Hong
Kong Med J 2018 Feb;24(1):56–62 | Epub 12 Jan 2018
DOI: 10.12809/hkmj176808
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Efficacy and tolerability of trastuzumab emtansine in
advanced human epidermal growth factor receptor 2–positive breast cancer
Winnie Yeo, FRCP, FHKAM (Medicine)1; MY
Luk, FHKCR, FHKAM (Radiology)2; Inda S Soong, FHKCR, FHKAM
(Radiology)3; Tony YS Yuen, FHKCR, FHKAM (Radiology)4;
TY Ng, FHKCR, FHKAM (Radiology)5; Frankie KF Mo, BSc, PhD6;
K Chan, FHKCR, FHKAM (Radiology)3; SY Wong, FHKCR, FHKAM
(Radiology)5; Janice Tsang, FHKCP, FHKAM (Medicine)7;
Carmen Leung, FHKCR, FHKAM (Radiology)4; Joyce JS Suen, FHKCR,
FHKAM (Radiology)8; Roger KC Ngan, FHKCR, FHKAM (Radiology)4
1 Department of Clinical Oncology, The
Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Clinical Oncology, Queen
Mary Hospital, Pokfulam, Hong Kong
3 Department of Clinical Oncology,
Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
4 Department of Clinical Oncology, Queen
Elizabeth Hospital, Jordan, Hong Kong
5 Department of Clinical Oncology, Tuen
Mun Hospital, Tuen Mun, Hong Kong
6 Comprehensive Clinical Trials Unit,
Department of Clinical Oncology, The Chinese University of Hong Kong,
Shatin, Hong Kong
7 Department of Medicine, The University
of Hong Kong, Pokfulam, Hong Kong
8 Department of Clinical Oncology,
Prince of Wales Hospital, Shatin, Hong Kong
Corresponding author: Prof Winnie Yeo (wyeo@cuhk.edu.hk)
Abstract
Introduction: The management of
human epidermal growth factor receptor 2 (HER2)–positive breast cancer
has changed dramatically with the introduction and widespread use of
HER2-targeted therapies. There is, however, relatively limited
real-world information about the effectiveness and safety of trastuzumab
emtansine (T-DM1) in Hong Kong Chinese patients. We assessed the
efficacy and toxicity profiles among local patients with HER2-positive
advanced breast cancer who had received T-DM1 therapy in the second-line
setting and beyond.
Methods: This retrospective
study involved five local centres that provide service for over 80% of
the breast cancer population in Hong Kong. The study period was from
December 2013 to December 2015. Patients were included if they had
recurrent or metastatic histologically confirmed HER2+ breast cancer who
had progressed after at least one line of anti-HER2 therapy including
trastuzumab. Patients were excluded if they received T-DM1 as first-line
treatment for recurrent or metastatic HER2+ breast cancer. Patient
charts including biochemical and haematological profiles were reviewed
for background information, T-DM1 response, and toxicity data. Adverse
events were documented during chemotherapy and 28 days after the last
dose of medication.
Results: Among 37 patients being
included in this study, 28 (75.7%) had two or more lines of anti-HER2
agents and 26 (70.3%) had received two or more lines of palliative
chemotherapy. Response assessment revealed that three (8.1%) patients
had a complete response, eight (21.6%) a partial response, 11 (29.7%) a
stable disease, and 12 (32.4%) a progressive disease; three patients
could not be assessed. The median duration of response was 17.3 (95%
confidence interval, 8.4-24.8) months. The clinical benefit rate
(complete response + partial response + stable disease, ≥12 weeks) was
37.8% (95% confidence interval, 22.2%-53.5%). The median
progression-free survival was 6.0 (95% confidence interval, 3.3-9.8)
months and the median overall survival had not been reached by the data
cut-off date. Grade 3 or 4 toxicities included thrombocytopaenia
(13.5%), raised alanine transaminase (8.1%), anaemia (5.4%), and
hypokalaemia (2.7%). No patient died as a result of toxicities.
