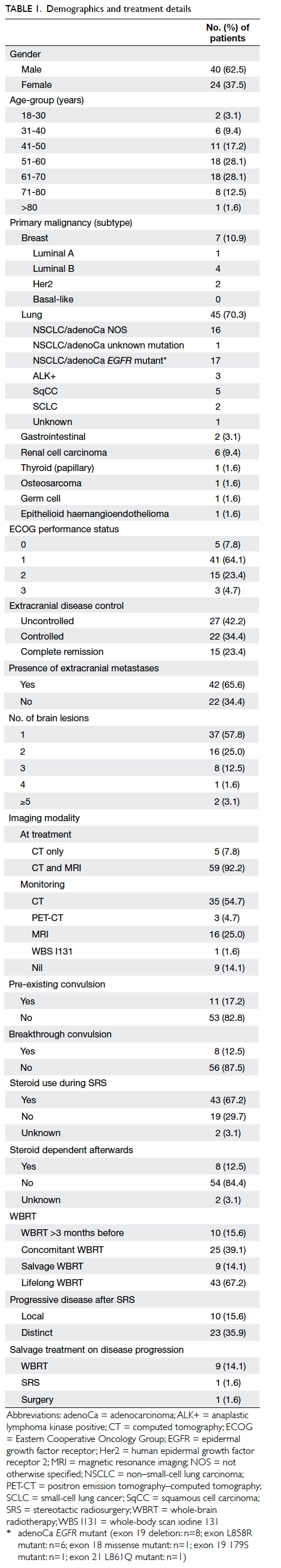
Hong
Kong Med J 2017 Dec;23(6):599–608 | Epub 10 Nov 2017
DOI: 10.12809/hkmj166138
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Frameless stereotactic radiosurgery for brain
metastases: a review of outcomes and prognostic scores evaluation
ST Mok, FHKCR, FHKAM (Radiology)1;
Michael KM Kam, FHKCR, FHKAM (Radiology)1; WK Tsang, FHKCR,
FHKAM (Radiology)1; Darren MC Poon, FHKCR, FHKAM (Radiology)1;
Herbert H Loong, FHKCP, FHKAM (Medicine)2; WM Yeung, FHKCR,
FHKAM (Radiology)1; TY Yeung, FHKCR, FHKAM (Radiology)1;
Jimmy Yu, BSc (Hons), MPhil1; Carlos KH Wong, PhD3
1 Department of Clinical Oncology,
Prince of Wales Hospital, Shatin, Hong Kong
2 Department of Clinical Oncology,
The Chinese University of Hong Kong, Shatin, Hong Kong
3 Department of Family Medicine and
Primary Care, The University of Hong Kong, Pokfulam, Hong Kong
This paper was presented at the MASCC/ISOO
meeting 2016, 23-25 June 2016, Adelaide, Australia.
Corresponding author: Dr ST Mok (mst216@ha.org.hk)
 A video clip showing frameless
stereotactic radiosurgery for brain metastases is available at www.hkmj.org
A video clip showing frameless
stereotactic radiosurgery for brain metastases is available at www.hkmj.orgAbstract
Introduction: Stereotactic brain
radiosurgery provides good local control in patients with limited brain
metastases. A newly developed frameless system allows pain-free
treatment. We reviewed the effectiveness of this frameless stereotactic
brain radiosurgery and identified prognostic factors that may aid better
patient selection.
Methods: Medical records of
patients with brain metastases treated with linear accelerator–based
frameless stereotactic brain radiosurgery between January 2010 and July
2015 in a university affiliated hospital in Hong Kong were reviewed.
Outcomes including local and distant brain control rate,
progression-free survival, and overall survival were analysed.
Prognostic factors were identified by univariable and multivariable
analyses. Association of outcomes with four common prognostic scores was
performed.
Results: In this study, 64
patients with 94 lesions were treated with a median dose of 18 Gy
(range, 12-22 Gy) in a single fraction. The median follow-up was 11.5
months. One-year actuarial local and distant brain control rates were
72% and 71%, respectively. The median overall survival was 13.0 months.
On multivariable analysis, Karnofsky performance status score (>50 vs
≤50) and number of lesions (1-2 vs ≥3) were found to associate
significantly with distinct brain progression-free survival (P=0.022,
hazard ratio=0.20, 95% confidence interval 0.05-0.80 and P=0.003, hazard
ratio=0.31, 95% confidence interval 0.14-0.68, respectively). Overall
survival was associated significantly with Basic Score for Brain
Metastases (P=0.031), Score Index for Radiosurgery in Brain Metastases
(P=0.007), and Graded Prognostic Assessment (P=0.003). Improvement in
overall survival was observed in all groups of different prognostic
scores.
