Hong Kong Med J 2018 Jun;24(3):218–25 | Epub 21 May 2018
DOI: 10.12809/hkmj176888
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Outcomes of salvage radiotherapy for recurrent prostate
cancer after radical prostatectomy
Eric KC Lee, MB, ChB, FHKAM (Radiology); WH Mui,
MB, BS, FHKAM (Radiology); Adrian W Chan, MB, BS, FRCR; Y Tung, MB, BS,
FHKAM (Radiology); Frank CS Wong, MB, ChB, FHKAM (Radiology)
Department of Clinical Oncology, Tuen Mun Hospital,
Tuen Mun, Hong Kong
Corresponding author: Dr Eric KC Lee (leekachai2000@yahoo.com.hk)
Abstract
Introduction: Salvage
radiotherapy (SRT) provides effective biochemical control for patients
with prostate cancer who have prostate-specific antigen (PSA) failure
after radical prostatectomy. However, the effect of SRT on long-term
clinical outcomes remains unknown. Therefore, we report the natural
history of patients treated with SRT.
Methods: We identified 84
Chinese patients with prostate cancer treated with SRT to the prostatic
fossa alone during 2006-2017 at Tuen Mun Hospital, Hong Kong. Survival
was calculated using Kaplan-Meier method. Log rank test and Cox
regression were used to determine significance of clinical parameters
with outcomes.
Results: Median SRT dose given
was 70 Gy (range, 64-76 Gy). Median pre-SRT PSA level was 0.4 ng/mL
(0.2-7.4 ng/mL). After SRT, 47 (56%) patients had undetectable (<0.1
ng/mL) PSA levels. After median follow-up of 48 months (2 months to
10 years), 25 (30%) patients had further biochemical
progression. Subsequently, 12 patients received androgen deprivation
therapy and nine (11%) developed distant metastasis. The 5-year
biochemical progression–free survival, androgen deprivation therapy–free
survival and metastasis-free survival were 62.7%, 83.5% and 86.7%,
respectively. Early PSA failure after radical prostatectomy (hazard
ratio=7.4), negative surgical margin (hazard ratio=2.7), positive
extracapsular extension (hazard ratio=4.6), and detectable PSA levels
after SRT (hazard ratio=17.3) were associated with lower biochemical
progression–free survival after SRT.
Conclusions: High-dose SRT with
intensity-modulated radiotherapy/volumetric modulated arc radiotherapy
is an effective local treatment that can prevent distant metastasis and
avoid the need for androgen deprivation therapy in Chinese patients who
have PSA failure after radical prostatectomy.
New knowledge added by this study
- Better biochemical progression–free survival after salvage radiotherapy (SRT) can be achieved through higher radiation doses and better selection of patients.
- Patients with prostate-specific antigen (PSA) failure ≤24 months after radical prostatectomy, negative surgical margin, positive extracapsular extension, or detectable PSA after SRT are more likely to develop biochemical progression after SRT.
- Distant metastasis is more likely to occur in patients with extracapsular extension, patients who cannot achieve biochemical complete response, and patients who develop biochemical progression within 1 year of SRT.
- For these patients, close monitoring for distant metastasis may be needed.
Introduction
Prostate cancer (PCa) is the most common
non-cutaneous malignancy among men in western countries, and is the third
most common cancer among men in Hong Kong.1
Increasing public awareness in the Chinese community, as well as the
common use of prostate-specific antigen (PSA) tests by primary health
physicians, have led to detection of PCa at an earlier stage, when it is
amenable to either radical surgery or radiotherapy (RT).2 Because of recent advancements in operative management,
such as robotic-assisted laparoscopic prostatectomy,3 many patients have found radical prostatectomy (RP) the
preferred treatment option. Nevertheless, adjuvant radiotherapy (ART) to
the prostatic fossa is indicated postoperatively in cases with positive
surgical margin (SM), or residual disease from extracapsular extension
(ECE). Alternatively, patients may receive salvage radiotherapy (SRT) when
there is PSA failure, defined as any detectable and rising PSA level after
RP.
Currently, ART is still being compared with SRT in
three randomised controlled trials (RADICALS, RAVES, GETUG-AFU 17).4 5 6 While the results of these European and Australasian
studies are still pending, the American Society for Radiation
Oncology/American Urological Association guidelines recommend that
physicians offer SRT to patients with PSA or local recurrence after RP in
whom there is no evidence of distant metastasis (DM).7 Patients should be advised that SRT should be
administered at the earliest sign of PSA recurrence. Approximately 60% of
patients who are treated with SRT before the PSA level rises to >0.5
ng/mL will achieve an undetectable PSA level, providing long-term PSA
control in nearly half of them.8
However, after SRT, some patients may still
experience further clinical progression, including DM and cancer-related
death. The effect of SRT on the long-term outcomes including
metastasis-free survival (MFS) and overall survival—especially in Chinese
patients—is not well understood. Herein we report the long-term survival
data of patients at a single institution in Hong Kong who received SRT to
the prostatic fossa using modern RT techniques.