Conclusions: In patients with
HER2-positive advanced breast cancer who have been heavily pretreated
with anti-HER2 agents and cytotoxic chemotherapy, T-DM1 is well
tolerated and provided a meaningful progression-free survival of 6
months and an overall survival that has not been reached. Further
studies to identify appropriate patient subgroups are warranted.
New knowledge added by this study
- This study confirms that the efficacy and toxicity profiles of trastuzumab emtansine (T-DM1) among Chinese patients are similar to the published data that have been based mainly on western populations.
- T-DM1 is effective in HER2-positive advanced breast cancer in the second-line setting and beyond. It has tolerable toxicity. Further research is warranted to enable identification of the appropriate patient population to enhance cost-effectiveness.
Introduction
Breast cancer is the most common female cancer in
Hong Kong. The human epidermal growth factor receptor HER2/neu
gene is amplified and overexpressed in 15% to 25% of breast cancers.1 The management of human epidermal growth factor
receptor 2 (HER2)–positive (HER2+) breast cancer has changed dramatically
with the introduction and widespread use of HER2-targeted therapies. The
landmark study reported by Slamon et al2
over a decade ago established the combination of trastuzumab with
chemotherapy as the standard of care for patients with HER2+ metastatic
breast cancer. The later CLEOPATRA trial showed that the combination of
pertuzumab with trastuzumab and chemotherapy (specifically, docetaxel)
could further improve survival when compared with the standard arm of
trastuzumab plus chemotherapy in the first-line setting.3
Trastuzumab emtansine (T-DM1) is an antibody-drug
conjugate that incorporates the HER2-targeted antitumour properties of
trastuzumab with the cytotoxic activity of the microtubule inhibitor DM1
(which is a derivative of maytansine). The high potency of the cytotoxic
DM1 moiety has been suggested as a key factor in the enhanced activity of
this compound.4 5 In the second-line setting, the pivotal EMILIA study
compared T-DM1 with lapatinib plus capecitabine among patients with HER2+
breast cancer who had previously been treated with trastuzumab and a
taxane; T-DM1 showed remarkable activity with an acceptable toxicity
profile.6 There is, however,
relatively limited real-world information about the effectiveness and
safety of T-DM1 in Hong Kong Chinese patients.
In this multicentre retrospective study, we
assessed the efficacy and toxicity profiles among local patients with
HER2+ advanced breast cancer who had received T-DM1 therapy in the
second-line setting and beyond.
Methods
This was a retrospective study that involved five
local centres that care for over 80% of the local breast cancer
population, and included the Pamela Youde Nethersole Eastern Hospital,
Prince of Wales Hospital, Queen Mary Hospital, Queen Elizabeth Hospital,
and Tuen Mun Hospital between December 2013 and December 2015, the period
when the relevant treatment was first started. The institutional ethics
committee of each participating centre approved the study.
Inclusion criteria included patients who had
recurrent or metastatic histologically confirmed HER2+ breast cancer who
either had progressed during trastuzumab with chemotherapy in the
first-line treatment setting, or had developed progressive disease after
at least one line of anti-HER2 agent including trastuzumab. Patients who
had received endocrine therapy for recurrent or metastatic disease were
included. Exclusion criteria included patients who received T-DM1 as
first-line treatment for recurrent or metastatic HER2+ breast cancer.
Patient charts were reviewed for background
information, T-DM1 response, and toxicity data by medical staff who were
not blinded to the study objectives. Biochemical and haematological
profiles were extracted from patient charts. Tumour response assessments
were recorded according to the Response Evaluation Criteria in Solid
Tumors Committee.7 Adverse events
were graded according to the National Cancer Institute’s Common
Terminology Criteria for Adverse Events (version 3.0). Adverse events were
also documented during chemotherapy and 28 days after the last dose of
study medication.
Statistical analysis
Outcomes in terms of tumour response,
progression-free survival (PFS), and overall survival (OS) were
determined. The PFS was assessed from day 1 of treatment cycle 1 to the
date when objective disease progression was observed, and OS was
calculated from day 1 of treatment cycle 1 to the date of death. Death was
regarded as a progression event in those subjects who died before disease
progression. Subjects without documented objective progression at the time
of the final analysis were censored at the date of their last tumour
assessment; data cut-off was on 31 August 2016. Survival curves were
constructed using the Kaplan-Meier method.
