Hong Kong Med J 2018 Jun;24(3):245–51 | Epub 31 May 2018
DOI: 10.12809/hkmj176846
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Three-dimensional versus two-dimensional laparoscopy
for ovarian cystectomy: a prospective randomised study
MW Lui, MB, BS, MRCOG; Vincent YT Cheung, MB, BS,
FRCOG
Department of Obstetrics and Gynaecology, Queen
Mary Hospital, The University of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Dr MW Lui (luimanwa@gmail.com)
Abstract
Introduction: Three-dimensional
(3D) laparoscopy is now available as an alternative to conventional
two-dimensional (2D) laparoscopy for ovarian cystectomy. However, the
clinical value of 3D laparoscopy in benign gynaecological surgery
remains uncertain. This study evaluated whether 3D laparoscopy had any
advantages over 2D laparoscopy for ovarian cystectomy for apparently
benign ovarian cysts.
Methods: This prospective
randomised study involved patients undergoing laparoscopic ovarian
cystectomy. The primary outcomes were the duration of cystectomy and
surgeon’s Global Operative Assessment of Laparoscopic Skills (GOALS)
score. The secondary outcomes were the preferences, perceptions, and
adverse effects reported by the participating surgeons.
Results: There were 38 patients
assigned to the 2D laparoscopy group and 37 patients assigned to the 3D
laparoscopy group. Participating surgeons in the 2D group reported more
efficient tissue handling than did those in the 3D group (mean [standard
deviation] rating score, 4.2 [0.8] vs 3.8 [0.8]; P=0.033). Duration of
cystectomy (47.6 [32.0] min vs 51.6 [36.2] min; P=0.198) and overall
GOALS score (20.8 [3.9] vs 20.1 [3.3]; P=0.393) were similar between
both groups. Participating surgeons in the 2D group reported nausea,
dizziness, ocular fatigue, and blurring of vision less frequently than
did those in the 3D group (5.3% vs 45.9%; P<0.001).
Conclusion: There were no
significant benefits to using 3D laparoscopy compared with conventional
2D laparoscopy for ovarian cystectomy, and 3D laparoscopy may cause more
frequent adverse effects in surgeons.
New knowledge added by this study
- For ovarian cystectomy, there is no significant benefit to using three-dimensional laparoscopy rather than conventional two-dimensional laparoscopy.
- Three-dimensional laparoscopy permits binocular vision and depth perception; however, surgeons using three-dimensional laparoscopy more frequently reported adverse effects such as ocular fatigue, nausea, dizziness, and blurring of vision.
- Clinical use of three-dimensional laparoscopy in more complex surgical procedures, such as laparoscopic suturing, or with more experienced surgeons may be beneficial; therefore, further investigation is worthwhile.
Introduction
Laparoscopy has replaced laparotomy in most
gynaecological procedures, and laparoscopic cystectomy is currently the
mainstay of treatment for apparently benign ovarian cysts. However, the
absence of depth perception and limited instrument dexterity are major
drawbacks of laparoscopy. Advances in three-dimensional (3D) video imaging
technology allow 3D laparoscopy to provide better precision than
conventional two-dimensional (2D) laparoscopy, especially in depth
perception and spatial orientation. This increased precision may help
improve surgeon’s performance during laparoscopic surgery.
Studies have shown that 3D laparoscopy objectively1 2
and subjectively3 4 improves surgical performance, especially during
complex tasks.5 In addition, 3D
laparoscopy lessens the learning curve for beginners.6 The durations of laparoscopic cholecystectomy and
pelvic lymphadenectomy have also been shortened when performed using 3D
technologies.7 8 However, the clinical value of 3D laparoscopy in benign
gynaecological surgery remains uncertain. This study aimed to evaluate any
advantages of using 3D laparoscopy over 2D laparoscopy for ovarian
cystectomy.
Methods
This prospective randomised study was conducted
from May 2014 to May 2016 at the Queen Mary Hospital, Hong Kong, a
teaching hospital affiliated with The University of Hong Kong. Women with
apparently benign ovarian cysts who were scheduled for elective
laparoscopic ovarian cystectomy and who were eligible for the study were
invited at the pre-admission clinic to enrol in the study. Inclusion
criteria were being older than 18 years; ability to understand Cantonese,
Putonghua, or English; and ability to understand the study information
during the consent process. Patients who were intra-operatively found to
have no ovarian cyst were excluded from further analysis.
