DOI: 10.12809/hkmj176296
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Emergency thrombectomy for acute ischaemic stroke:
current evidence, international guidelines, and local clinical practice
Anderson CO Tsang, MB, BS, MRCSEd1; Ryo
WL Yeung2; Mona MY Tse, MB, BS, FHKAM (Medicine)3;
Raymand Lee, MB, BS, FHKAM (Radiology)4; WM Lui, MB, BS, FCSHK1
1 Division of Neurosurgery, Department
of Surgery, Queen Mary Hospital, The University of Hong Kong, Pokfulam,
Hong Kong
2 Li Ka Shing Faculty of Medicine, The
University of Hong Kong, Pokfulam, Hong Kong
3 Division of Neurology, Department of
Medicine, Queen Mary Hospital, Pokfulam, Hong Kong
4 Department of Radiology, Queen Mary
Hospital, Pokfulam, Hong Kong
Corresponding author: Dr Anderson CO Tsang (acotsang@hku.hk)
Abstract
Acute ischaemic stroke due to large vessel
occlusion leads to grave neurological morbidity and mortality.
Conventional intravenous thrombolysis is ineffective in achieving timely
reperfusion in this group of patients. The publication of five positive
randomised controlled trials of emergency thrombectomy for acute
ischaemic stroke in 2015 provided strong evidence to support
endovascular reperfusion therapy and represented a paradigm shift in
acute stroke management. In this article, we review the current evidence
and international guidelines, and report on the findings of a survey
study of the clinical practice and opinions of local neurologists,
neurosurgeons, and interventional radiologists in emergency
thrombectomy. We also discuss the controversies around thrombectomy
treatment, local experience, and suggestions to incorporate thrombectomy
in acute stroke treatment.
Introduction
Before 2015, the standard of care for emergency
ischaemic stroke treatment was intravenous thrombolysis with tissue
plasminogen activator (IV-tPA). This stemmed from the National Institute
of Neurological Disorders and Stroke trial in 1995, which showed that
compared with placebo, patients who were given IV-tPA within 3 hours from
symptom onset were 30% more likely to have minimal or no disability at 3
months.1 The therapeutic window was
further extended to 4.5 hours from symptom onset by the European
Cooperative Acute Stroke Study III, which demonstrated similar benefits
when IV-tPA was administered between 3 and 4.5 hours to selected patients.2
Nonetheless, in patients with ischaemia due to
occlusion of a major cerebral artery, such as the intracranial internal
carotid artery or the first and second segment of middle cerebral artery
(M1, M2), the efficacy of intravenous thrombolysis was limited, with a
recanalisation rate of only 4% to 30%.3
This group of patients frequently had a grave neurological prognosis and
high mortality owing to the large infarct territory, and could develop
malignant cerebral oedema that required decompressive craniectomy.4
To improve the recanalisation rate, endovascular
mechanical thrombectomy to remove the occluding clot was proposed. Early
studies of this technique showed conflicting results and were attributed
to poor patient selection and suboptimal endovascular devices that
resulted in a low recanalisation rate, thus failing to show the expected
benefits of endovascular thrombectomy.5
6 7
With modern improved thrombectomy devices, the
publication of five positive randomised trials of acute mechanical
thrombectomy for ischaemic stroke resulting from anterior circulation
large-vessel occlusion in the New England Journal of Medicine in
2015 marked a paradigm shift in stroke treatment.8
9 10
11 12
Since then, emergency endovascular thrombectomy has been internationally
regarded as the new standard of care for acute ischaemic stroke caused by
major vessel occlusion, and is recommended by all major stroke guidelines.
Summary of current evidence
The five independent randomised controlled trials
that provided strong evidence to support endovascular thrombectomy were MR
CLEAN,8 REVASCAT,9 ESCAPE,10
EXTEND-IA,11 and SWIFT PRIME,12 conducted in the US, Europe, and Australia from 2010
to 2015. Although there were differences in terms of inclusion and
exclusion criteria, all five studies recruited only acute stroke patients
with angiogram-proven major vessel occlusion in the anterior circulation,
and used newer-generation thrombectomy devices (mostly stent retrievers)
that achieved higher recanalisation rates than earlier devices used in
previous trials.