Results
Patient characteristics
Patient characteristics are shown in Table
1. Of a total of 37 patients, 33 (89.2%) had an Eastern Cooperative
Oncology Group performance status of 0 or 1.
Of the 37 patients, tumour biology studies at
initial disease presentation showed that 15 (40.5%) patients were
oestrogen receptor (ER)–positive, 10 (27.0%) were progesterone receptor
(PR)–positive, and 31 (83.8%) had HER2+ breast cancer. Overall, 21
patients had tumour re-biopsy at the time of developing metastatic
disease, 10 (47.6%) patients were ER-positive, nine (42.9%) PR-positive,
and 21 (100%) had HER2+ (which included six patients who were found to
have HER2+ tumours only when anti-HER2 therapy was considered for
metastatic disease).
At the time of initiating T-DM1 therapy, 21
patients had three or more disease sites involved; the most common sites
included lymph nodes (n=27, 73.0%), lungs (n=20, 54.1%), and bones (n=19,
51.4%).
Prior treatments
Prior treatments that patients received are listed
in Table 1. With regard to adjuvant treatments, 13
(35.1%) patients had prior adjuvant trastuzumab, 21 (56.8%) had adjuvant
chemotherapy, eight (21.6%) had adjuvant endocrine therapy, and 19 (51.4%)
had adjuvant radiotherapy.
With regard to treatment for recurrent/ metastatic
disease, nine (24.3%) patients had one line of prior trastuzumab with
chemotherapy including three who had trastuzumab in combination with
pertuzumab and chemotherapy; 11 (29.7%) had two lines while 17 (45.9%) had
three or more lines of anti-HER2 therapy. Overall, 22 (59.5%) patients had
received prior lapatinib, and five (13.5%) had received pertuzumab beyond
the first-line setting.
A total of 26 (70.3%) patients had received two or
more lines of palliative chemotherapy, with the majority having received
taxanes (n=33, 89.2%), capecitabine (n=23, 62.2%) and vinorelbine (n=17,
45.9%). Nineteen patients had received one or more lines of palliative
endocrine therapy, these included eight (21.6%) with tamoxifen, 15 (40.5%)
with aromatase inhibitors, and seven (18.9%) with ovarian ablation.
Trastuzumab emtansine dose and dose interruptions
The median number of days from last anti-HER2
therapy to the first dose of T-DM1 was 32 days (range, 14-274 days).
The median number of cycles was six (range, 1-43).
The follow-up data were frozen on 31 August 2016. The median follow-up
period was 15.6 months (95% confidence interval [CI], 8.1-20.4 months).
Overall, 33 patients were started on the standard dose of 3.6 mg/kg, given
once every 3 weeks; 13 patients had dose delay, 10 patients had dose
reduction for subsequent cycles, and six patients had both dose delay and
dose reductions for subsequent cycles. A total of 326 cycles were
administered; 44 (13.5%) cycles were delayed, 11 (3.4%) cycles had further
dose reductions in the subsequent cycles, and 51 (15.6%) cycles had both
dose delay and dose reductions.
At the time of data cut-off, 28 had discontinued
T-DM1 treatment: 20 (71.4%) due to progressive disease, four (14.3%) were
lost to follow-up, one (3.6%) due to patient withdrawal, and three (10.7%)
due to unspecified causes. No patient discontinued treatment due to
intolerable toxicities.
Response and survival
Among the 37 patients, there were three (8.1%)
complete response (CR), eight (21.6%) partial response (PR), 11 (29.7%)
stable disease (SD), and 12 (32.4%) progressive disease; three patients
could not be assessed (ie they did not have response assessment documented
during their treatment). The median duration of response was 17.3 months
(interquartile range, 9.4-24.5; 95% confidence interval, 8.4-24.8 months).