Patients were allocated by block randomisation to
undergo surgery with 2D laparoscopy (2D group) or 3D laparoscopy (3D
group) according to a computer-generated random sequence, in blocks of
five. The group allocation for each patient was disclosed to the surgeon
on the day before the surgery using a consecutively numbered, opaque,
sealed envelope. Demographic data of patients and duration of surgeries
were collected by a research nurse.
A pneumoperitoneum was created using a Veress
needle to provide visually guided closed access. For 3D laparoscopy, a
10-mm 3D telescopic videoscope was used (Endoeye Flex 3D; Olympus, Center
Valley [PA], US). All surgeons were trained for 3D laparoscopy using a
pelvic trainer with standardised tasks including peg transfer, precision
cutting, duct cannulation, and suturing with knot tying. The 3D
laparoscopy training was continued until the surgeons could confidently
operate using 3D images. All non-specialist surgeons were supervised by a
laparoscopist accredited at the advanced level in gynaecological
laparoscopic surgery, according to the Hong Kong College of Obstetricians
and Gynaecologists.9 At their
discretion, surgeons were allowed to switch from 3D laparoscopy to
traditional 2D laparoscopy if difficulty was encountered during surgery.
All 2D laparoscopies were performed using a 10-mm laparoscope (26033AP;
Karl Storz Endoscopy-America Inc, Culver City [CA], US). The same 32-inch
high-definition monitor (LMD-3215MT; Sony Corporation, Tokyo, Japan) was
used for all operations. In the 2D and 3D groups, cystectomy was performed
in the usual manner, using two or three 5-mm accessory ports inserted in
the lower abdomen under direct vision. The start time of the operation
(first skin incision), insertion of primary trocar, completion of
cystectomy, and end of operation (final skin closure) were recorded by the
research nurse.
After the operation, all surgeons were required to
self-evaluate their performance by using the Global Operative Assessment
of Laparoscopic Skills (GOALS) assessment tool.10
The five-item GOALS score includes assessment of depth perception,
bimanual dexterity, efficiency, tissue handling, and autonomy. Any
operator discomfort encountered during the surgery, any need to convert to
2D laparoscopy, and the surgeon’s preference for the type of laparoscopy
based on experience were also recorded. Demographic data and operative
findings, such as size and laterality of cysts, operative duration, and
presence of adhesions were analysed. Duration of cystectomy was defined as
the time from completion of primary port insertion to separation of the
cyst from the ovary and completion of haemostasis. The time spent on
specimen retrieval was not included, owing to variations in the specimen
retrieval method with or without use of a specimen bag.
The primary outcome of the present study was the
difference between the GOALS score of 2D and 3D groups. The secondary
outcomes were the duration of cystectomy and surgeon’s preferences and
reported adverse effects. Subgroup analysis was performed to compare the
outcomes for different experience levels among the surgeons. The surgeons
were categorised according to their experience in performing laparoscopic
surgery (≤5 years or >5 years). Surgeons with more than 5 years of
experience had achieved competency in gynaecological laparoscopic surgery
to at least an intermediate level, according to the Hong Kong College of
Obstetricians and Gynaecologists, and had completed a required number of
laparoscopic operations as requested by the College.9
A sample size of 36 patients was required in each
group, as calculated using an alpha of 0.05 and a beta of 0.2 for
detection of a difference in the sum of four items of the GOALS score
(excluding tissue handling) of 13 (interquartile range [IQR], 11-16) in
the 2D group and 16 (IQR, 12-18) in the 3D group, as based on a previous
study,11 using a two-sided test.
To allow for a 10% dropout rate, 40 patients were recruited into each
group. For randomised patients whose operations were subsequently
rescheduled outside the study period, treatment assignment numbers were
reallocated to subsequent eligible patients who provided consent.
Statistical analysis was performed using SPSS Windows version 21.0 (IBM Corp,
Armonk [NY], US). Data were presented as proportions or mean and standard
deviation. Student’s t test and Chi squared test were used for
statistical analyses. A P value of <0.05 was considered statistically
significant.