All patients in the five trials were given IV-tPA
as standard treatment when eligible, and were then randomised to receive
endovascular thrombectomy or standard care alone. All these trials
unanimously showed significant benefit in the thrombectomy group in terms
of improved functional outcome at 3 months, as measured by the modified
Rankin scale (mRS) [Table 18 9 10
11 12
13].
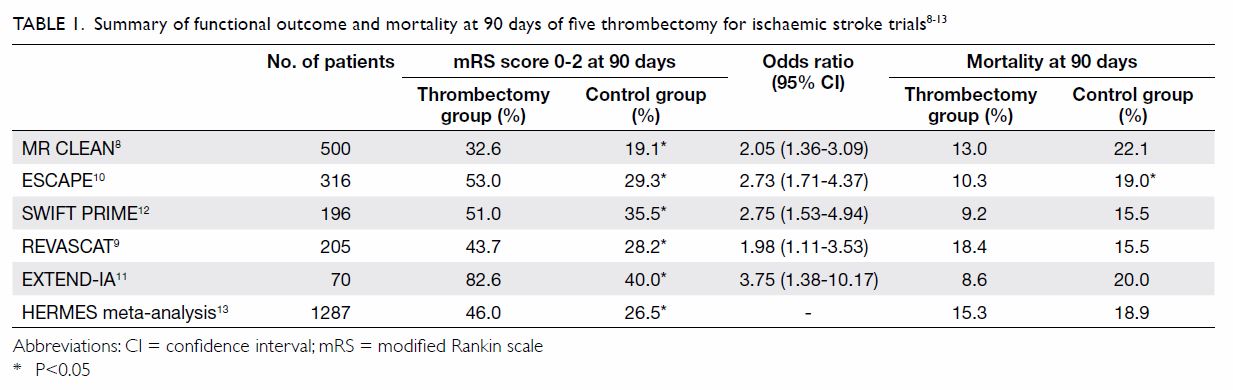
Table 1. Summary of functional outcome and mortality at 90 days of five thrombectomy for ischaemic stroke trials8 9 10 11 12 13
Functional outcome benefit
A meta-analysis of the above five trials with a
total of 1287 eligible patients showed that 46% of patients treated by
endovascular thrombectomy achieved functional independence (mRS score,
0-2) at 90 days, compared with 26.5% of patients in the control group13 (Table 1). The number needed-to-treat was 2.6 for one
patient to improve functionally by at least 1 point on mRS. In terms of
safety, there was no significant difference between the intervention group
and the control group in symptomatic intracranial haemorrhage (4.4% vs
4.3%; P=0.81) or mortality rate at 90 days (15.3% vs 18.9%; P=0.15).13
Moreover, the clinical benefit was maintained
whether the patient was eligible to receive IV-tPA in the first place, and
across all age-groups including the patients older than 80 years. Patients
improved after thrombectomy regardless of the initial severity of stroke,
as documented by the National Institutes of Health Stroke Scale (NIHSS)
and the initial Alberta Stroke Program Early CT Score.
Therapeutic time frame
Regarding the treatment time frame, thrombectomy
within 6 hours from symptom onset was consistently beneficial across all
five thrombectomy trials. In the REVASCAT trial that included patients
within 8 hours of symptom onset, the median onset-to-reperfusion time was
still within 6 hours in the thrombectomy group, although no separate data
were provided for those who presented between 6 and 8 hours.9 Similarly, in the ESCAPE trial, which included patients
up to 12 hours from stroke onset, the median time to reperfusion was 4
hours from symptom onset and very few patients beyond 6 hours were
recruited.10 At present, two
clinical trials are underway to investigate the benefits of endovascular
thrombectomy for anterior circulation stroke beyond 6 hours, and both
require computed tomography (CT) perfusion or magnetic resonance imaging
(MRI) to assess infarct core and perfusion mismatch to determine
eligibility (NCT02586415, NCT02142283). Therefore, the efficacy and risk
of anterior circulation thrombectomy beyond 6 hours without advanced
imaging selection criteria are uncertain, and should be performed with
discretion or in a clinical research setting.
Posterior circulation and the paediatric population
At present, there is no evidence from randomised
trials regarding thrombectomy for posterior circulation large-vessel
occlusion or paediatric patients.
Contemporary thrombectomy devices
One major improvement of these five trials8 9 10 11 12 compared with previous negative thrombectomy trials5 6
7 was the exclusive use of newer
stent retriever devices and a consequent higher recanalisation rate.