Conclusion: Frameless
stereotactic brain radiosurgery is effective in patients with
oligo-metastases of brain and should be increasingly considered in
patients with favourable prognostic scoring.
New knowledge added by this study
- Survival of patients with brain metastases has significantly improved over the past decade.
- Frameless stereotactic brain radiosurgery is effective and has acceptable toxicities.
- Calculation of a prognostic score can aid clinicians in the identification of patients who will benefit most from stereotactic brain radiosurgery.
Introduction
Patients with brain metastases have previously had
poor survival of only 3 to 4 months with non-surgical treatment.1 2 Substantial
improvement has been achieved in recent years with the advance of systemic
treatment and radiation techniques. Stereotactic radiosurgery (SRS) was
first delivered with the Cobalt-60 Gamma Knife system by Leksell in 1951.3 Today, SRS can also be delivered
via the linear accelerator (LINAC) system and proton beam system. It is
usually indicated in patients with oligo-brain metastases (≤4) with a
diameter of less than 4 cm.4 It is
particularly advantageous for lesions in the deep brain parenchyma that
are not easily accessible by surgery. A frame-based system was initially
used to immobilise the patient. A frameless system was later developed to
minimise patient suffering and was reported to have comparable outcomes to
the framed-based system.5 Since the
introduction of a frameless system, SRS or even fractionated stereotactic
radiotherapy has been increasingly used to treat patients with oligo-brain
metastases. Patients do not have to undergo painful frame placement.
Rather, they undergo simple planning procedures over 2 consecutive days.
The patient is required to return only for mould fitting and planning of
computed tomography. Together with diagnostic fine-cut magnetic resonance
imaging co-registration, oncologists can easily contour the target on the
radiotherapy planning system. With the use of the ExacTrac system
(Brainlab AG, Germany) to verify treatment position, the magnitude of
error is reported to be only 0.7 mm, and the mean deviation between
frame-based and image-guided initial positioning is just 1.0 mm (standard
deviation, 0.5 mm).6 A frameless
system became one of the choices of treatment in SRS and was included in
the ASTRO policy.7 The recommended
dosages according to the RTOG 9005 trial are 24 Gy, 18 Gy, and 15 Gy for
tumours of ≤20 mm, 21-30 mm, and 31-40 mm in maximum diameter,
respectively.8 For framed SRS,
1-year local progression-free survival (PFS) was reported to be up to 70%
to 90%, and median overall survival (OS) of 6 to 12 months.9 10 11 12 13 14 15 The outcomes of frameless SRS have been reported only
in limited series, with 1-year local control of 79% to 95%.5 16 17 18
Patient selection and tailor-made management are
indeed challenging. Several scoring systems have been modelled to predict
survival of patients with brain metastases, including the Radiation
Therapy Oncology Group Recursive Partitioning Analysis (RTOG RPA),19 Basic Score for Brain Metastases (BSBM),20 the Score Index for Radiosurgery in Brain Metastases
(SIR),21 Graded Prognostic
Assessment (GPA),22 and
Disease-Specific Graded Prognostic Assessment (DS-GPA).23 These scoring systems were developed at a time when
treatment strategies were also rapidly evolving with the availability of
more accurate diagnostic imaging, better radiotherapy techniques, and more
effective systemic and targeted agents. A paradigm shift to more
aggressive treatment of oligo-metastasis as a result of longer cancer
survivorship now requires further validation of these scoring systems.
In this study, we reviewed the outcomes of patients
who underwent LINAC-based frameless SRS and identified prognostic factors
that affect survival. By doing so, we hope to gain a better understanding
of which patients will benefit from SRS without jeopardising their quality
of life.
Methods
Records of patients who underwent frameless SRS for
limited brain metastases in a university-affiliated hospital between
January 2010 and July 2015 were retrospectively reviewed. Patient data
were extracted from paper records and the Clinical Management System of
the Hospital Authority, Hong Kong by investigators in charge of the study.
Data extracted included gender, age, type of primary malignancy, date of
diagnosis of malignancy and brain metastases, extracranial disease status
and control at treatment time, diagnostic and monitoring modalities,
presence of convulsions, and steroid and anticonvulsant use before and
after treatment period. Treatment details including immobilisation
technique, number of lesions, dose and fractionation, and volume of
lesions were reviewed from department records and the Brainlab iplan
system (Brainlab AG, Germany). Prognostic scoring including RTOG RPA,
BSBM, SIR, and GPA were calculated (Appendix 1 19
20 21
22 23).