Results
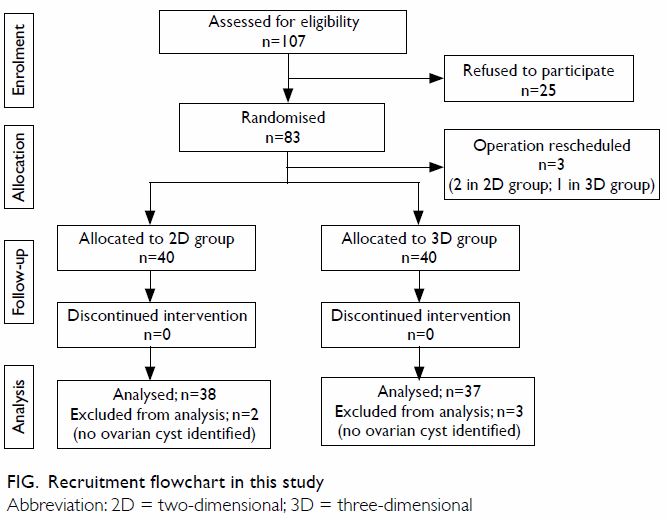
Of the 83 patients recruited into the study from
May 2014 to May 2016, operations were rescheduled for three patients who
were therefore withdrawn from the study; 80 patients completed the trial (Fig). Of these 80 patients, two from the 2D group
and three from the 3D group were excluded from analysis because no cysts
were identified. Finally, 38 patients in the 2D group and 37 patients in
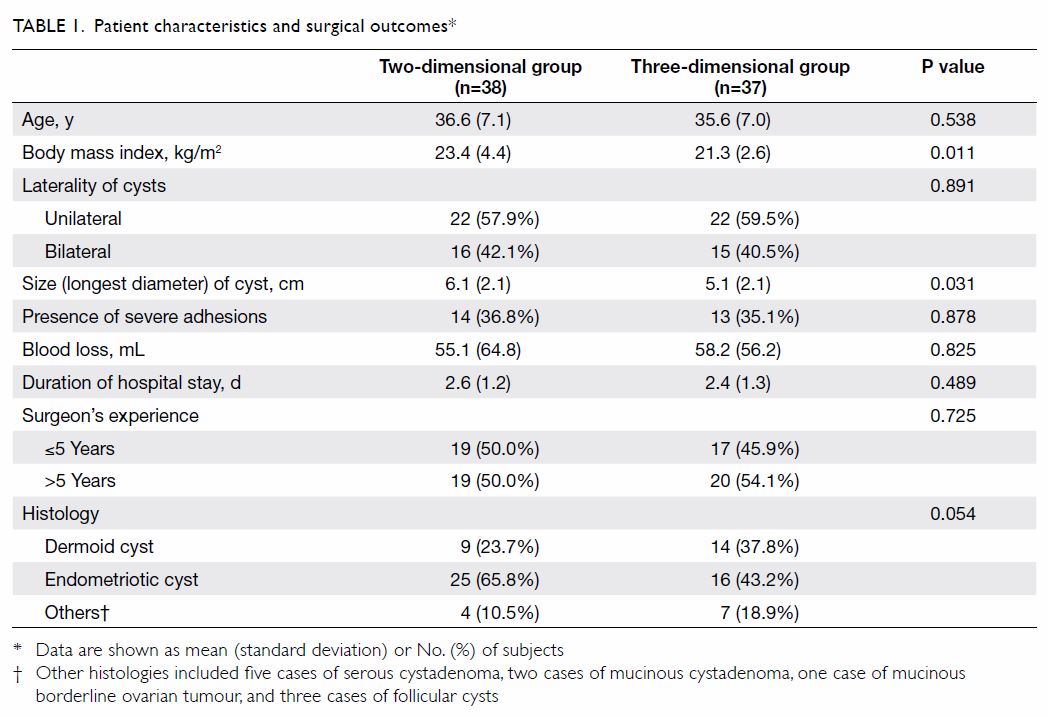
the 3D group were included for analysis. Patient characteristics and
surgical outcomes are presented in Table 1. There were no significant differences
between the 2D and 3D groups in terms of patient age, laterality of the
ovarian cyst, histological diagnosis of the cyst, presence of severe
adhesions, volume of blood loss, and experience level of the surgeon.