Near-complete/complete (TICI 2b/3) recanalisation was achieved in 59% to
88% of patients, compared with only 25% to 41% in early studies that used
intra-arterial tPA and first-generation devices.14
Another contemporary thrombectomy device was the
direct aspiration catheter (ADAPT technique),15
which applied suction to remove the clot via a large-bore endovascular
catheter (Fig 1). This technique was supported by multiple
single-centre series that showed comparable and sometimes superior results
over stent retrievers, although none of these were head-to-head
comparative trials.16 17 18 The 2016
THERAPY trial was the only randomised study to compare aspiration
thrombectomy versus IV-tPA alone.19
It was terminated prematurely with a limited sample size after publication
of the positive stent retriever trials, and as such failed to demonstrate
a statistically significant benefit for aspiration thrombectomy over
intravenous thrombolysis alone.
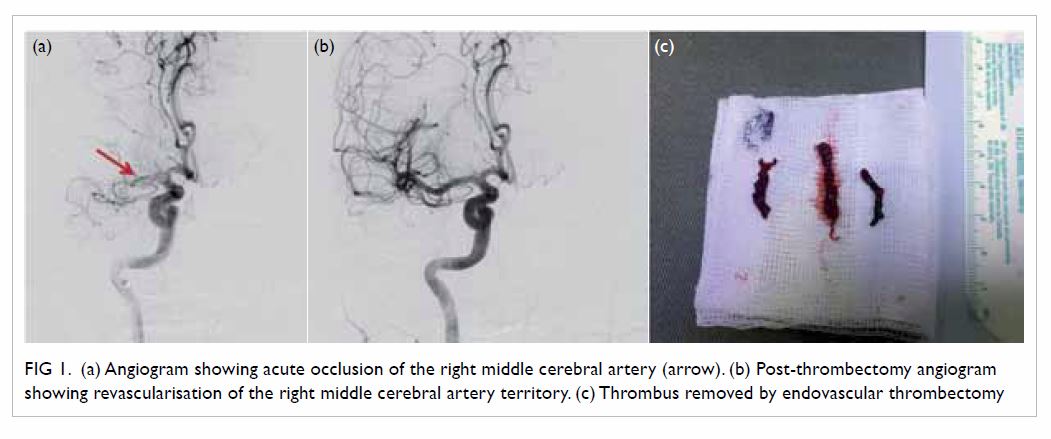
Figure 1. (a) Angiogram showing acute occlusion of the right middle cerebral artery (arrow). (b) Post-thrombectomy angiogram showing revascularisation of the right middle cerebral artery territory. (c) Thrombus removed by endovascular thrombectomy
Cost-effectiveness of thrombectomy
Cost-utility analyses have been performed in
several health care systems around the world and all have shown the
cost-effectiveness of endovascular thrombectomy. The cost per
quality-adjusted life year (QALY) gained for thrombectomy over standard
thrombolysis treatment varies between US$7988 (Sweden), US$9386 (US),
US$11 651 (UK), and US$11 990 (Canada).18
20 21
22 Using internationally accepted
willingness-to-pay per QALY threshold of the gross domestic product per
capita, all of the above health care systems found endovascular
thrombectomy to be cost-effective.