Methods
Patient selection
Using the MOSAIQ system (version 2.62, IMPAC
Medical Systems, Inc.; Sunnyvale [CA], US), we identified 91 Chinese
patients treated with postoperative RT to the prostatic fossa at Tuen Mun
Hospital, Hong Kong, between 2006 and 2017. The treatment records and
clinical data of these patients were reviewed. Two patients who received
ART with undetectable PSA were excluded. Patients who had received
androgen deprivation therapy (ADT) prior to SRT were also excluded. These
selection criteria yielded 84 evaluable individuals who received SRT to
the prostatic fossa alone for PSA failure (defined as detection of PSA
concentration at 0.2 ng/mL, with a second confirmatory level detected at
0.2 ng/mL) more than 3 months after RP.
Radiation therapy techniques
A planning computed tomographic scan was performed
for each patient with 3-mm slice thickness, and the clinical target volume
was determined with reference to one of the published consensus
guidelines.9 10 11 The usual
boundaries of the clinical target volume are: inferiorly, 5 mm below the
urethral anastomosis; anteriorly, the posterior aspect of the symphysis
pubis or the posterior third of the bladder; laterally, the medial border
of the obturator internus and levator ani muscles; posteriorly, the
anterior mesorectal fascia; and superiorly, 5 mm above the surgical bed.
The planning target volume was defined as clinical target volume with a
margin of 4 to 5 mm posteriorly and 0.7 to 1 cm in all other directions.
Organs at risk, including the rectum, bladder, and bilateral femoral heads
were contoured. Conformal radiotherapy or inverse planning techniques with
intensity-modulated radiotherapy (IMRT) using seven to nine static beams
were used before October 2010. After that, volumetric modulated arc
radiotherapy (VMAT) was employed using the Pinnacle treatment planning
system (Philips Medical Systems, Fitchburg [WI], US) with treatment
delivered through one to two dynamic cone arcs.
Variable definition
Clinical data included age at SRT, time from
surgery to RT (≤24 months vs >24 months), SRT dose, pre-SRT PSA level,
and post-SRT nadir PSA. Pathological data consisted of pathological T
stages (T2a vs T2b vs T2c vs T3a or T3b), ECE, seminal vesicle invasion,
SM, and pathological Gleason scores (≤7 or ≥8).
Outcome definition
After SRT, patients were followed up with PSA level
checks every 3 months in the first 2 years, every 6 months from year 3 to
year 5, then annually. A complete response was defined as an undetectable
nadir PSA (<0.1 ng/mL). Biochemical progression (PSA failure) was
defined as a rise of PSA level by 0.2 ng/mL above the nadir with a second
confirmation at least 1 week apart.12
Biochemical progression-free survival (bPFS) was defined as the date from
SRT completion to the first date of biochemical progression. Patients who
showed biochemical progression or symptoms suggestive of metastasis
received imaging studies at the discretion of the oncologist.
Metastasis-free survival was defined as the date from SRT completion to
the date of occurrence of metastasis on imaging. Patients who showed
biochemical progression with or without metastasis were counselled on the
use of ADT; ADT-free survival was defined as the date of SRT completion to
the first date of ADT administration.
Statistical analyses
The Kaplan-Meier method was used to estimate bPFS,
MFS, and ADT-free survival. Log-rank tests and Cox regression analysis
were used to test the association between groups and oncologic outcomes.
Covariates consisted of continuous variables, including patient age at
SRT, SRT dose, and pre-SRT PSA, and discrete variables including post-SRT
nadir PSA (detectable vs undetectable), pathological T stages (T2a vs T2b
vs T2c vs T3a vs T3b), pathological Gleason score (≤7 vs ≥8), SM (negative
vs positive), ECE (negative vs positive), seminal vesicle invasion
(negative vs positive), and time of SRT (≤24 months after RP or >24
months after RP). Only variables that were significantly associated with
outcomes on univariate analyses were further tested for association in
multivariate analyses.
Statistical analyses were performed using IBM SPSS
Statistics for Windows, version 24.0 (IBM Corp, Armonk [NY], US), and
numerical data were presented according to Cole.13
Results
Patients
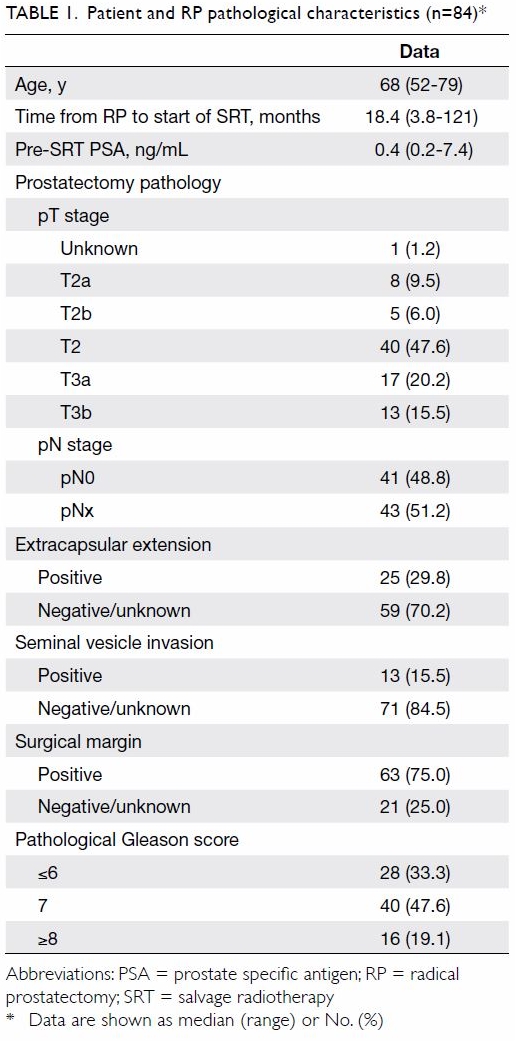
The median age of the 84 patients was 68 years
(range, 52-79 years) when they received SRT. The patients’ median pre-SRT
PSA level was 0.4 ng/mL (range, 0.2-7.4 ng/mL). Of the patients, 63 (75%)
had positive SM in their prostatectomy specimens. Extracapsular extension
was detected in 25 (29.8%) patients. Pelvic lymph nodes of 41 patients
were sampled during RP and were all found to be negative for malignancy.