Three accessory ports were used in four patients in the 2D group and in
five patients in the 3D group. In all other patients, two accessory ports
were used. The mean (standard deviation) diameter of the ovarian cyst was
smaller in the 3D group than that in the 2D group (5.1 [2.1] cm vs 6.1 cm
[2.1] cm; P=0.031). Body mass index in the 2D group was significantly
higher than that in the 3D group (23.4 [4.4] kg/m2 vs 21.3
[2.6] kg/m2; P=0.011). Severe adhesion was defined as a score
of >20 for adnexal adhesion unilaterally12
or a score of >40 for endometriosis,13
according to the American Society for Reproductive Medicine
classifications.
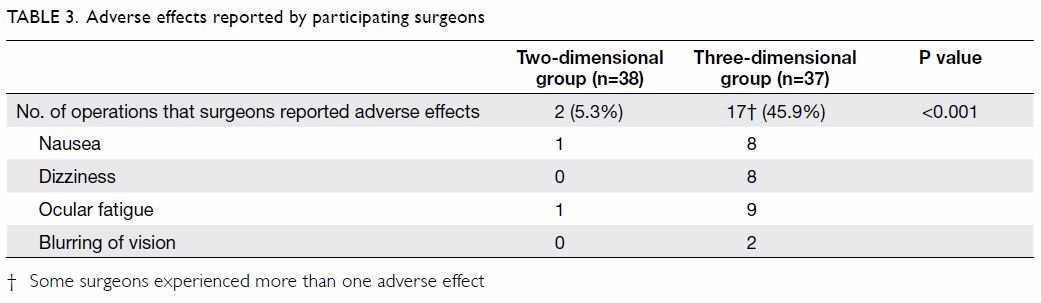
The differences between 2D and 3D groups in terms
of GOALS score and duration of cystectomy are presented in Table
2. A total of 15 surgeons participated in the study and there were
13 in each group: 11 in both, while two for each were involved in 2D and
3D groups, respectively. Participating surgeons in the 2D group reported
more efficient tissue handling than did those in the 3D group. Adverse
effects, including nausea, dizziness, ocular fatigue, and blurring of
vision were reported less frequently by participating surgeons in 2D group
than those in 3D group (Table 3). However, none of the participating
surgeons requested intra-operative conversion from 3D to 2D laparoscopy.
At the end of surgery, more participating surgeons in the 3D group
expressed a preference for 2D laparoscopy (43.3%) than for 3D laparoscopy
(18.9%), whereas 37.8% had no preference. A subgroup analysis of
participating surgeons in the two groups did not show statistically
significant differences in terms of GOALS score (2D vs 3D; 28.9 [5.1] vs
28.2 [46.0]; P=0.585), tissue handling (4.2 [0.8] vs 3.9 [0.8]; P=0.060),
and duration of cystectomy (93.7 [46.1] min vs 97.7 [52.2] min; P=0.737).
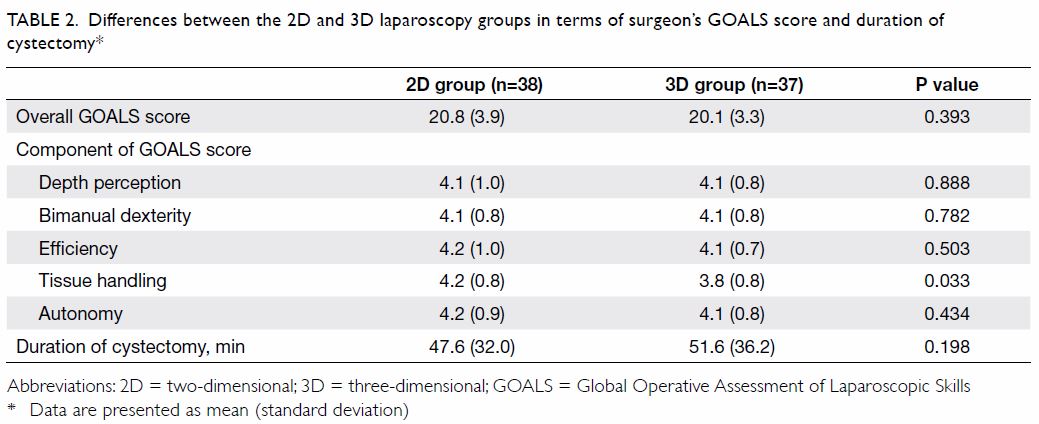
Table 2. Differences between the 2D and 3D laparoscopy groups in terms of surgeon’s GOALS score and duration of cystectomy
Subgroup analyses according to the experience level
of the surgeon and the presence of dense adhesions are shown in Tables
4 and 5, respectively. Two of the surgeons in the 3D group
and three of the surgeons in the 2D laparoscopy are accredited at the
advanced level in gynaecological laparoscopic surgery by the Hong Kong
College of Obstetricians and Gynaecologists. Surgeons with more than 5
years of laparoscopic experience reported lower scores in tissue handling
and efficiency when using 3D laparoscopy. There were no differences in
terms of GOALS score and duration of cystectomy in the subgroup with dense
adhesions.