International guidelines
The overwhelming clinical evidence to support
application of thrombectomy in anterior circulation stroke prompted the
European Stroke Organisation (ESO) and subsequently the American Heart
Association/American Stroke Association (AHA/ASA) to release a focused
update of early stroke treatment guidelines in 2015, recommending
endovascular thrombectomy with stent retriever device if onset of symptoms
was within 6 hours and was due to a major vessel occlusion of the anterior
circulation, and when suitable criteria were met (Class I, Level of
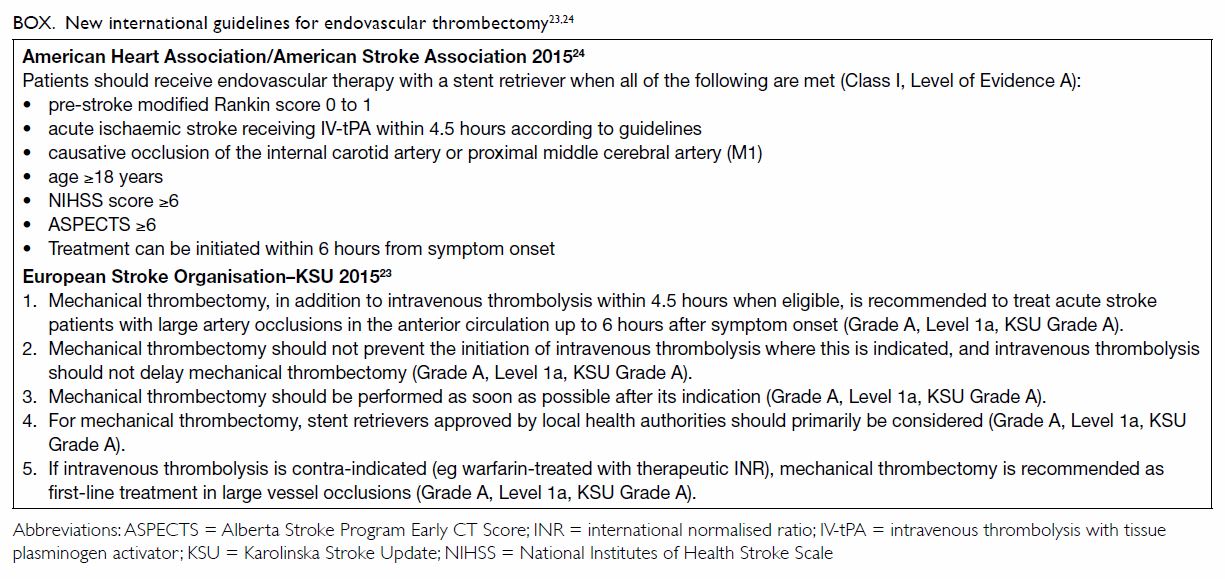
Evidence A).23 24 The Box lists the latest AHA/ASA and ESO recommendations
regarding endovascular thrombectomy.23
24
Emergency thrombectomy in Hong Kong
Endovascular thrombectomy for ischaemic stroke is a
relatively new procedure in Hong Kong and is not routinely available.
Although it was practised in certain centres prior to 2015 for selected
cases,25 there was no consensus on
treatment indications, patient selection criteria, or operative
techniques. With acute mechanical thrombectomy now becoming the
international standard of care for acute anterior circulation ischaemic
stroke, we undertook a survey to identify the availability, clinical
practice, and potential obstacles of an acute stroke thrombectomy service
in Hong Kong.
Design of the survey
We designed a survey which addressed the
availability of expertise, the indications, patient selection for stroke
treatment, interventional techniques, and perceived obstacles to timely
stroke treatment. This was a web-based survey consisting of 43 questions
and was administered from May to August 2016. Respondents were drawn from
the Hong Kong Stroke Society, Hong Kong Neurosurgical Society, and Hong
Kong Society of Interventional and Therapeutic Neuroradiology. Invitations
to participate were sent by email. Participants did not receive any
incentive to complete this survey. We received 44 responses from the three
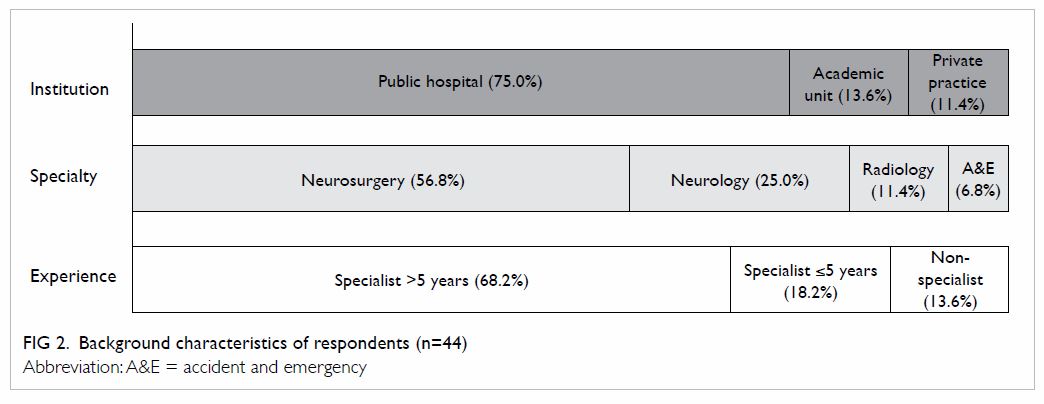
societies. Background characteristics of the respondents are shown in Figure 2.
