DOI: 10.12809/hkmj166231
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Intravenous stroke thrombolysis after reversal of
dabigatran effect by idarucizumab: first reported case in Hong Kong
WT Lo, MRCP, FHKAM (Medicine)1; KF Ng,
MRCS (Edin), FHKAM (Emergency Medicine)2; Simon CH Chan, MRCP1;
Vivian WY Kwok, MSc1; CS Fong, MSc1; ST Chan, MSc1;
Gordon CK Wong, MRCP, FHKAM (Emergency Medicine)2; WC Fong,
FRCP, FHKAM (Medicine)1
Departments of 1Medicine and 2Accident
and Emergency, Queen Elizabeth Hospital, Jordan, Hong Kong
Corresponding author: Dr WC Fong (fwcz01@ha.org.hk)
Case presentation
A 78-year-old woman was diagnosed with atrial
fibrillation in September 2015. An echocardiogram showed no evidence of
valvular heart disease. She was prescribed dabigatran 110 mg twice a day.
In December 2016, she was admitted to our hospital for acute ischaemic
stroke, presenting with a sudden onset of decreased level of
consciousness. Apart from atrial fibrillation, she also had hypertension,
diabetes mellitus, hyperlipidaemia, and a history of hip fracture with
bilateral hip implants, requiring a rollator for walking.
On the day of admission, she was reported by her
carer to be poorly responsive, with no verbal response and no spontaneous
limb movement. She was last seen well 30 minutes previously. Physical
examination revealed bilateral pinpoint pupils and no verbal response. She
had slight flexion of her four limbs to pain and her National Institutes
of Health Stroke Scale (NIHSS) score was 34. The blood pressure was 142/64
mm Hg. There was no hypoglycaemia. Naloxone was given with no improvement.
Cerebral computed tomography (CT) showed no early infarct changes but
bilateral subcortical white matter hypodensities, compatible with small
vessel disease (Fig a). A clinical diagnosis was made of acute
ischaemic brainstem stroke. The family confirmed that the patient had been
taking dabigatran regularly as prescribed, with the last dose taken about
2 hours before symptom onset. She had taken no sedative medications.
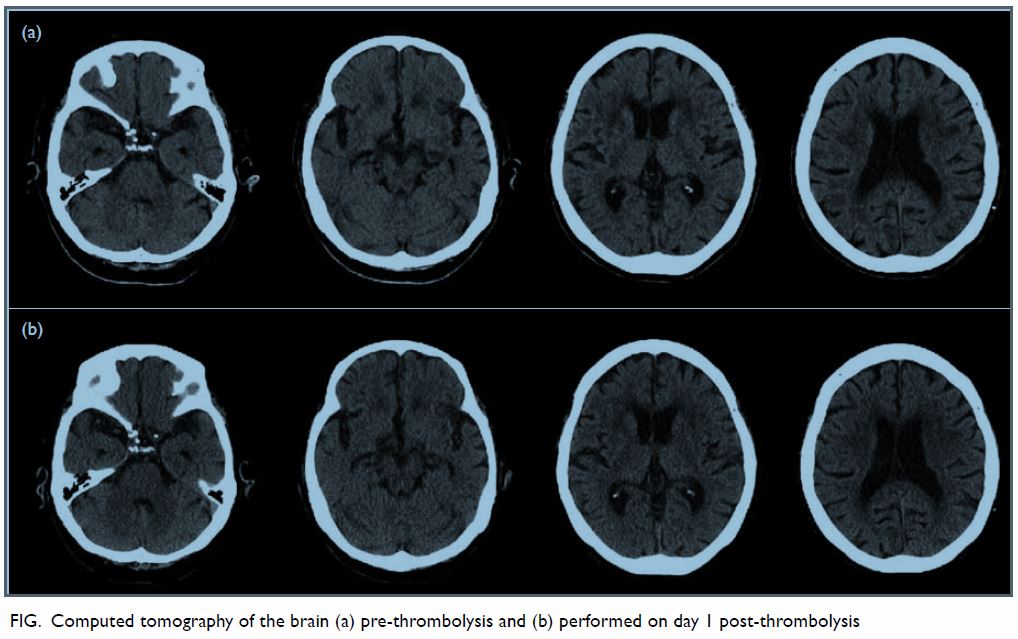
Figure. Computed tomography of the brain (a) pre-thrombolysis and (b) performed on day 1 post-thrombolysis
After obtaining informed consent, an intravenous
bolus of 5 g idarucizumab was given. Blood for clotting profile, including
thrombin time, was taken before treatment and 5 minutes afterwards.
Intravenous recombinant tissue plasminogen activator (r-tPA) was then
given at 0.6 mg/kg body weight 10 minutes after the start of idarucizumab
injection, 2 hours from symptom onset. The prolonged activated partial
thromboplastin time (APTT) and thrombin time (TT) normalised after
administration of idarucizumab. The Table shows the clotting variables before and after
idarucizumab. By the next morning, the patient had regained her speech
although her response was slow with dysarthria and dysphagia. She could
raise her four limbs against gravity, power being grade 3. Follow-up CT at
24 hours post r-tPA showed no significant infarct and no bleeding (Fig
b). Transcranial Doppler ultrasonography showed no evidence of
significant vertebrobasilar occlusive disease. By day 2, she had recovered
further neurologically, with improvement in alertness, dysarthria and
dysphagia, and could tolerate oral feeding. Limb power was grade 4+ over
four limbs. Pupils were no longer pinpoint. She was recommenced on
dabigatran 150 mg twice a day on day 2 based on her age and creatinine
clearance. The patient was discharged on day 7 with her neurological
function returned to baseline.