These and other pathological characteristics are summarised in Table
1. The median time from surgery to start of SRT was 18.4 months
(range, 3.8-121 months).
Treatment delivery
Before October 2010, one patient was treated with
conformal RT and 10 patients were treated with IMRT. Subsequently the
other 73 patients were treated with VMAT. The median dose given to the
prostatic fossa was 70 Gy (range, 64-76 Gy), with 66 (79%) patients
receiving a dose of ≥70 Gy. The mean dose delivered using VMAT (69.5 Gy)
was slightly higher than that delivered using IMRT/conformal RT (68.1 Gy)
[independent-samples t test, t=2.1; P=0.028].
Treatment outcome
Of 84 patients, 47 (56%) had undetectable PSA
levels (complete response; <0.1 ng/mL) after SRT. After a median
follow-up of 48 months (range, 2-120 months), 25 (30%) patients had
biochemical progression with an estimated 5-year bPFS of 62.7% (95%
confidence interval [CI], 50.1-75.3%) [Fig 1a]. Among the 25 patients who developed
biochemical progression after SRT, seven were found to have DM and
subsequently received ADT, and five started ADT in the absence of DM, two
of whom later developed DM and had their disease became
castration-resistant. Overall, 12 patients received ADT and nine (11%)
patients developed DM. The 5-year ADT-free survival and MFS were 83.5%
(95% CI, 73.7-93.3%) and 86.7% (95% CI, 77.7-95.7%), respectively (Fig