Service availability
Overall, 24 (54.5%) respondents from six public
hospitals and five private practices were regularly involved in the
decision and provision of mechanical thrombectomy for patients with acute
ischaemic stroke. Most were neurosurgeons (75.0%), and the remainder were
neurologists and interventional radiologists. In most centres, the
interventionist capable of performing thrombectomy was a neurosurgeon or
interventional radiologist, whereas in half of the responding centres
there were also neurologists who were able to perform this procedure. For
the majority of interventionists (62.5%), mechanical thrombectomy was
offered only on an ‘ad hoc’ basis with no official service hours. The mean
number of interventionists currently available was 4.3 per centre. The
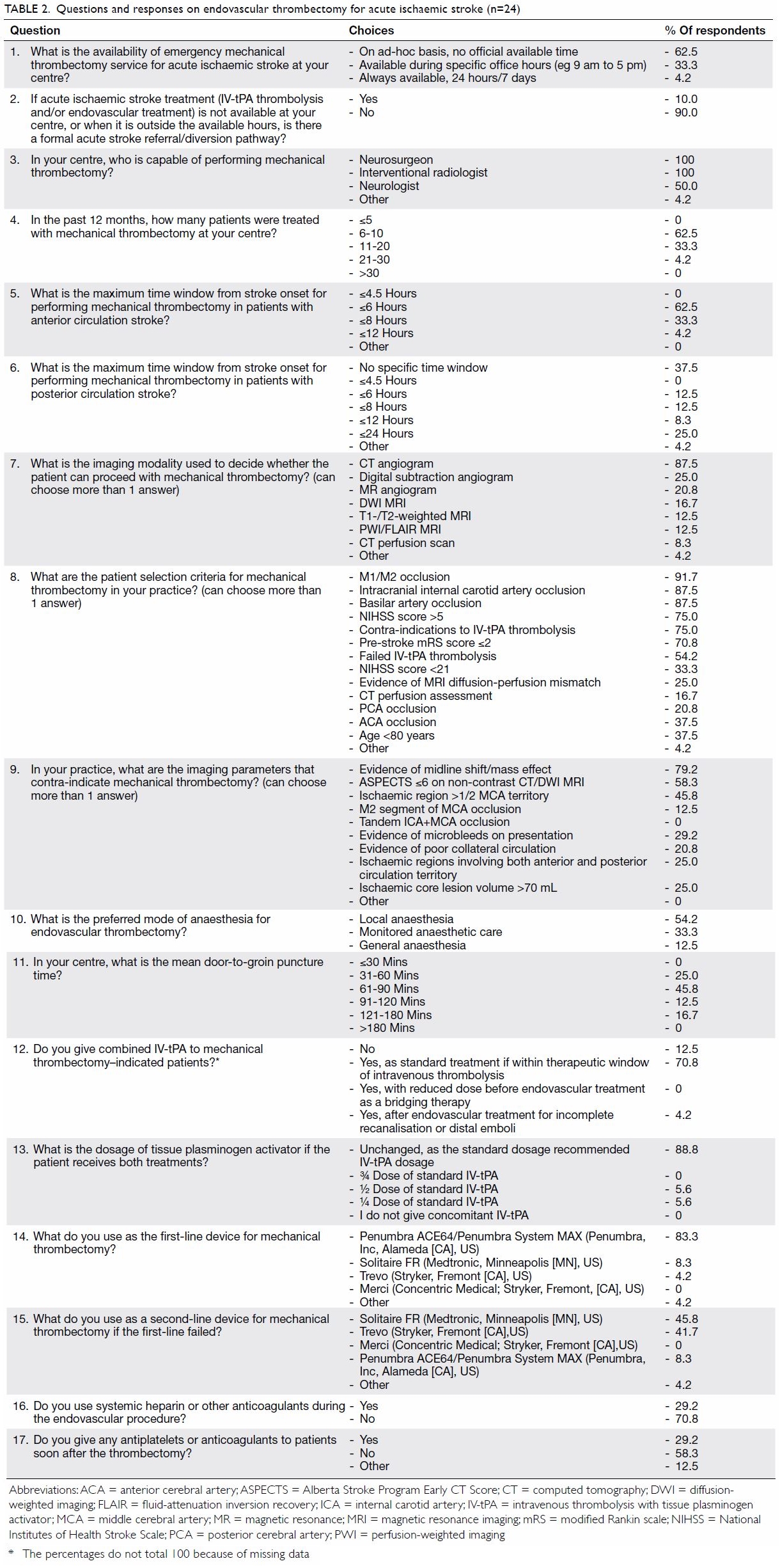
questions and responses of the survey are shown in Table 2.