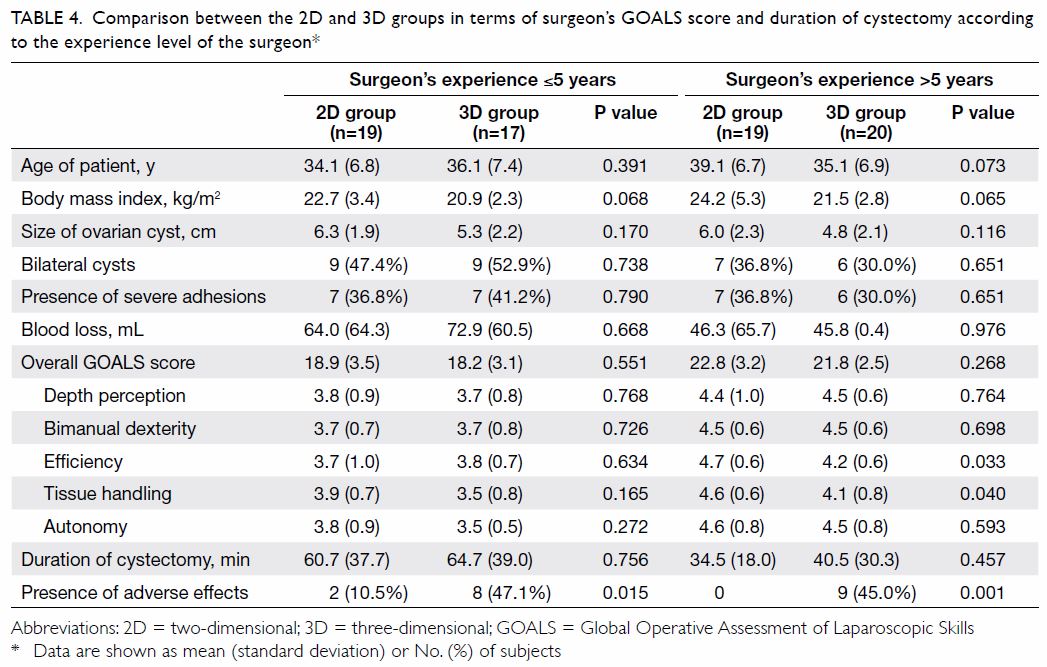
Table 4. Comparison between the 2D and 3D groups in terms of surgeon’s GOALS score and duration of cystectomy according to the experience level of the surgeon
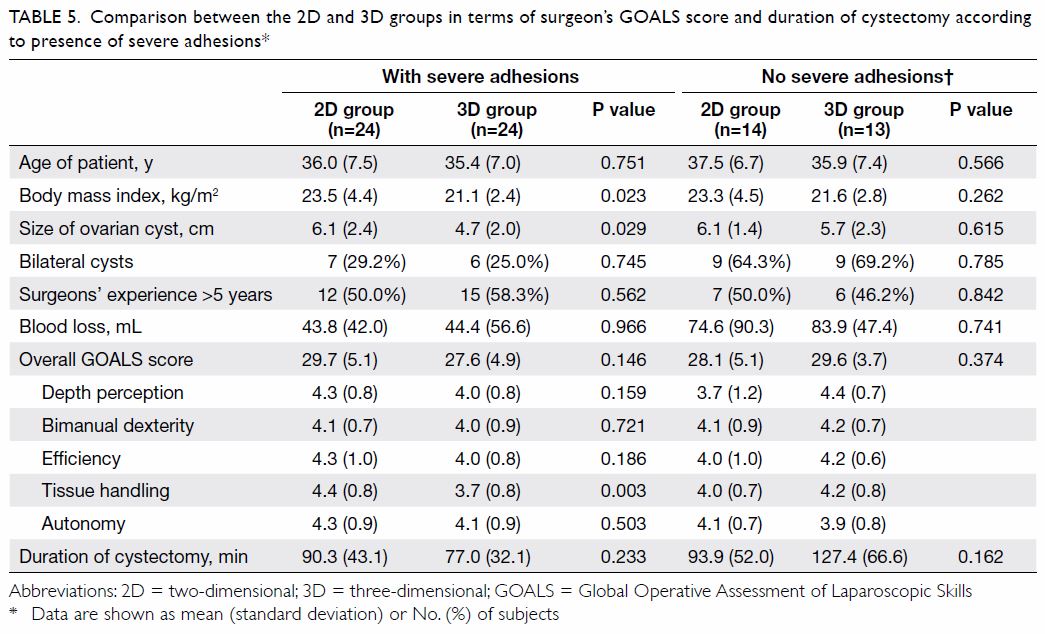
Table 5. Comparison between the 2D and 3D groups in terms of surgeon’s GOALS score and duration of cystectomy according to presence of severe adhesions
Discussion
Three-dimensional laparoscopy is gaining popularity
in modern gynaecological surgery owing to improved depth perception and
spatial orientation compared with 2D laparoscopy. Improved effectiveness
using 3D laparoscopy has been shown extensively in training models,
especially when performing complex tasks5
and in beginners.6 8 14 However,
our study was unable to show an improvement in terms of GOALS score and
duration of operation (Table 2) despite the 3D laparoscopy group having a
smaller mean ovarian cyst diameter (Table 1). This finding contradicts a recent
meta-analysis that 3D laparoscopy was associated with shortened surgical
time and hospital study, less blood loss, and fewer perioperative
complications.15
The addition of binocular vision and depth
perception in 3D laparoscopy is associated with more frequent adverse
effects such as ocular fatigue, nausea, and dizziness.16 In the present study, participating surgeons in the
3D group more frequently reported nausea, dizziness, ocular fatigue, and
blurring of vision than did those in the 2D group. However, this result
may be because the participating surgeons were unfamiliar with 3D images;
with experience, this discomfort may be lessened. Maintaining stability of
the telescope is of utmost importance during 3D laparoscopy; therefore,
familiarity with 3D images is important for assistants to mitigate adverse
effects. Furthermore, maintaining an appropriate distance between the
screen and the surgeon also alleviates nausea and ocular fatigue.16