1b, c). Notably, only six patients died, all from causes other than
PCa.
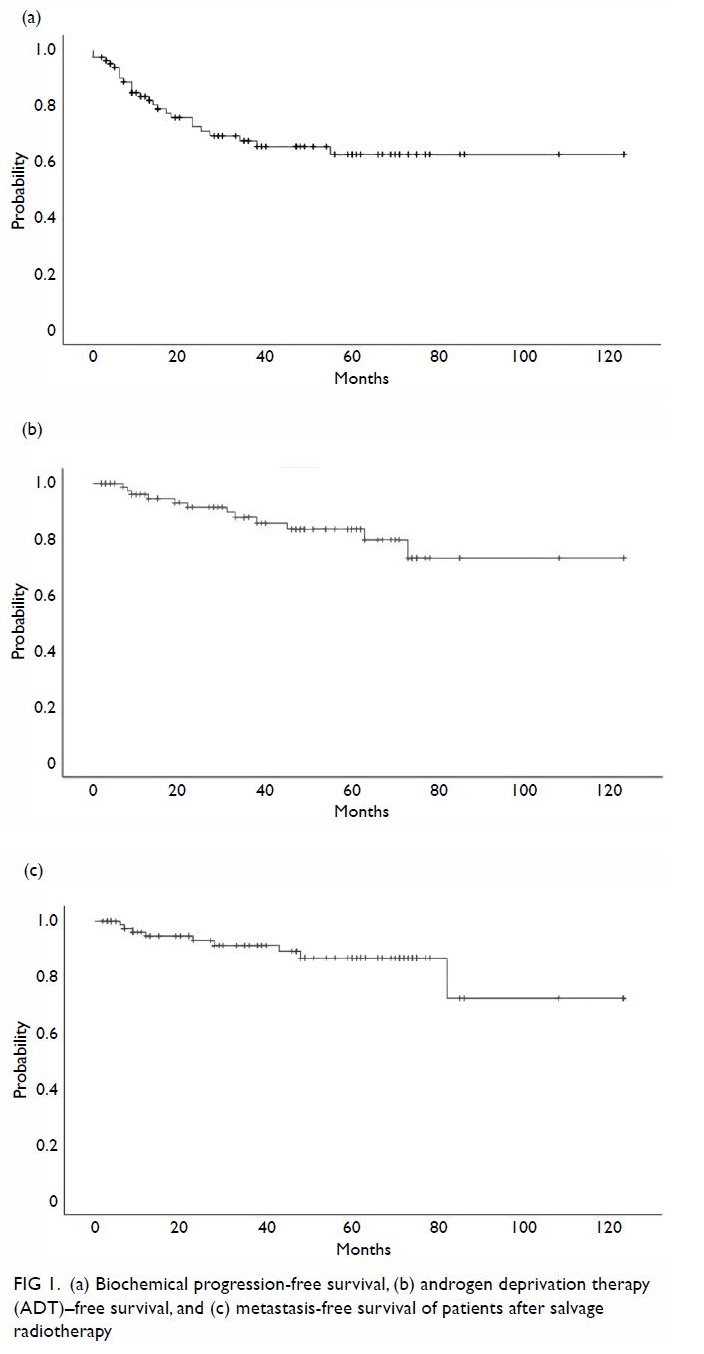
Figure 1. (a) Biochemical progression–free survival, (b) androgen deprivation therapy–free survival, and (c) metastasis-free survival of patients after salvage radiotherapy
Biochemical progression–free survival and
metastasis-free survival
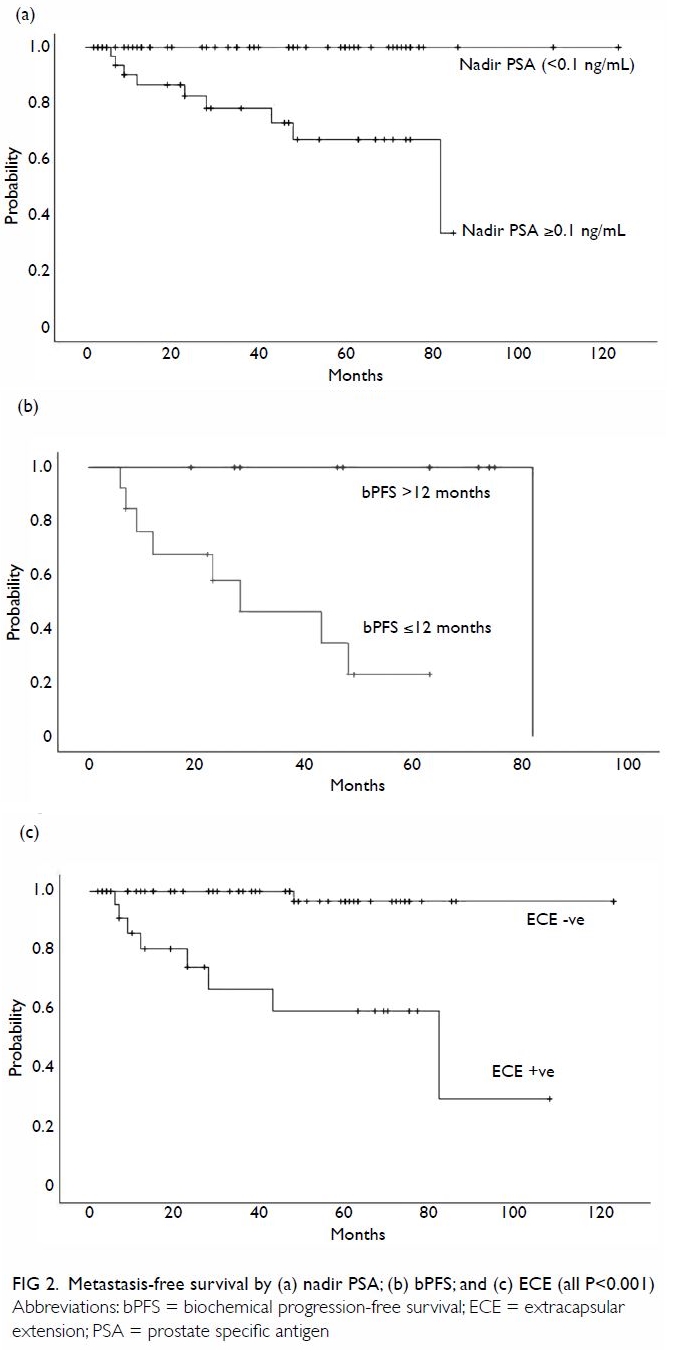
On univariate analysis, a post-SRT nadir PSA ≥0.1
ng/mL, positive ECE, and bPFS ≤12 months were significantly associated
with a shorter MFS (all P<0.001; Fig 2). Similarly, a post-SRT nadir PSA ≥0.1 ng/mL
(P<0.001), positive ECE (P<0.001), negative SM (P=0.045),
pathological Gleason score ≥8 (P=0.002), and time from surgery to SRT ≤24
months (P=0.008) were significant predictors of a shorter bPFS (Fig
3). The pre-SRT PSA level, age, and SRT dose were not associated
with either MFS or bPFS in this cohort on univariate analysis. On
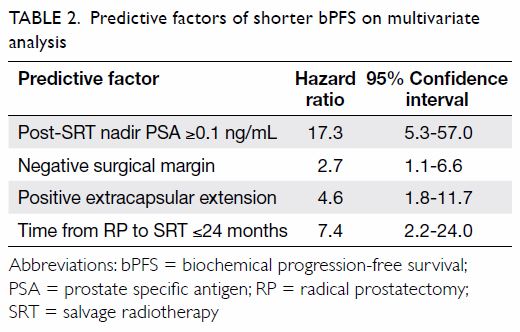
multivariate analysis using the Cox regression method, negative SM (hazard
ratio [HR]=2.7; 95% confidence interval [CI], 1.1-6.6), positive ECE
(HR=4.6; 95% CI, 1.8-11.7), post-SRT nadir PSA ≥0.1 ng/mL (HR=17.3; 95%
CI, 5.3-57.0), and time from surgery to SRT ≤24 months (HR=7.4; 95% CI,
2.2-24.0) retained significant association with a shorter bPFS (Table
2). There was no variable significantly associated with MFS after
multivariate analysis.