Clinical practice
Direct aspiration technique (ADAPT15) was the most popular first-line thrombectomy
technique, adopted by 83.3% of respondents. A stent retriever was chosen
as a second-line thrombectomy device by 91.7% of interventionists. The
majority of local practitioners used 6 hours as the time limit for
anterior circulation thrombectomy in accordance with current guidelines,
but a significant number of respondents (37.5%) adopted a more liberal
limit of 8 hours or beyond. Most practitioners would prescribe intravenous
thrombolysis using the standard dosage in patients who fulfilled
thrombolysis indications, regardless of thrombectomy decision, and in line
with current guidelines.
For patient selection, most local interventionists
used CT angiography as the predominant imaging modality. This finding is
unsurprising considering CT perfusion and MRI scanners were not routinely
available in an emergency setting in many public hospitals where most
acute stroke patients were treated. Apart from angiographic evidence of
major vessel occlusion (intracranial internal carotid artery, M1/M2
segment of middle cerebral artery and basilar artery), stroke symptom
severity of NIHSS score of >5 and pre-stroke functional status (mRS
score ≤2) were regarded as important selection criteria by over 70% of
respondents.
Up to 87.5% of respondents performed thrombectomy
without general anaesthesia. This practice has been recommended after a
recent meta-analysis that confirmed better outcome in patients treated
under conscious sedation than in those under general anaesthesia.26 A more recent randomised trial, however, showed no
difference in outcome whether general anaesthesia or conscious sedation
was used.27
Incorporating emergency thrombectomy in acute stroke
care in Hong Kong
Locally, an acute ischaemic stroke service is
heavily dependent on a public health system that handles over 80% of
emergency hospital admissions in Hong Kong.28
Our neurologists and stroke physicians in public hospitals have made major
contributions over the past two decades in implementing intravenous
thrombolysis and spearheading 24-hour acute stroke care. As a result of
much effort, currently seven of 17 emergency hospitals in the public
hospital system provide 24-hour IV-tPA thrombolysis service.29 Future enhancement of an acute stroke service should
aim to provide timely and universal access for patients with acute
ischaemic stroke, and divert potentially eligible thrombectomy patients to
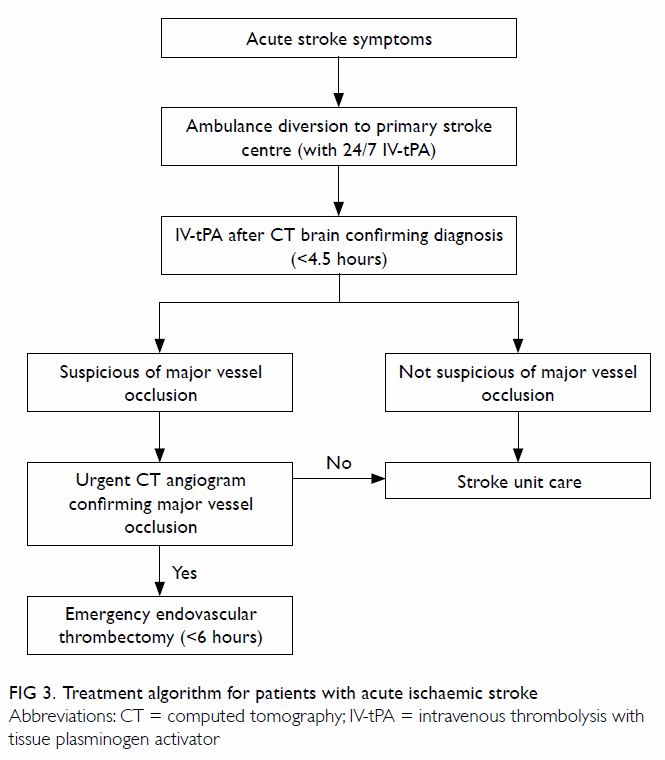
centres that can provide such service (Fig 3).
