working Group. N
Engl J Med 2015;372:2353-8. Crossref
34. Miller AB, Wall C, Baines CJ, Sun P,
To T, Narod SA. Twenty five year follow-up for breast cancer incidence and
mortality of the Canadian National Breast Screening Study: randomised
screening trial. BMJ 2014;348:g366. Crossref
35. Autier P, Boniol M, Gavin A, Vatten
LJ. Breast cancer mortality in neighbouring European countries with
different levels of screening but similar access to treatment: trend
analysis of WHO mortality database. BMJ 2011;343:d4411. Crossref
36. Gøtzsche PC, Jørgensen KJ. Screening
for breast cancer with mammography. Cochrane Database Syst Rev
2013;(6):CD001877. Crossref
37. Marmot MG, Altman DG, Cameron DA,
Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer
screening: an independent review. Br J Cancer 2013;108:2205-40. Crossref
38. Swiss Medical Board. Systematic
mammography screening. December 2013. Available from:
http://www.medical-board.ch/fileadmin/docs/public/mb/fachberichte/2013-12-15_bericht_mammographie_final_kurzfassung_e.pdf.
Accessed
19 Sep 2017.
39. National Cancer Institute.
International Cancer Screening Network. Breast cancer screening programs
in 26 ICSN Countries, 2012: organization, policies, and program reach.
December 2016. Available from:
https://healthcaredelivery.cancer.gov/icsn/breast/screening.html. Accessed
31 Jan 2018.
40. Epidemiology and Disease Control
Division. Singapore Ministry of Health. National Health Survey 2010.
Available from:
https://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2011/NHS2010%20-%20low%20res.pdf.
Accessed 31 Jan 2018.
41. Health Promotion Administration,
Taiwan Ministry of Health and Welfare. Breast cancer screening rate:
Percentage of women aged 45-69 reporting a mammography in the past 2
years. Available from: http://210.71.254.151/dataset/143070580718.
Accessed 31 Jan 2018.
42. Jørgensen KJ, Zahl PH, Gøtzsche PC.
Breast cancer mortality in organised mammography screening in Denmark:
comparative study. BMJ 2010;340:c1241. Crossref
43. Wong IO, Schooling CM, Cowling BJ,
Leung GM. Breast cancer incidence and mortality in a transitioning Chinese
population: current and future trends. Br J Cancer 2015;112:167-70. Crossref
44. Kalager M, Zelen M, Langmark F, Adami
HO. Effect of screening mammography on breast-cancer mortality in Norway.
N Engl J Med 2010;363:1203-10. Crossref
45. Lui CY, Lam HS, Chan LK, et al.
Opportunistic breast cancer screening in Hong Kong; a revisit of the Kwong
Wah Hospital experience. Hong Kong Med J 2007;13:106-13.
46. Wong IO, Kuntz KM, Cowling BJ, Lam CL,
Leung GM. Cost-effectiveness analysis of mammography screening in Hong
Kong Chinese using state-transition Markov modelling. Hong Kong Med J
2010;16 Suppl 3:38-41.
47. Wong IO, Tsang JW, Cowling BJ, Leung
GM. Optimizing resource allocation for breast cancer prevention and care
among Hong Kong Chinese women. Cancer 2012;118:4394-403. Crossref
48. Maurice A, Evans DG, Shenton A, et al.
Screening younger women with a family history of breast cancer—does early
detection improve outcome? Eur J Cancer 2006;42:1385-90. Crossref
49. Kerlikowske K, Carney PA, Geller B, et
al. Performance of screening mammography among women with and without a
first-degree relative with breast cancer. Ann Intern Med 2000;133:855-63.
Crossref
50. Gui GP, Kadayaprath G, Darhouse N, et
al. Clinical outcome and service implications of screening women at
increased breast cancer risk from a family history. Eur J Surg Oncol
2006;32:719-24. Crossref
51. Cortesi L, Turchetti D, Marchi I, et
al. Breast cancer screening in women at increased risk according to
different family histories: an update of the Modena Study Group
experience. BMC Cancer 2006;6:210. Crossref
52. Lord SJ, Lei W, Craft P, et al. A
systematic review of the effectiveness of magnetic resonance imaging (MRI)
as an addition to mammography and ultrasound in screening young women at
high risk of breast cancer. Eur J Cancer 2007;43:1905-17. Crossref
53. Warner E, Messersmith H, Causer P,
Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance
imaging to screen women at high risk for breast cancer. Ann Intern Med
2008;148:671-9. Crossref
54. Passaperuma K, Warner E, Causer PA, et
al. Long-term results of screening with magnetic resonance imaging in
women with BRCA mutations. Br J Cancer 2012;107:24-30. Crossref
55. Kuhl C, Weigel S, Schrading S, et al.
Prospective multicenter cohort study to refine management recommendations
for women at elevated familial risk of breast cancer: the EVA trial. J
Clin Oncol 2010;28:1450-7. Crossref
56. Sardanelli F, Podo F, Santoro F, et
al. Multicenter surveillance of women at high genetic breast cancer risk
using mammography, ultrasonography, and contrast-enhanced magnetic
resonance imaging (the high breast cancer risk italian 1 study): final
results. Invest Radiol 2011;46:94-105. Crossref
57. Warner E, Hill K, Causer P, et al.
Prospective study of breast cancer incidence in women with a BRCA1
or BRCA2 mutation under surveillance with and without magnetic
resonance imaging. J Clin Oncol 2011;29:1664-9. Crossref
58. Heijnsdijk EA, Warner E, Gilbert FJ,
et al. Differences in natural history between breast cancers in BRCA1
and BRCA2 mutation carriers and effects of MRI screening-MRISC,
MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev
2012;21:1458-68. Crossref
59. Evans DG, Kesavan N, Lim Y, et al. MRI
breast screening in high-risk women: cancer detection and survival
analysis. Breast Cancer Res Treat 2014;145:663-72. Crossref
60. Lowry KP, Lee JM, Kong CY, et al.
Annual screening strategies in BRCA1 and BRCA2 gene
mutation carriers: a comparative effectiveness analysis. Cancer
2012;118:2021-30. Crossref