The clinical benefit rate, defined as CR, PR, or SD of 12 weeks or longer,
was 37.8% (95% CI, 22.2%-53.5%).
Overall, based on the Kaplan-Meier method, the
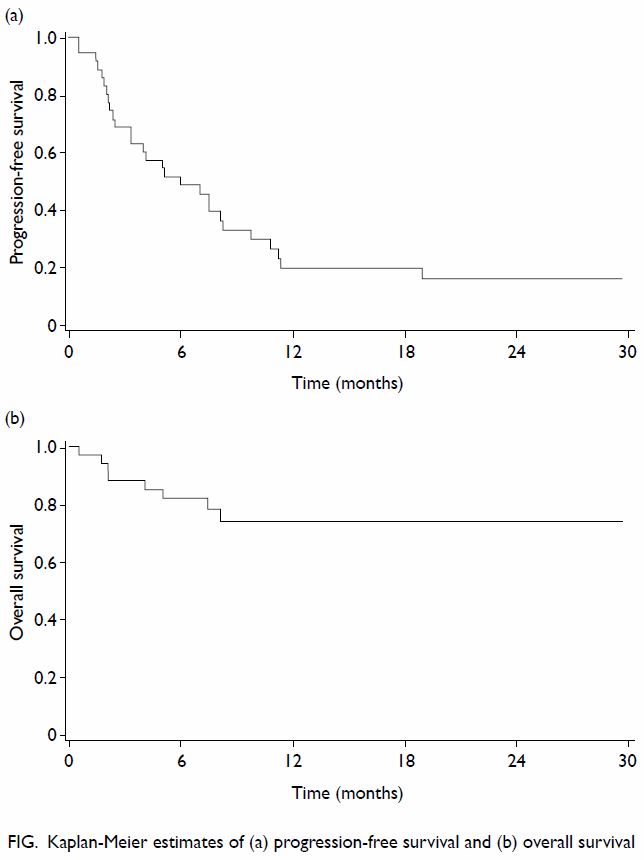
median PFS was 6.0 (95% CI, 3.3-9.8) months; the 6-month and 12-month PFSs
were 51.6% and 23.1%, respectively (Fig a). The median duration of follow-up for PFS was
5.0 (interquartile range, 2.2-10.3) months. The median OS was not reached;
the 6-month and 12-month OSs were 82.1% and 74.4%; respectively (Fig
b).
Toxicity
Haematological and non-haematological toxicities
are listed in Table 2. Grade 3 or 4 toxicities that occurred in
one or more patients included thrombocytopenia (n=5, 13.5%), raised
alanine transaminase (n=3, 8.1%), anaemia (n=2, 5.4%), and hypokalaemia
(n=1, 2.7%). Apart from these, other toxicities that occurred in more than
10% of patients included raised alkaline phosphatase, hyponatraemia,
neutropenia, leukopenia, fatigue, raised serum creatinine, and diarrhoea.
There was no cardiac toxicity and no patients died as a result of
toxicities.