Our survey identified the most common reported
obstacles in implementing prompt stroke interventions as poor inter- and
in-hospital logistics of patient transfer and triage, delayed
presentation, and insufficient interventionists, as well as difficulty in
obtaining emergency CT angiogram in an urgent setting.
The two-tier primary and comprehensive stroke
centre model that aims to give priority access to patients with suspected
acute stroke may be seen as a framework for acute stroke service.30 31 32 The first-tier stroke centre should be capable of
providing 24-hour urgent CT and CT angiogram with round-the-clock IV-tPA
service. All patients with suspicious stroke symptoms, such as acute
hemiplegia or dysphasia, should be directly transferred to one of these
first-tier stroke centres, bypassing other non-stroke centres in emergency
hospitals to avoid unnecessary delay in diagnosis. When major vessel
occlusion is suspected, immediate CT angiography should be performed in
the same setting to determine thrombectomy eligibility. Second-tier stroke
centres should additionally be capable of 24-hour endovascular
thrombectomy, and be equipped with full-time neurosurgery, neurocritical
care, and advanced radiological imaging support.31
Among the local stroke specialists, a median number of four second-tier
comprehensive stroke centres is believed to be appropriate in Hong Kong.
Support from the health administration, structured training for
endovascular techniques, and efficient use of existing resources are
required to effectively incorporate emergency thrombectomy into routine
clinical service locally.
Conclusion
There is a strong body of clinical evidence and
international guidelines to support emergency endovascular thrombectomy
for acute ischaemic stroke due to anterior circulation major vessel
occlusion. As the local stroke community embraces this new treatment
modality, efforts should be directed towards providing universal and
timely access to emergency ischaemic stroke therapy for the Hong Kong
population.
Declaration
All authors have disclosed no conflicts of
interest.
Acknowledgements
This paper is supported by the Hong Kong Stroke
Society Research Scholarship 2015 and the Health and Medical Research Fund
(01150027). We thank Dr GKK Leung for his valuable advice in the course of
this study. We also thank the Hong Kong Stroke Society, Hong Kong
Neurosurgical Society, and Hong Kong Society of Interventional and
Therapeutic Neuroradiology for their support in the survey study.
References
1. National Institute of Neurological
Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen
activator for acute ischemic stroke. N Engl J Med 1995;333:1581-7. Crossref
2. Hacke W, Kaste M, Bluhmki E, et al.
Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N
Engl J Med 2008;359:1317-29. Crossref
3. Bhatia R, Hill MD, Shobha N, et al. Low
rates of acute recanalization with intravenous recombinant tissue
plasminogen activator in ischemic stroke: real-world experience and a call
for action. Stroke 2010;41:2254-8. Crossref
4. Jüttler E, Schwab S, Schmiedek P, et al.
Decompressive surgery for the treatment of malignant infarction of the
middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke
2007;38:2518-25. Crossref
5. Broderick JP, Palesch YY, Demchuk AM, et
al. Endovascular therapy after intravenous t-PA versus t-PA alone for
stroke. N Engl J Med 2013;368:893-903. Crossref
6. Ciccone A, Valvassori L, Nichelatti M,
et al. Endovascular treatment for acute ischemic stroke. N Engl J Med
2013;368:904-13. Crossref
7. Kidwell CS, Jahan R, Gornbein J, et al.
A trial of imaging selection and endovascular treatment for ischemic
stroke. N Engl J Med 2013;368:914-23. Crossref
8. Berkhemer OA, Fransen PS, Beumer D, et
al. A randomized trial of intraarterial treatment for acute ischemic
stroke. N Engl J Med 2015;372:11-20. Crossref
9. Jovin TG, Chamorro A, Cobo E, et al.
Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl
J Med 2015;372:2296-306. Crossref
10. Goyal M, Demchuk AM, Menon BK, et al.
Randomized assessment of rapid endovascular treatment of ischemic stroke.
N Engl J Med 2015;372:1019-30. Crossref
11. Campbell BC, Mitchell PJ, Kleinig TJ,
et al. Endovascular therapy for ischemic stroke with perfusion-imaging
selection. N Engl J Med 2015;372:1009-18. Crossref
12. Saver JL, Goyal M, Bonafe A, et al.
Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in
stroke. N Engl J Med 2015;372:2285-95. Crossref
13. Goyal M, Menon BK, van Zwam WH, et al.
Endovascular thrombectomy after large-vessel ischaemic stroke: a
meta-analysis of individual patient data from five randomised trials.