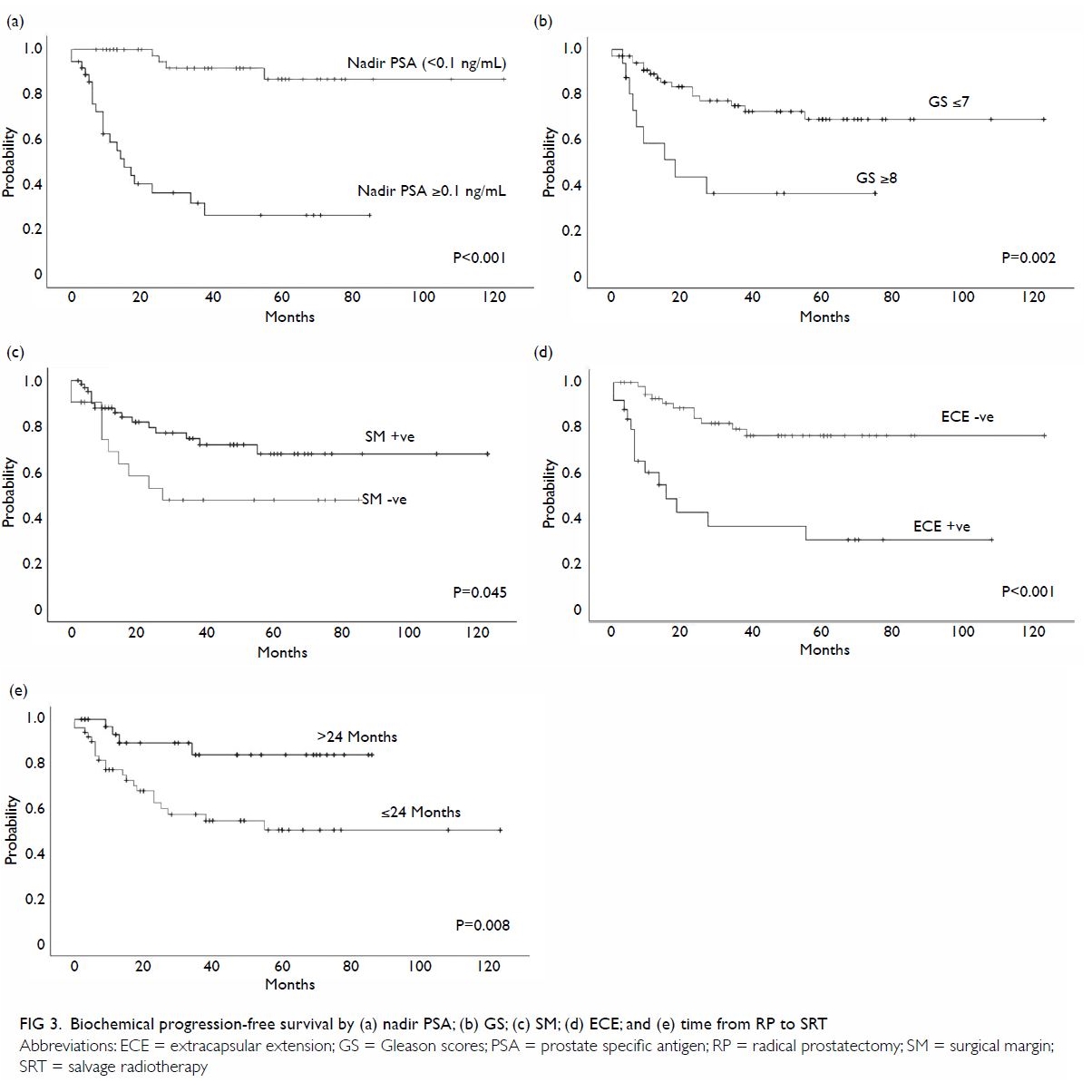
Figure 3. Biochemical progression–free survival by (a) nadir PSA; (b) GS; (c) SM; (d) ECE; and (e) time from RP to SRT
Discussion
Most patients who develop biochemical recurrence
after RP for localised PCa remain asymptomatic for many years.14 However, patients with increasing PSA level are at
high risk of developing DM. Salvage radiotherapy is effective in terms of
biochemical control when it is administered at low PSA level. Stephenson
et al12 reported a 6-year
progression-free probability of 32% after SRT. In their
multi-institutional retrospective cohort of 1603 consecutive patients from
17 North American tertiary referral centres who received SRT after RP for
PSA recurrence between 1987 and 2005, the median dose was only 64.8 Gy
(interquartile range, 63-66 Gy) delivered using older techniques. The
5-year bPFS of 62.7% in the present study is similar or better than those
reported in western countries.12 15 16
This might be due to better selection of patients (most patients started
SRT when their PSA level was ≤0.5 ng/mL), or the higher dose of SRT to the
prostatic fossa (median 70 Gy). In our cohort, all patients but one were
treated using IMRT/VMAT. Intensity-modulated radiotherapy was introduced
in the 1990s and it has since enabled radiation oncologists to deliver
higher doses of radiation to treat patients with PCa—including patients
with residual disease at the prostatic fossa—without causing excessive
radiation damage to healthy tissue.17
18 19
Volumetric modulated arc radiotherapy has recently attracted much interest
because it can dynamically deliver a radiation dose during rotation of the
gantry; this is also superior to IMRT in terms of its plan qualities and
efficiency in the treatment of PCa.20
21
Pisansky et al22
reported that SRT doses of ≥66.0 Gy were associated with reduced
cumulative incidence of biochemical progression. A systemic review by King23 provides level 2a evidence for
escalated SRT dose of at least 70 Gy. A 2% improvement in relapse-free
survival can be achieved for each additional Gy from 60 Gy to 70 Gy.23 However, higher SRT dose was not shown to be
associated with better bPFS/MFS in our 84 patients by univariate analysis,
because most (79%) had been treated with an SRT dose of ≥70 Gy, and the
follow-up time may still be too short to demonstrate any further
dose-response relationship. We postulated that such high-dose SRT can be
delivered safely with modern techniques using VMAT, therefore our current
usual prescribed dose is 70 Gy to the prostatic fossa, unless limited by
dose constraints of the organ at risk. We have previously shown the
efficiency and low toxicities using VMAT for SRT to the prostatic fossa.24 Longer follow-up is necessary to
ensure that late complications are within safety limits.
Despite the success of SRT in biochemical control,