Outcome parameters including local and distant
brain control, PFS, and OS were generated using SPSS (Windows version
22.0; IBM Corp, Armonk [NY], US). Univariable analysis with Cox
proportional hazards model was performed to generate prognostic factors
for local and distinct brain PFS (defined as the time from treatment to
documented local progression/distinct brain progression or death) and OS.
For each outcome, statistically significant non-modifiable patient and
disease factors in univariable analysis together with important treatment
factors were included in respective multivariable analysis using Cox
proportional hazards model. The enter method was used for variable
selection process. Kaplan-Meier survival curve for OS was generated for
different prognostic scoring groups and log rank significance was
calculated. The study was approved by clinical research ethics committee
of the NTEC-CUHK Cluster, Hospital Authority, Hong Kong, with patient
informed consent waived.
Results
Demographics
A total of 68 patients were screened during the
study period. Four patients who were treated with fractionated
stereotactic radiotherapy and single-fraction SRS in the same treatment
were excluded, and thus 64 patients were included. All patients were
treated with frameless LINAC-based SRS with ExacTrac system verification,
while contouring and dosimetry with the Brainlab iplan system. Dose
administered was based on tumour diameter: 22 Gy to lesions of ≤2 cm, 18
Gy to lesions of 2.1-3.0 cm, and 15 Gy to lesions of 3.1-4.0 cm. A 1.5-mm
margin was allowed from gross tumour volume to planning target volume.
Deviation of dose prescription from departmental protocol was permitted at
the individual physician’s discretion.
Among the 64 patients, there were 40 men and 24
women. The median age at the time of treatment was 58 years (range, 22-95
years). The median Eastern Cooperative Oncology Group performance status
was 1 (range, 0-3), and Karnofsky Performance Status (KPS) score was 80
(range, 40-100). Primary disease included carcinoma of breast (n=7), lung
(n=45), gastrointestinal (n=2), renal cell (n=6), thyroid (n=1),
osteosarcoma (n=1), germ cell (n=1), and epithelioid haemangioendothelioma
(n=1). Further details of demographics are summarised in Table
1.
Treatment
A total of 94 lesions were treated with a dose of
12 Gy to 22 Gy according to size (12 Gy, n=6; 15 Gy, n=12; 16 Gy, n=2; 18
Gy, n=48; 20 Gy, n=14; 22 Gy, n=12). The median dose was 18 Gy. The median
size of lesion treated was 19 mm (range, 3-43 mm).
Outcomes
The median follow-up time was 11.5 (range,
0.4-56.4) months. One-year actuarial local control rate was 72% (95%
confidence interval [CI], 57%-83%) and distant control rate was 71% (95%
CI, 56%-82%). The median local PFS was 11.2 (95% CI, 8.4-11.2) months. The
median distinct brain PFS was 10.8 (95% CI, 8.4-13.1) months. The median
OS was 13.0 (95% CI, 10.6-11.3) months.
Toxicities
Four (6.3%) patients had acute toxicities, mainly
brain oedema, and one patient had a seizure for 3 days after treatment.
Eight (12.5%) patients had delayed seizure after a median time of 10.5
months. One patient had radionecrosis confirmed pathologically after
surgical resection. There were 43 (67.2%) patients who were prescribed
steroid before treatment, and eight (12.5%) patients became steroid
dependent until their demise. Steroid prescription was not found to affect
OS significantly. Nonetheless among the steroid group, becoming steroid
dependent was associated with poorer prognosis, with a median OS in the
steroid-dependent group of 0.92 months versus 13.6 months in the
non–steroid-dependent group (P<0.005, log rank; Appendix 2, Fig a). The worse survival of steroid-dependent
patients was independent of volume of brain metastases.
