DOI: 10.12809/hkmj176236
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Cystic artery pseudoaneurysm with haemobilia after
laparoscopic cholecystectomy
K To, BA (Hons); Eric CH Lai, MB, ChB, MRCSEd,
FRACS; Daniel TM Chung, MB, ChB, MRCSEd, FRCS; Oliver CY Chan, MB ChB,
MRCSEd, FRCS; CN Tang, MB, BS, FRCS
Department of Surgery, Pamela Youde Nethersole
Eastern Hospital, Chai Wan, Hong Kong
Corresponding author: Dr Eric CH Lai (elaichun@gmail.com)
Case presentation
A 56-year-old man underwent laparoscopic
cholecystectomy for acute cholecystitis at another hospital in December
2013. The cholecystectomy was uneventful and the patient was discharged
home 3 days later. However, after hospital discharge, the patient
presented with recurring upper abdominal pain, tarry stool, and fever. He
was admitted to another hospital 4 weeks after the cholecystectomy because
of fever, right upper quadrant pain, and haematemesis. Emergency
oesophagogastroduodenoscopy and colonoscopy were performed. No bleeding
source was identified. Computed tomography (CT) revealed subhepatic fluid
collection; old-blood–stained fluid was drained by image-guided catheter
drainage. The patient was transferred to the Department of Surgery, Pamela
Youde Nethersole Eastern Hospital, Hong Kong, for further treatment.
When the patient arrived at hospital, his blood
pressure was approximately 90/60 mm Hg and his pulse rate was 110 beats
per minute. Laboratory studies revealed the following values: haemoglobin
level, 72 g/L; white blood cell count, 20.3 × 109 /L; platelet
count, 388 × 109 /L; total bilirubin, 164 μmol/L; alanine
aminotransferase, 187 IU/L; and alkaline phosphatase, 337 IU/L. The
patient was treated with intravenous fluid hydration and was transfused
with three units of packed red blood cells. He was also given a course of
antibiotics. Abdominal CT showed a cystic artery pseudoaneurysm of 1.22 ×
1.96 × 1.38 cm (anterior-posterior × transverse × longitudinal
dimensions). Two subhepatic collections with haematoma were also visible,
over the gallbladder fossa and below hepatic segment 6. Selective right
hepatic artery angiography revealed a pseudoaneurysm at the cystic artery.
This aneurysm was embolised with stainless steel coils (Fig
1). The catheter for subhepatic collection drainage was then
replaced with one with better positioning. Endoscopic retrograde
cholangiopancreatography was performed the next day. The cholangiogram
showed a dilated biliary tree with haemobilia; most of the blood clots
were extracted using a balloon. A cystic duct stump leak was observed
after blood clot removal, and a 10-cm-long 11.5-F biliary stent was
inserted for biliary drainage (Fig 2). Liver function improved gradually. The
patient was discharged from hospital 2 weeks after admission.
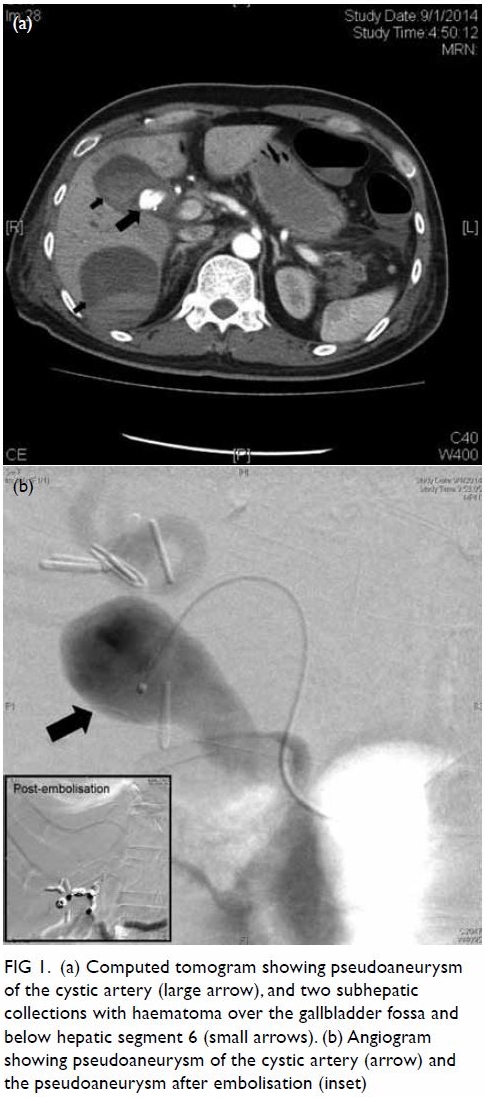