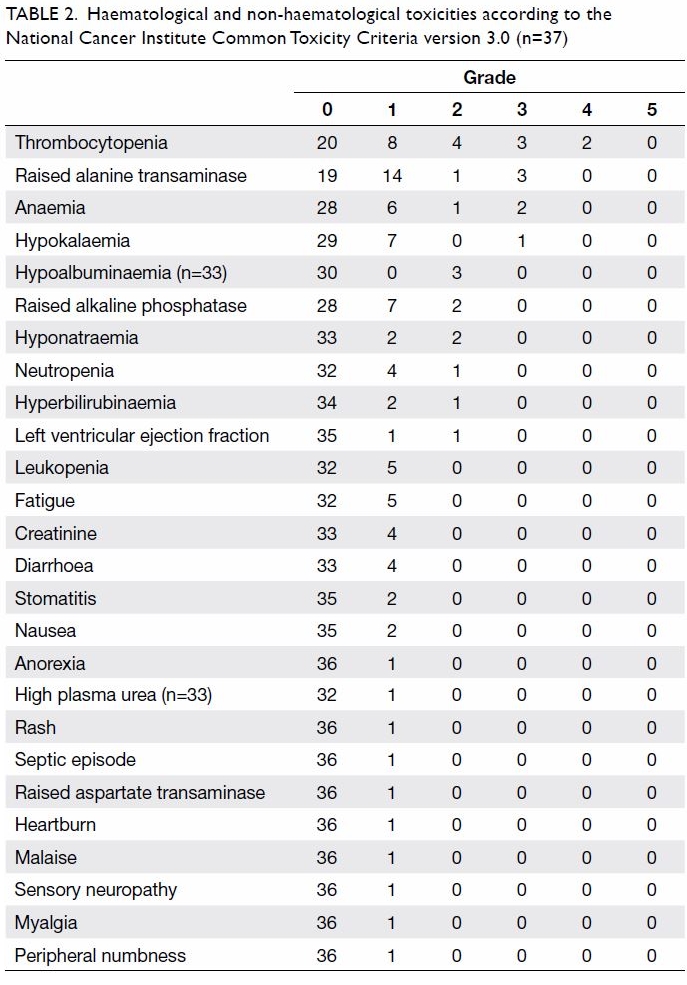
Table 2. Haematological and non-haematological toxicities according to the National Cancer Institute Common Toxicity Criteria version 3.0 (n=37)
Discussion
During the past decade, the treatment of HER2+
breast cancer has rapidly evolved, and patients with HER2+ metastatic
breast cancer have experienced a remarkable improvement in clinical
outcomes in terms of OS.8
The efficacy of T-DM1 was well demonstrated in the
pivotal EMILIA study that compared T-DM1 with lapatinib plus capecitabine
among HER2+ breast cancer patients in the second-line setting. The studied
patients had previously been treated with trastuzumab and a taxane. For
the T-DM1–treated patients, the objective response rate was 44%, the
median PFS was 9.6 months, and the median OS was 30.9 months.6
In the current multicentre retrospective study
among the Chinese patients with breast cancer, over 70% were heavily
pretreated with anti-HER2 agents as well as cytotoxic chemotherapy. The
efficacy results are consistent with previous findings from the TH3RESA
study.9 The latter involved over
600 HER2+ patients with advanced breast cancer who had received two or
more anti-HER2–containing regimens, including trastuzumab and lapatinib,
and previous taxane therapy. At a median follow-up of 6.5 months, the
TH3RESA study reported that among the T-DM1–treated patients, the
objective response rate was 31%, the median duration of response was 9.7
months, the median PFS was 6.2 months, and the median OS was not reached.9 Similarly, the safety profile in
the current study was consistent with the reported clinical trials, where
grade 3 or worse thrombocytopenia was the most commonly reported adverse
event (13.5%), followed by raised alanine transaminase (8.1%), anaemia
(5.4%), and hypokalaemia (2.7%). Notably there was no grade 3 or worse
neutropenia, no febrile neutropenia, and no cardiac toxicity noted in the
current study.
In heavily pretreated patient populations, two
studies, namely the TH3RESA study9
and the EGF104900 study10 (which
assessed combination of trastuzumab and lapatinib in the absence of
chemotherapy), have shown that even after a median of four prior regimens,
the use of anti-HER2 therapy can lead to meaningful clinical benefits. In
the TH3RESA study, the PFS benefit with T-DM1 was observed in subgroups
including hormone receptor–positive tumours and non-visceral disease, as
well as asymptomatic or treated brain metastases. An exploratory analysis
conducted in the present study revealed that the median PFSs for patients
with hormone receptor–positive disease and hormone receptor–negative
disease were 7.5 and 6.0 months, respectively. Owing to small patient
numbers, the finding was not significant (P=0.78) but nonetheless lends
support to the published data.