Discussion
This is the first report in Hong Kong of the
successful use of idarucizumab to reverse the anticoagulant effect of
dabigatran, followed by intravenous thrombolysis with r-tPA for the
treatment of ischaemic stroke. Novel oral anticoagulants (NOACs) are now
increasingly used for the prevention of cardioembolic stroke in patients
with non-valvular atrial fibrillation. Dabigatran, which acts as a
thrombin inhibitor, is one such NOAC. Idarucizumab is a monoclonal
antibody that has a high affinity for binding to the immunoglobulin G
fragment of dabigatran. A 5-g dose was shown to be able to reverse the
anticoagulant effect of dabigatran within minutes and had a good safety
profile in the study of Reversal Effects of Idarucizumab on Active
Dabigatran (RE-VERSE AD trial).1
Patients who develop an acute ischaemic stroke
while taking dabigatran are often excluded from treatment with intravenous
thrombolysis owing to their bleeding risk. For patients who are not
candidates for mechanical thrombectomy or who are in institutions where an
endovascular thrombectomy service is not routinely available, giving
intravenous thrombolysis after reversal of the anticoagulant effect is a
treatment option. There is no pro-anticoagulant effect from idarucizumab
itself, and an in-vitro study demonstrated no interaction between
idarucizumab and r-tPA–induced thrombolysis.2
As far as we know, there have been five case reports at the time of
writing of this article, all from German centres, that have reported the
successful use of idarucizumab for this indication; none had any
haemorrhagic complications.2 3 Four of these studies reported successful thrombolysis
with good neurological recovery, whereas one study reported failed
clinical improvement in a patient with infarcts in multiple territories.3 Diener et al4 have published an expert opinion on the management of
these ischaemic strokes based on their experience in Germany. They
proposed that for patients who have taken dabigatran in the preceding 24
hours from the time of assessment (or 96 hours if the creatinine clearance
is <30 mL/min), or for patients in whom time of last dabigatran dose is
unknown and who have a prolonged APTT or TT, idarucizumab should be given
if the patient is not a candidate for mechanical thrombectomy. In our
institution, we do not have point-of-care devices to test clotting
function, the turnaround time for APTT and TT results may be hours, and
dabigatran concentrations cannot be measured in our laboratory. We
therefore propose that for patients in whom time of last dabigatran dose
is unknown, idarucizumab can still be considered with the family’s consent
to enable early intravenous thrombolysis. For the same reason, we did not
wait for the results of APTT or TT to confirm reversal of dabigatran’s
effect before initiating intravenous thrombolysis. Idarucizumab rapidly
and completely reversed the anticoagulant activity of dabigatran in 88% to
98% of patients in the RE-VERSE AD trial.1
Safety (avoidance of symptomatic intracranial
haemorrhages) was our top concern for this patient. As she had multiple
risk factors for intracranial haemorrhage (old age, high NIHSS score,
cerebral white matter disease and on anticoagulant therapy), we gave r-tPA
at a dose of 0.6 mg/kg body weight. This dose was associated with
significantly fewer symptomatic intracranial haemorrhages in the recent
ENCHANTED trial.5
Following reversal of effect, dabigatran can be
resumed as early as 24 hours afterwards. Although our patient had improved
by the next day, she still had significant dysphagia and could not
tolerate oral feeding. By the second day, she had improved further so
dabigatran was resumed at a higher dose based on her age and renal
function.
In conclusion, idarucizumab can be considered to
reverse the anticoagulant effect of dabigatran in patients with ischaemic
stroke within a therapeutic window, so that they may benefit from
intravenous thrombolytic therapy.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Pollack CV Jr, Reilly PA, Eikelboom J,
et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511-20. Crossref
2. Berrouschot J, Stoll A, Hogh T,
Eschenfelder CC. Intravenous thrombolysis with recombinant tissue-type
plasminogen activator in a stroke patient receiving dabigatran
anticoagulant after antagonization with idarucizumab. Stroke
2016;47:1936-8. Crossref
3. Kafke W, Kraft P. Intravenous
thrombolysis after reversal of dabigatran by idarucizumab: a case report.
Case Rep Neurol 2016;8:140-4. Crossref
4. Diener HC, Bernstein R, Butcher K, et
al. Thrombolysis and thrombectomy in patients treated with dabigatran with
acute ischemic stroke: Expert opinion. Int J Stroke 2017;12:9-12. Crossref
5. Anderson CS, Robinson T, Lindley RI, et
al. Low-dose versus standard-dose intravenous alteplase in acute ischemic
stroke. N Engl J Med 2016;374:2313-23. Crossref