Lancet 2016;387:1723-31. Crossref
14. Higashida RT, Furlan AJ, Roberts H, et
al. Trial design and reporting standards for intra-arterial cerebral
thrombolysis for acute ischemic stroke. Stroke 2003;34:e109-37. Crossref
15. Turk AS, Frei D, Fiorella D, et al.
ADAPT FAST study: a direct aspiration first pass technique for acute
stroke thrombectomy. J Neurointerv Surg 2014;6:260-4. Crossref
16. Hentschel KA, Daou B, Chalouhi N, et
al. Comparison of non-stent retriever and stent retriever mechanical
thrombectomy devices for the endovascular treatment of acute ischemic
stroke. J Neurosurg 2017;126:1123-30. Crossref
17. Lapergue B, Blanc R, Guedin P, et al.
A direct aspiration, first pass technique (ADAPT) versus stent retrievers
for acute stroke therapy: an observational comparative study. AJNR Am J
Neuroradiol 2016;37:1860-5. Crossref
18. Turk AS, Turner R, Spiotta A, et al.
Comparison of endovascular treatment approaches for acute ischemic stroke:
cost effectiveness, technical success, and clinical outcomes. J
Neurointerv Surg 2015;7:666-70. Crossref
19. Mocco J, Zaidat OO, von Kummer R, et
al. Aspiration thrombectomy after intravenous alteplase versus intravenous
alteplase alone. Stroke 2016;47:2331-8. Crossref
20. Aronsson M, Persson J, Blomstrand C,
Wester P, Levin LÅ. Cost-effectiveness of endovascular thrombectomy in
patients with acute ischemic stroke. Neurology 2016;86:1053-9. Crossref
21. Nguyen-Huynh MN, Johnston SC. Is
mechanical clot removal or disruption a cost-effective treatment for acute
stroke? AJNR Am J Neuroradiol 2011;32:244-9. Crossref
22. Ganesalingam J, Pizzo E, Morris S,
Sunderland T, Ames D, Lobotesis K. Cost-utility analysis of mechanical
thrombectomy using stent retrievers in acute ischemic stroke. Stroke
2015;46:2591-8. Crossref
23. Wahlgren N, Moreira T, Michel P, et
al. Mechanical thrombectomy in acute ischemic stroke: Consensus statement
by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT, ESNR
and EAN. Int J Stroke 2016;11:134-47. Crossref
24. Powers WJ, Derdeyn CP, Biller J, et
al. 2015 American Heart Association/American Stroke Association focused
update of the 2013 guidelines for the early management of patients with
acute ischemic stroke regarding endovascular treatment: a guideline for
healthcare professionals from the American Heart Association/American
Stroke Association. Stroke 2015;46:3020-35. Crossref
25. Wong EH, Yu SC, Lau AY, et al.
Intra-arterial revascularisation therapy for acute ischaemic stroke:
initial experience in a Hong Kong hospital. Hong Kong Med J
2013;19:135-41.
26. Brinjikji W, Murad MH, Rabinstein AA,
Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general
anesthesia during endovascular acute ischemic stroke treatment: a
systematic review and meta-analysis. AJNR Am J Neuroradiol 2015;36:525-9.
Crossref
27. Schönenberger S, Uhlmann L, Hacke W,
et al. Effect of conscious sedation vs general anesthesia on early
neurological improvement among patients with ischemic stroke undergoing
endovascular thrombectomy: a randomized clinical trial. JAMA
2016;316:1986-96. Crossref
28. Public health. Hong Kong: the facts.
Information Services Department, Hong Kong SAR Government. February 2016.
29. Hospital Authority Annual plan
2015-2016. Hong Kong: The Hospital Authority; 2015.
30. Alberts MJ, Latchaw RE, Jagoda A, et
al. Revised and updated recommendations for the establishment of primary
stroke centers: a summary statement from the brain attack coalition.
Stroke 2011;42:2651-65. Crossref
31. Alberts MJ, Latchaw RE, Selman WR, et
al. Recommendations for comprehensive stroke centers: a consensus
statement from the Brain Attack Coalition. Stroke 2005;36:1597-616. Crossref
32. Gorelick PB. Primary and comprehensive
stroke centers: history, value and certification criteria. J Stroke
2013;15:78-89. Crossref