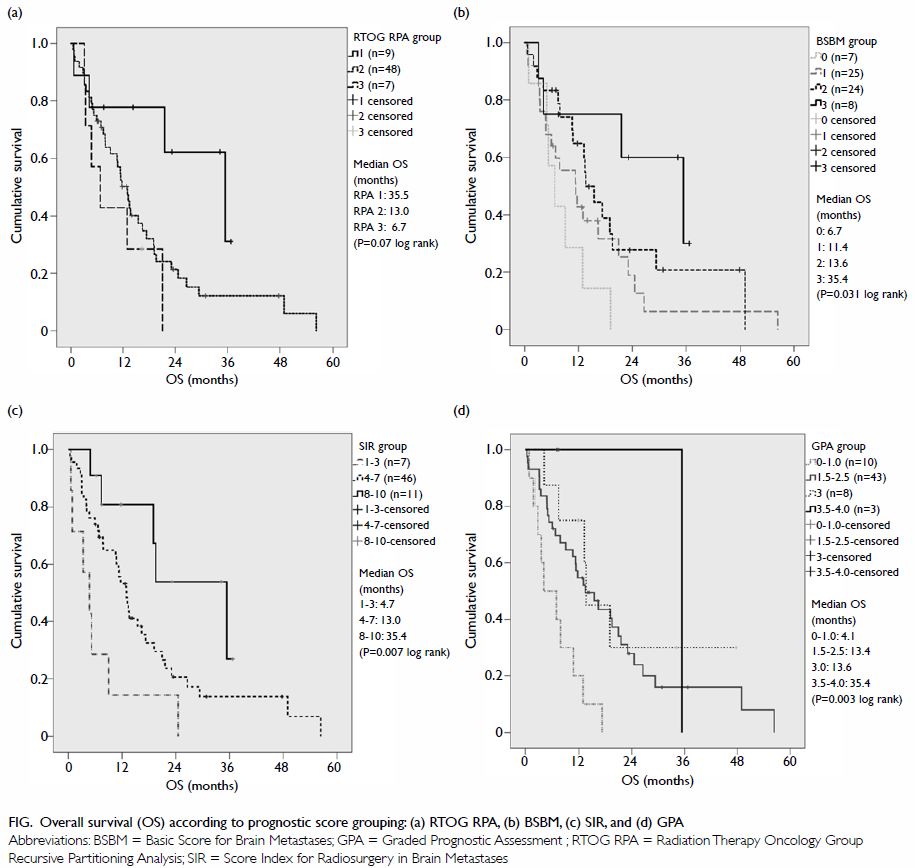
Figure. Overall survival (OS) according to prognostic score grouping: (a) RTOG RPA, (b) BSBM, (c) SIR, and (d) GPA
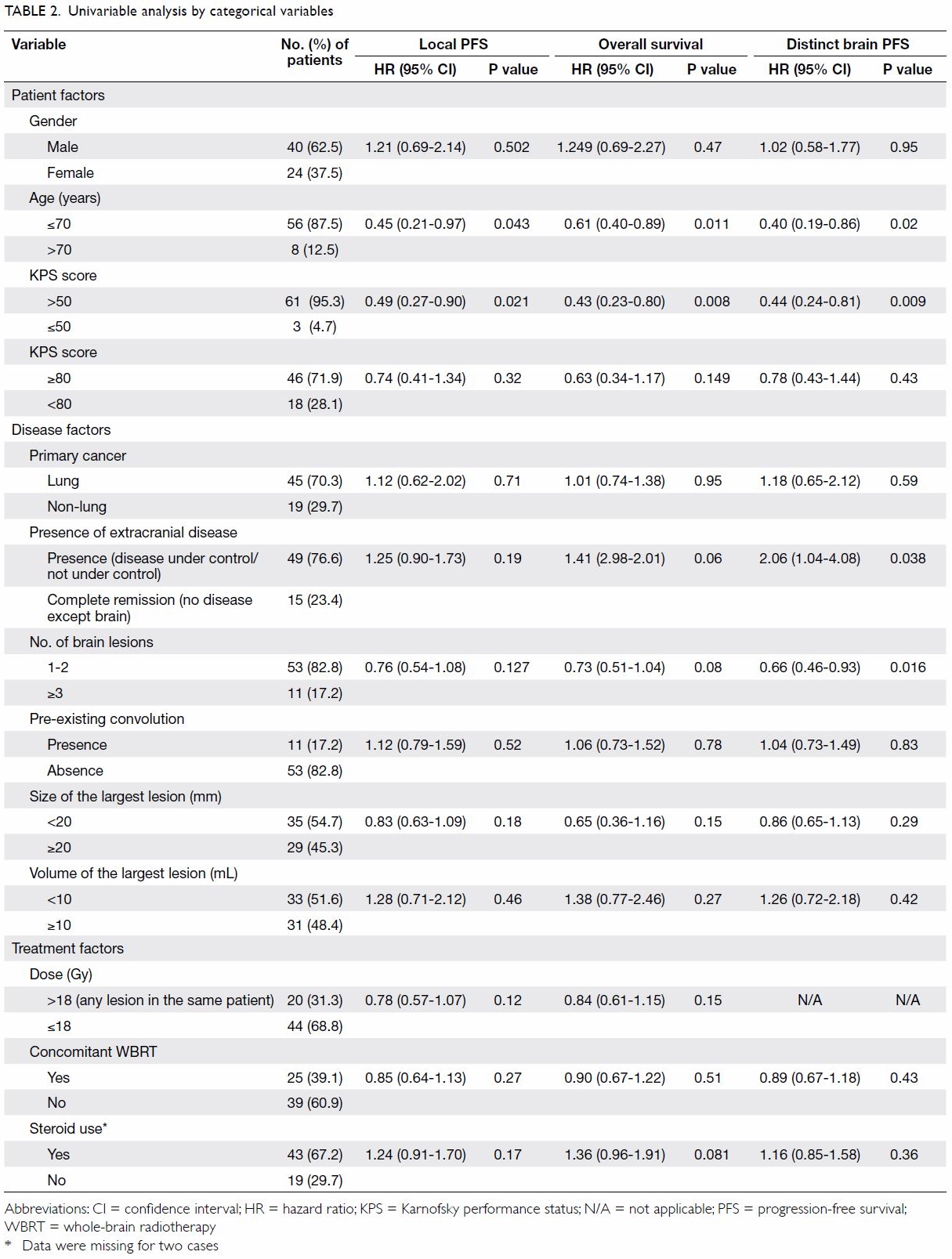
Prognostic patient and disease factors
Potential prognostic factors of survival including
patient factors such as gender, age, and performance status; and disease
factors such as primary cancer, presence of extracranial disease,
pre-existing convulsion, number of brain lesions, and size and volume of
the largest lesion were examined with reference to decision for SRS
treatment by univariable analysis using Cox proportional hazards model. It
was found that OS was associated significantly with age (≤70 vs >70
years; P=0.011) and KPS score (>50 vs ≤50; P=0.008). Local PFS was
associated significantly with age (≤70 vs >70 years; P=0.043) and KPS
score (>50 vs ≤50; P=0.021). Distinct brain PFS was associated
significantly with age (≤70 vs >70 years; P=0.02), presence of
extracranial disease (presence vs absence; P=0.038), KPS score (>50 vs
≤50; P=0.009), and number of brain lesions (<1-2 vs ≥3; P=0.016).
Results of univariable analysis are summarised in Table 2.
Treatment factors
Dose relationship
Dose relationship for each lesion was analysed
separately. Lesions prescribed >18 Gy had statistically significant
superior time to progression (radiologically documented local progression)
than those given ≤18 Gy, with a 1-year local control rate of 88% vs 60% (Appendix 2, Fig b). Some patients had more than one lesion
treated with different doses. Nonetheless after taking into account the
highest dose given in the same patient, dose did not affect local PFS or
OS significantly (Table 2); dose was not analysed in distinct brain
PFS as it should not affect distant brain progression.
Effect of whole-brain radiotherapy
With particular reference to the effect of
whole-brain radiotherapy (WBRT), it was found that concomitant WBRT
(within 3 months of treatment with SRS) did not have a statistically
significant impact on OS, local PFS, or distant brain PFS (Table
2).