some patients may develop further biochemical progression. In our present
study, patients whose surgical pathology revealed negative margin and
positive ECE had a shorter bPFS (HRs of 2.7 and 4.6, respectively).
Patients who start SRT within 2 years of RP may also have a shorter PSA
doubling time, leading to earlier detection of recurrence. These patients
have a greater than 7-fold higher risk of biochemical regression after SRT
than those with later recurrence. Salvage radiotherapy to the prostatic
fossa alone cannot eradicate cancer that has spread outside the surgical
bed after RP. In fact, negative SM, positive ECE, and shorter PSA doubling
time are three of the many adverse factors which predict a shorter bPFS
after SRT, using the nomogram proposed by Stephenson et al.25 However, we cannot demonstrate the importance of
pre-SRT PSA level in our patient cohort because more than 65% of the
patients had started SRT when their PSA level was ≤0.5 ng/mL.
Overall, the role of SRT in improving MFS and
overall survival is less certain, because the disease can be indolent and
mortality due to causes other than PCa is more likely in older patients.
Patients also have other complications related to disease progression,
such as painful bone metastasis. Efforts have been made to identify
surrogate endpoints that can predict further disease progression,
metastasis, and even cancer-related death after SRT. In a single
institution review, Johnson et al26
reported approximately 50% of men experience further biochemical
progression after SRT. Those who have a short interval to biochemical
progression of ≤18 months after SRT are most likely to experience DM,
PCa-specific mortality, and overall mortality. Bartkowiak et al27 reported on the long-term outcomes of patients with a
median follow-up of 7 years (maximum, 14 years) after SRT. They found that
a post-SRT nadir PSA <0.1 ng/mL was associated with improved bPFS and
overall survival. The results of our univariate analysis support the
abovementioned findings27 (Fig
2a, b). On multivariate analysis, we found that undetectable nadir
PSA (<0.1 ng/mL) is the most important factor for predicting longer
bPFS (Table 2). In the present study, of the 47 patients
who achieved biochemical complete response after SRT, none developed DM.
In contrast, among the 25 patients who had biochemical progression, nine
whose disease progressed within 1 year after SRT eventually developed DM.
Although our result of a 5-year MFS of nearly 90% is encouraging, with the
median follow-up of only 4 years, we can hypothesise only that better
biochemical control is correlated with improvements in other clinical
outcomes. For patients whose PSA level does not become undetectable and
rapidly rises within 1 year after SRT (bPFS ≤12 months), close monitoring
for DM may be needed.