Among the 37 patients in the current study, five
had prior pertuzumab therapy in addition to trastuzumab (including one who
also had lapatinib). One of these patients achieved PR and had a total of
eight cycles of T-DM1 treatment. The efficacy of T-DM1 among patients
previously treated with trastuzumab and pertuzumab has recently been
reported in a retrospective study.11
Although the response rate was relatively low at 18%, 30% of the patients
had received prolonged T-DM1 therapy, defined as treatment duration of 6
months or longer.
It has to be noted that despite the efficacy shown
in the second-line and beyond setting among HER2+ patients with advanced
breast cancer, the MARIANNE study, which tested three different anti-HER2
regimens in the first-line setting, did not show T-DM1 to be superior to
standard treatment.12 In that
study, previously untreated patients with HER2+ metastatic breast cancer
were randomised to one of the three arms: control (trastuzumab plus
taxane), T-DM1 alone, or T-DM1 plus pertuzumab. Although the results
revealed that grade 3 or higher adverse events were lower in the T-DM1
arm, efficacy data on PFS were similar in all three arms, at 13.7 months,
14.1 months, and 15.2 months, respectively. In another exploratory
analysis in the present study, the PFS of those patients who had undergone
only one line of prior anti-HER2 therapy was compared with those who had
two or more lines of anti-HER2 therapy revealed corresponding figures of
8.2 and 5.1 months, respectively (P=0.34).
In addition, cost-effective analysis has been
conducted in a number of countries with regard to the use of T-DM1. For
patients with HER2+ metastatic breast cancer, the Canadian analysis
demonstrated that utilising T-DM1 could lead to substantial savings for
the public health care system when the costs of treatment-related adverse
events incurred by other anti-cancer agents were taken into account.13 Nonetheless, analyses based in the United Kingdom and
the United States have not supported such findings.14 15 16
The identification of an appropriate patient
population for the utilisation of T-DM1 may enable better resource
allocation. Yet to date, no biomarkers have been identified that can
predict better outcome among patients with HER2+ advanced breast cancer
treated with T-DM1. Based on the biomarker analyses from EMILIA and
TH3RESA studies, T-DM1 was similarly effective in the presence of PI3K
wild-type or mutated tumours, and the benefit with T-DM1 was seen
irrespective of HER2 mRNA, HER3 mRNA, or PTEN protein level.17 18
The current study is limited by its retrospective
design, possible information bias during data retrieval/extraction/coding,
as well as the small number of patients (especially for subgroup analysis)
and inadequate follow-up period for OS. Although the results could not be
compared directly with reported prospective trials, patients were
representative, and treatment and outcomes reflect routine clinical
practice. The T-DM1 therapy provided a meaningful PFS with a favourable
toxicity profile among heavily pretreated patients with HER2+ advanced
breast cancer. Research is needed to identify biomarkers that will predict
sensitivity and resistance to individual anti-HER2 agents, and thereby
enable identification of those patients most likely to respond to T-DM1
and appropriate treatment to optimise patient benefit, reduce excessive
toxicities, and minimise costs.
Conclusions
The T-DM1 therapy has a tolerable toxicity profile
among local patients with recurrent or metastatic HER2+ breast cancer. For
patients who responded to T-DM1 therapy, there was a durable response. In
our study, T-DM1 is associated with a PFS of 6 months and an OS that has
not been reached. Further biomarker study is needed to enable appropriate
patient selection for this treatment.
Acknowledgements
We thank Dr Vicky TC Chan of the Department of
Clinical Oncology, Prince of Wales Hospital, and Drs Carol Kwok and
Raymond KY Wong of the Department of Oncology, Princess Margaret Hospital,
for their support in this study.
Declaration
This study has been supported by the Hong Kong
Breast Oncology Group. W Yeo has received honoraria for expert opinion
from Novartis and Pfizer and has received a research grant from
Mundipharma in relation to breast cancer research over the past 12 months.
The funder had no role in study selection, quality assessment, data
analysis, or writing the manuscript. All other authors have disclosed no
conflicts of interest.