Previous studies have shown that 3D laparoscopy is
beneficial for less experienced surgeons6
8 14
and for any surgeon performing complex tasks.5
However, in our subgroup analysis, we were unable to confirm any benefits
of 3D laparoscopy in relation to the experience level of the surgeons. All
participating surgeons were much more familiar with 2D laparoscopy and,
thus, the difference between groups might simply reflect the surgeon’s
assessment of what they are used to. This familiarity effect may explain
the lower scores in tissue handling and efficiency with 3D laparoscopy
attained by the more experienced surgeons.
The surgeon’s preference for 2D laparoscopy and the
heterogeneity of the participating surgeons and patients make the subgroup
analyses underpowered and represents a constitute limitation of the
present study. The differences in mean diameter of the ovarian cysts and
body mass index between the two groups also suggest ineffective
randomisation. Other limitations include ineffective randomisation,
withdrawal of patients after randomisation, and surgeon’s lack of
experience with 3D laparoscopy. During data analysis, there were also no
controls for possible confounding factors, such as experience of each
surgeon with 3D laparoscopy or significant differences in patient
characteristics between the groups.
In conclusion, the results show that there is no
significant benefit to using 3D laparoscopy for ovarian cystectomy
compared with conventional 2D laparoscopy. Moreover, 3D laparoscopy is
associated with more frequent adverse effects for surgeons. However, it is
possible that more complex procedures, such as those involving
laparoscopic suturing and knot tying, might be easier to perform with 3D
laparoscopy than with 2D laparoscopy. Therefore, further evaluation of the
clinical performance of 3D laparoscopy in operations of different
complexities and of surgeons with different experience levels are
warranted.