Figure 1. (a) Computed tomogram showing pseudoaneurysm of the cystic artery (large arrow), and two subhepatic collections with haematoma over the gallbladder fossa and below hepatic segment 6 (small arrows). (b) Angiogram showing pseudoaneurysm of the cystic artery (arrow) and the pseudoaneurysm after embolisation (inset)
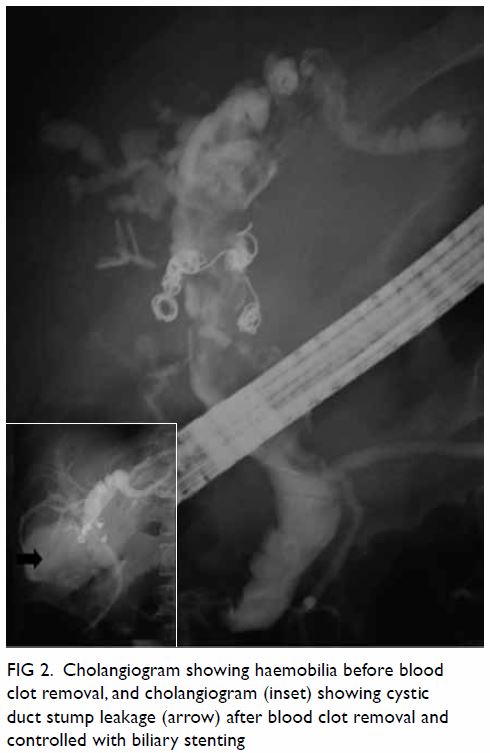
Figure 2. Cholangiogram showing haemobilia before blood clot removal, and cholangiogram (inset) showing cystic duct stump leakage (arrow) after blood clot removal and controlled with biliary stenting
Follow-up CT no longer showed pseudoaneurysm and
instead showed a resolving collection. Endoscopic retrograde
cholangiopancreatography with stent removal was performed 3 months later.
The cholangiogram showed a normal biliary tree. The patient recovered and
liver function test results were normal.
Discussion
Hepatic artery or cystic artery pseudoaneurysms are
rare complications of laparoscopic cholecystectomy, with cystic artery
involvement being reported much less frequently in the literature.
Pseudoaneurysm formation is a consequence of vascular injury; important
causes include arterial access procedures, accident trauma, and surgical
trauma.1 Two-thirds of cases are
iatrogenic.1 With the advent of
laparoscopic cholecystectomy, iatrogenic hepatobiliary injury is now
another cause. Concomitant formation of cystic artery pseudoaneurysm and
cystic duct stump leak is a rare complication of laparoscopic
cholecystectomy. The majority of pseudoaneurysms present within 6 weeks
after the operation.2 3
We have reported a case of laparoscopic
cholecystectomy that was complicated by a cystic artery pseudo-aneurysm
and a cystic duct stump bile leak, which were managed with angiographic
coil embolisation and endoscopic biliary drainage, respectively. The
patient presented with the classic Quincke’s triad of haemobilia, namely
upper gastrointestinal bleeding, right upper quadrant pain, and
obstructive jaundice. The aetiology most likely originated from the
infected fluid collection after cholecystectomy, which caused a series of
events, including cystic duct stump leak, cystic artery pseudoaneurysm,
and haemobilia, in that order. First, bile leakage is a potential
complication of cholecystectomy and the cystic duct stump is the most
common site of leakage.4 The
contributing factor of cystic duct stump leak in the current case was
likely cystic duct stump necrosis secondary to mechanical or thermal
injury during cholecystectomy, as well as adjacent infection. Second,
haemobilia can occur secondary to a cystic artery pseudoaneurysm, although
extremely rarely. Artery pseudoaneurysm is a continuous inflammatory
process that leads to erosion in the elastic and muscular components of
the arterial wall, ultimately resulting in pseudoaneurysm formation. The
likely precipitating factors in the current case include initial clip
encroachment of the vasculature, mechanical or thermal injury, and
continuous inflammation due to the adjacent infected bile or collection.