The improvement in overall survival and MFS of
adjuvant ADT with SRT has been demonstrated by Shipley et al28 in a phase III study. However, ADT is not routinely
recommended to our patients because of the known metabolic and
cardiovascular toxicities and the negative impact on patients’ quality of
life. In addition, most of our patients have fewer adverse features than
those reported by Shipley et al.28
For patients with biochemical regression alone after SRT, we suggest
monitoring for any site of disease recurrence such that further SRT could
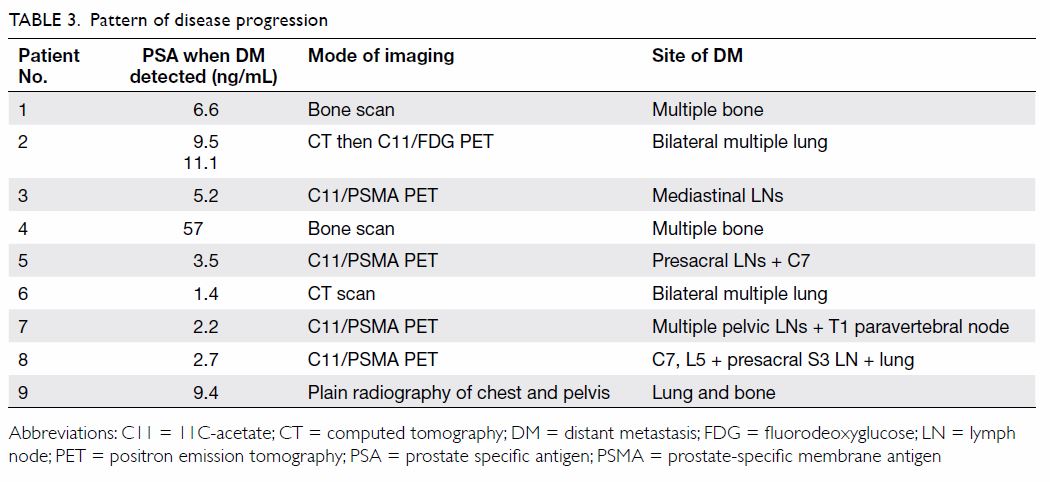
still be feasible. Nonetheless, we applied positron emission tomography
with 68 Ga-labelled prostate-specific membrane antigen (PET-PSMA) to
identify the site of recurrence in four of our patients when their PSA
levels increased to ≥2.2 ng/mL (Table 3). All four patients were found to have DM
which was not amenable to further local treatment and ADT had become their
only option. It remains unclear whether PET-PSMA or other imaging studies
at lower PSA levels are sensitive or useful enough to alter the management
decision.29 Further research to
study the use of novel radiological examinations in this situation is
needed.
Conclusions
This is the first report to demonstrate the
therapeutic effects in terms of bPFS and MFS of SRT in Chinese patients in
a Hong Kong centre. Salvage radiotherapy is an effective local treatment
that can prevent DM and avoid the need for ADT in most patients who have
PSA failure after RP in Chinese patients. Our results appear to be better
than those of some studies in western countries, in which older
radiotherapy techniques and lower radiation doses were used. The
limitations of our study include the retrospective design with lack of
evaluation of patients’ reported outcome, small sample size, and short
duration of follow-up. A multi-institutional study is recommended to
collect more local data and experiences.
Author contributions
Concept or design: EKC Lee, Y Tung.
Acquisition of data: EKC Lee, AW Chan.
Analysis or interpretation of data: EKC Lee.
Drafting of the article: EKC Lee, WH Mui, FCS Wong.
Critical revision for important intellectual content: EKC Lee, WH Mui, FCS Wong.
Acquisition of data: EKC Lee, AW Chan.
Analysis or interpretation of data: EKC Lee.
Drafting of the article: EKC Lee, WH Mui, FCS Wong.
Critical revision for important intellectual content: EKC Lee, WH Mui, FCS Wong.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Declaration
All authors have no conflicts of interest to
disclose. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity. An earlier version of this paper was
presented as poster presentation at the 9th European Multidisciplinary
Meeting on Urological Cancers, 16-19 November 2017, Barcelona, Spain.
Ethical approval
The study was conducted with approval from the New
Territories West Cluster Clinical and Research Ethics Committee.
References
1. Hospital Authority, HKSAR Government.
Leading cancer sites in Hong Kong in 2014. Available from:
http://www3.ha.org.hk/cancereg/pdf/top10/rank_2014.pdf. Accessed Jul 2017.
2. Poon DM, Chan SL, Leung CM, et al.
Efficacy and toxicity of intensity-modulated radiation therapy for
prostate cancer in Chinese patients. Hong Kong Med J 2013;19:407-15. Crossref
3. Ng AT, Tam PC. Current status of
robot-assisted surgery. Hong Kong Med J 2014;20:241-50. Crossref
4. Parker C, Clarke N, Logue J, et al.
RADICALS (Radiotherapy and Androgen Deprivation In Combination After Local
Surgery). Clin Oncol (R Coll Radiol) 2007;19:167-71. Crossref
5. Richaud P, Sargos P, Henriques de
Figueiredo B, et al. Postoperative radiotherapy of prostate cancer [in
French]. Cancer Radiother 2010;14:500-3. Crossref
6. Trans Tasman Radiation Oncology Group.
RAVES trial: radiotherapy—adjuvant versus early salvage. Available from:
http://www.clinicaltrial.gov/ct2/show/NCT00860652. Accessed Jul 2017.