References
1. Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and
survival with amplification of the HER-2/neu oncogene. Science
1987;235:177-82. Crossref
2. Slamon DJ, Leyland-Jones B, Shak S, et
al. Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med
2001;344:783-92. Crossref
3. Baselga J, Cortés J, Kim SB, et al.
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N
Engl J Med 2012;366:109-19. Crossref
4. Lewis Phillips GD, Li G, Dugger DL, et
al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an
antibody-cytotoxic drug conjugate. Cancer Res 2008;68:9280-90. Crossref
5. Lopus M, Oroudjev E, Wilson L, et al.
Maytansine and cellular metabolites of antibody-maytansinoid conjugates
strongly suppress microtubule dynamics by binding to microtubules. Mol
Cancer Ther 2010;9:2689-99. Crossref
6. Verma S, Miles D, Gianni L, et al.
Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J
Med 2012;367:1783-91. Crossref
7. Therasse P, Arbuck SG, Eisenhauer EA, et
al. New guidelines to evaluate the response to treatment in solid tumors.
J Natl Cancer Inst 2000;92:205-16. Crossref
8. Lobbezoo DJ, van Kampen RJ, Voogd AC, et
al. Prognosis of metastatic breast cancer subtypes: The hormone
receptor/HER2-positive subtype is associated with the most favorable
outcome. Breast Cancer Res Treat 2013;141:507-14. Crossref
9. Krop IE, Kim SB, González-Martín A, et
al. Trastuzumab emtansine versus treatment of physician’s choice for
pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised,
open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. Crossref
10. Blackwell KL, Burstein HJ, Storniolo
AM, et al. Randomized study of lapatinib alone or in combination with
trastuzumab in women with ErbB2-positive, trastuzumab-refractory
metastatic breast cancer. J Clin Oncol 2010;28:1124-30. Crossref
11. Dzimitrowicz H, Berger M, Vargo C, et
al. T-DM1 activity in metastatic human epidermal growth factor receptor
2–positive breast cancers that received prior therapy with trastuzumab and
pertuzumab. J Clin Oncol 2016;34:3511-7. Crossref
12. Perez EA, Barrios C, Eiermann W, et
al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab
plus taxane for human epidermal growth factor receptor 2-positive,
advanced breast cancer: primary results from the phase III MARIANNE study.
J Clin Oncol 2017;35:141-8. Crossref
13. Piwko C, Prady C, Yunger S, Pollex E,
Moser A. Safety profile and costs of related adverse events of trastuzumab
emtansine for the treatment of HER2-positive locally advanced or
metastatic breast cancer compared to capecitabine plus lapatinib from the
perspective of the Canadian health-care system. Clin Drug Investig
2015;35:487-93. Crossref
14. Diaby V, Adunlin G, Ali AA, et al.
Cost-effectiveness analysis of 1st through 3rd line sequential targeted
therapy in HER2-positive metastatic breast cancer in the United States.
Breast Cancer Res Treat 2016;160:187-96. Crossref
15. Le QA, Bae YH, Kang JH.
Cost-effectiveness analysis of trastuzumab emtansine (T-DM1) in human
epidermal growth factor receptor 2 (HER2): positive advanced breast
cancer. Breast Cancer Res Treat 2016;159:565-73. Crossref
16. Squires H, Stevenson M, Simpson E,
Harvey R, Stevens J. Trastuzumab emtansine for treating HER2-positive,
unresectable, locally advanced or metastatic breast cancer after treatment
with trastuzumab and a taxane: an evidence review group perspective of a
NICE single technology appraisal. Pharmacoeconomics 2016;34:673-80. Crossref
17. Baselga J, Lewis Phillips GD, Verma S,
et al. Relationship between tumor biomarkers and efficacy in EMILIA, a
phase III study of trastuzumab emtansine in HER2-positive metastatic
breast cancer. Clin Cancer Res 2016;22:3755-63. Crossref
18. Kim SB, Wildiers H, Krop IE, et al.
Relationship between tumor biomarkers and efficacy in TH3RESA, a phase III
study of trastuzumab emtansine (T-DM1) vs. treatment of physician’s choice
in previously treated HER2-positive advanced breast cancer. Int J Cancer
2016;139:2336-42. Crossref