Pseudoaneurysm can present with bleeding in the
form of haemobilia, haematemesis, or melaena. In the current case, upper
gastrointestinal bleeding from haemobilia resulted from the cystic artery
pseudoaneurysm’s communication with the cystic duct. The resulting
symptoms were typical of Quincke’s triad of upper abdominal pain, upper
gastrointestinal haemorrhage, and jaundice.5
However, these symptoms are present collectively only in a minority
(32%-40%) of patients.5 Thus,
detection relies heavily on both clinical reasoning and imaging
techniques. If intra-abdominal collection or haemorrhage is suspected
clinically, arterial-phase CT is appropriate to detect any pseudoaneurysm.
Since gastrointestinal haemorrhage is one of the presentations, urgent
oesophagogastroduodenoscopy may be arranged first to rule out any
suspected upper gastrointestinal pathology. However, as in the current
case, if that procedure fails to show any bleeding source, urgent CT
should be considered. Close observation and timely arrangement of
appropriate procedures are essential.
Prompt recognition with adequate management was
very important in the current case. The treatment of our patient included
five objectives: achieving haemostasis, controlling the cystic duct stump
leak, relieving obstructive jaundice, controlling the infection with
antibiotics, and draining the intra-abdominal collection. Untreated
haemobilia poses an immediate threat to life. It can lead to acute
haemodynamic instability, necessitating detection, access, and control of
the pseudoaneurysm. Arterial-phase CT is a good initial non-invasive mode
of detection of laparoscopic cholecystectomy complications. It can be used
to evaluate intra-abdominal collection, biliary tree dilatation, and
possible bile duct injury, and to visualise pseudoaneurysms or
haemorrhage. Arterial-phase CT also allows a three-dimensional assessment
of the bile duct and vasculature. Selective arterial angiography can
provide a real-time evaluation of pseudoaneurysms and bleeding. At the
same time, it can provide the chance of immediate therapeutic
intervention. Transarterial embolisation is the treatment of choice for
haemostasis, and a high success rate, of 75% to 100%, has been
reported.2 3 5 When bleeding control by embolisation fails, repeated
sessions of transarterial embolisation for haemostasis or surgical
intervention to repair or ligate the artery is necessary. Endoscopic
retrograde cholangiopancreatography with stent placement or sphincterotomy
is highly effective in diagnosing haemobilia, controlling cystic duct
stump leakage, and relieving obstructive jaundice. We favoured stenting
over sphincterotomy because of a presumed lower risk of immediate
complications.
The lessons to be learnt from this case include the
importance of (1) meticulous surgical techniques (such as good
haemostasis, careful use of powered devices, proper use of endoclips, and
adequate drainage of the operative field), and (2) early recognition and
prompt management.
In conclusion, cystic artery pseudoaneurysm is a
rare, potentially life-threatening complication of laparoscopic
cholecystectomy, and prompt recognition and treatment are essential.
Haemobilia may be present many weeks after the initial injury.
Declaration
The authors have no conflicts of interest to
disclose.
References
1. Green MH, Duell RM, Johnson CD, et al.
Haemobilia. Br J Surg 2001;88:773-86.
2. Senthilkumar MP, Battula N, Perera M, et
al. Management of a pseudo-aneurysm in the hepatic artery after a
laparoscopic cholecystectomy. Ann R Coll Surg Engl 2016;98:456-60. Crossref
3. Nicholson T, Travis S, Ettles D, et al.
Hepatic artery angiography and embolization for hemobilia following
laparoscopic cholecystectomy. Cardiovasc Intervent Radiol 1999;22:20-4. Crossref
4. Lau WY, Lai EC, Lau SH. Management of
bile duct injury after laparoscopic cholecystectomy: a review. ANZ J Surg
2010;80:75-81. Crossref
5. Merrell SW, Schneider PD.
Hemobilia—evolution of current diagnosis and treatment. West J Med
1991;155:621- 5.