7. Valicenti RK, Thompson I Jr, Albertsen
P, et al. Adjuvant and salvage radiation therapy after prostatectomy:
American Society for Radiation Oncology/American Urological Association
guidelines. Int J Radiat Oncol Biol Phys 2013;86:822-8. Crossref
8. Wiegel T, Lohm G, Bottke D, et al.
Achieving an undetectable PSA after radiotherapy for biochemical
progression after radical prostatectomy is an independent predictor of
biochemical outcome—results of a retrospective study. Int J Radiat Oncol
Biol Phys 2009;73:1009-16. Crossref
9. Michalski JM, Lawton C, El Naqa I, et
al. Development of RTOG consensus guidelines for the definition of the
clinical target volume for postoperative conformal radiation therapy for
prostate cancer. Int J Radiat Oncol Biol Phys 2010;76:361-8. Crossref
10. Poortmans P, Bossi A, Vandeputte K, et
al. Guidelines for target volume definition in post-operative radiotherapy
for prostate cancer, on behalf of the EORTC Radiation Oncology Group.
Radiother Oncol 2007;84:121-7. Crossref
11. Sidhom MA, Kneebone AB, Lehman M, et
al. Post-prostatectomy radiation therapy: consensus guidelines of the
Australian and New Zealand Radiation Oncology Genito-Urinary Group.
Radiother Oncol 2008;88:10-9. Crossref
12. Stephenson AJ, Scardion PT, Kattan MW,
et al. Predicting the outcome of salvage radiation therapy for recurrent
prostate cancer after radical prostatectomy. J Clin Oncol 2007;25:2035-41.
Crossref
13. Cole TJ. Too many digits: the
presentation of numerical data. Arch Dis Child 2015;100:608-9. Crossref
14. Pound CR, Partin AW, Eisenberger MA,
Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA
elevation following radical prostatectomy. JAMA 1999;281:1591-7. Crossref
15. Geinitz H, Riegel MG, Thamm R, et al.
Outcome after conformal salvage radiotherapy in patients with rising
prostate-specific antigen levels after radical prostatectomy. Int J Radiat
Biol Oncol Phys 2012;82:1930-7. Crossref
16. Fossati N, Karnes RJ, Boorjian SA, et
al. Long-term impact of adjuvant versus early salvage radiation therapy in
pT3N0 prostate cancer patients treated with radical prostatectomy: results
from a multi-institutional series. Eur Urol 2017;71:886-93. Crossref
17. Goldin GH, Sheets NC, Meyer A, et al.
Patterns of intensity modulated radiation therapy (IMRT) use for the
definitive and postoperative treatments of prostate cancer: a
SEER-medicare analysis. Int J Radiat Oncol Biol Phys 2011;81(2
Suppl):S408. Crossref
18. Nath SK, Sandhu AP, Rose BS, et al.
Toxicity analysis of postoperative image-guided intensity-modulated
radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys
2010;78:435-41. Crossref
19. Ost P, De Troyer B, Fonteyne V,
Oosterlinck W, De Meerleer G. A matched control analysis of adjuvant and
salvage high-dose postoperative intensity-modulated radiotherapy for
prostate cancer. Int J Radiat Oncol Biol Phys 2011;80:1316-22. Crossref
20. Kopp RW, Duff M, Catalfamo F, Shah D,
Rajecki M, Ahmad K. VMAT vs. 7-field-IMRT: assessing the dosimetric
parameters of prostate cancer treatment with a 292-patient sample. Med
Dosim 2011;36:365-72. Crossref
21. Palma D, Vollans E, James K, et al.
Volumetric modulated arc therapy for delivery of prostate radiotherapy:
reduction in treatment time and monitor unit requirements compared to
intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008;72(1
Suppl):S312. Crossref
22. Pisansky TM, Agrawal S, Hamstra DA, et
al. Salvage radiation therapy dose response for biochemical failure of
prostate cancer after prostatectomy—A multi-institutional observational
study. Int J Radiat Oncol Biol Phys 2016;96:1046-53. Crossref
23. King CR. The dose-response of salvage
radiotherapy following radical prostatectomy: a systemic review and
meta-analysis. Radiother Oncol 2016;121:199-203. Crossref
24. Lee EK, Yuen KK, Mui WH, et al.
Salvage radiotherapy to the prostatic fossa using volumetric-modulated arc
therapy: early results. Hong Kong J Radiol 2013;16:191-7. Crossref
25. Stephenson AJ, Shariat SF, Zelefsky
MJ, et al. Salvage radiotherapy for recurrent prostate cancer after
radical prostatectomy. JAMA 2004;291:1325-32. Crossref
26. Johnson S, Jackson W, Li D, et al. The
interval to biochemical failure is prognostic for metastatic, prostate
cancer-specific mortality, and overall mortality after salvage radiation
therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2013;86:554-61.
Crossref
27. Bartkowiak D, Bottke D, Thamm R,
Siegmann A, Hinkelbein W, Wiegel T. The PSA-response to salvage
radiotherapy after radical prostatectomy correlates with freedom from
progression and overall survival. Radiother Oncol 2016;118:131-5. Crossref
28. Shipley WU, Seiferheld W, Lukka HR, et
al. Radiation with or without antiandrogen therapy in recurrent prostate
cancer. N Engl J Med 2017;376:417-28. Crossref
29. Perera M, Papa N, Christidis D, et al.
Sensitivity, specificity, and predictors of positive
68Ga-prostate-specific membrane antigen positron emission tomography in
advanced prostate cancer: a systemic review and meta-analysis. Eur Urol
2016;70:926-37. Crossref