Author contributions
All authors have made substantial contributions to
the concept of this study; acquisition of data; analysis or interpretation
of data; drafting of the article; and critical revision for important
intellectual content.
Acknowledgement
We wish to thank Ms Wai-ki Choi for helping in
patient recruitment and data collection.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Declaration
The authors have no conflicts of interest to
disclose. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity. The study was presented as oral
presentation in the 25th Asian and Oceanic Congress of Obstetrics and
Gynaecology, 16 June 2017, Hong Kong.
Ethical approval
Ethical approval was obtained from the
Institutional Review Board of the University of Hong Kong/Hospital
Authority Hong Kong West Cluster. Written informed consent was obtained
from all participating patients and surgeons. The study was registered
with ClinicalTrials.gov (NCT02775344).
References
1. Storz P, Buess GF, Kunert W, Kirschniak
A. 3D HD versus 2D HD: surgical task efficiency in standardised phantom
tasks. Surg Endosc 2012;26:1454-60. Crossref
2. Lusch A, Bucur PL, Menhadji AD, et al.
Evaluation of the impact of three-dimensional vision on laparoscopic
performance. J Endourol 2014;28:261-6. Crossref
3. Tanagho YS, Andriole GL, Paradis AG, et
al. 2D versus 3D visualization: impact on laparoscopic proficiency using
the fundamentals of laparoscopic surgery skill set. J Laparoendosc Adv
Surg Tech A 2012;22:865-70. Crossref
4. Sørensen SM, Savran MM, Konge L, Bjerrum
F. Three-dimensional versus two-dimensional vision in laparoscopy: a
systematic review. Surg Endosc 2016;30:11-23. Crossref
5. Wagner OJ, Hagen M, Kurmann A, Horgan S,
Candinas D, Vorburger SA. Three-dimensional vision enhances task
performance independently of the surgical method. Surg Endosc
2012;26:2961-8. Crossref
6. Cicione A, Autorino R, Breda A, et al.
Three-dimensional vs standard laparoscopy: comparative assessment using a
validated program for laparoscopic urologic skills. Urology
2013;82:1444-50. Crossref
7. Bilgen K, Ustun M, Karakahya M, et al.
Comparison of 3D imaging and 2D imaging for performance time of
laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech
2013;23:180-3. Crossref
8. Fanfani F, Rossitto C, Restaino S, et
al. How technology can impact surgeon performance: a randomized trial
comparing 3-dimensional versus 2-dimensional laparoscopy in gynecology
oncology. J Minim Invasive Gynecol 2016;23:810-7. Crossref
9. Hong Kong College of Obstetricians and
Gynaecologists. Endoscopic surgery: accreditation of gynaecological
laparoscopic surgery. Available from:
http://www.hkcog.org.hk/hkcog/pages_2_64.html. Accessed 4 Jun 2017.
10. Vassiliou MC, Feldman LS, Andrew CG,
et al. A global assessment tool for evaluation of intraoperative
laparoscopic skills. Am J Surg 2005;190:107-13. Crossref
11. Ko JK, Li RH, Cheung VY.
Two-dimensional versus three-dimensional laparoscopy: evaluation of
physicians’ performance and preference using a pelvic trainer. J Minim
Invasive Gynecol 2015;22:421-7. Crossref
12. Hulka JF, Omran K, Berger GS.
Classification of adnexal adhesions: a proposal and evaluation of its
prognostic value. Fertil Steril 1978;30:661-5. Crossref
13. Revised American Fertility Society
classification of endometriosis: 1985. Fertil Steril 1985;43:351-2. Crossref
14. Alaraimi B, El Bakbak W, Sarker S, et
al. A randomized prospective study comparing acquisition of laparoscopic
skills in three-dimensional (3D) vs. two-dimensional (2D) laparoscopy.
World J Surg 2014;38:2746-52. Crossref
15. Cheng J, Gao J, Shuai X, Wang G, Tao
K. Two-dimensional versus three-dimensional laparoscopy in surgical
efficacy: a systematic review and meta-analysis. Oncotarget
2016;7:70979-90. Crossref
16. Kunert W, Storz P, Kirschniak A. For
3D laparoscopy: a step toward advanced surgical navigation: how to get
maximum benefit from 3D vision. Surg Endosc 2013;27:696-9. Crossref