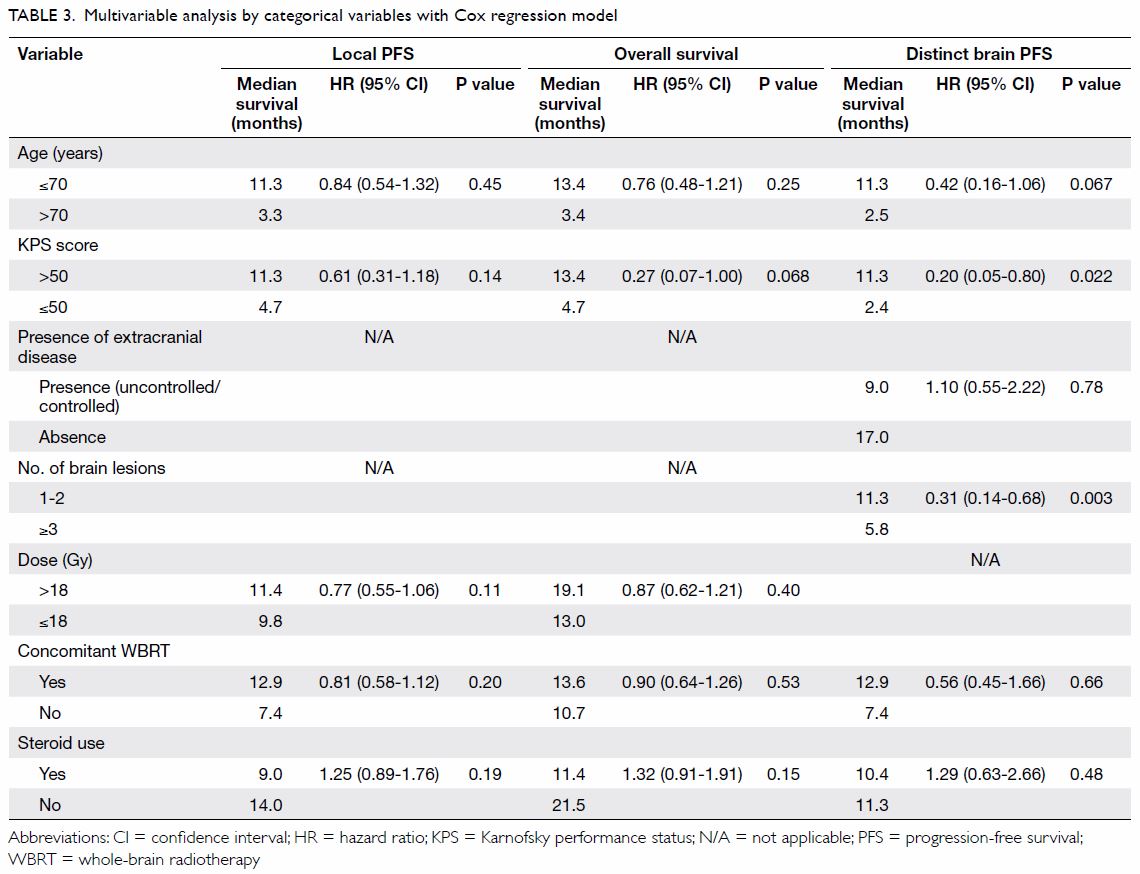
Multivariable analysis
Multivariable analysis using Cox proportional
hazards model and taking patient, disease, and treatment factors into
account identified that statistically significant factors associated with
distinct brain PFS were KPS score (>50 vs ≤50; P=0.022, hazard ratio
[HR]=0.20, 95% CI=0.05-0.80) and number of brain lesions (1-2 vs ≥3;
P=0.003, HR=0.31, 95% CI=0.14-0.68) [Table 3].
Primary lung cancer
Of note, a large number of patients in the group
had primary lung cancer (n=45), most of which were non–small-cell lung
cancer (NSCLC) [n=42]. Among NSCLC patients, a sensitive activating EGFR
mutation (exon 19 deletion or exon 21 L858R mutation) was present in 14.
Three other patients carried a less common mutation: exon 21 861Q (n=1),
exon 18 missense (n=1), and exon 18 179S (n=1). Patients with an exon 19
deletion or exon 21 L858R mutation had superior OS compared with the
non-mutational group (P=0.019, HR=0.281, 95% CI=0.097-0.814) but there was
no statistically significant difference in local or distant brain control.
Among the 14 patients with sensitive activating EGFR mutation,
three patients who were diagnosed with brain metastases received WBRT
before SRS treatment, and six patients were given SRS together with WBRT.
Again, concomitant WBRT was not shown to affect local/distinct brain PFS
or OS. For epidermal growth factor receptor (EGFR)–tyrosine kinase
inhibitor (TKI) treatment, 12 of 14 patients had lifelong EGFR-TKI
treatment, with a median survival of 19.5 months; one with exon 19
deletion and one with exon 18 missense deletion did not have EGFR-TKI
treatment. There were seven patients who were prescribed EGFR-TKI before
SRS treatment (range of duration, 5.7-21.4 months), and eight patients who
were started on or continued on more lines of EGFR-TKI after SRS
treatment.
Association with available prognostic scoring
Overall survival was significantly associated with
BSBM (P=0.031, log-rank), SIR (P=0.007, log-rank), and GPA (P=0.003,
log-rank) [Fig]. A comparison of median survival of the current
study with the other original studies is shown in Table 4.19 20 21
22 Of note, DS-GPA was not
analysed due to the small number of patients with breast,
gastrointestinal, and renal cell primaries. The calculation of GPA and
DS-GPA of lung primary was the same.
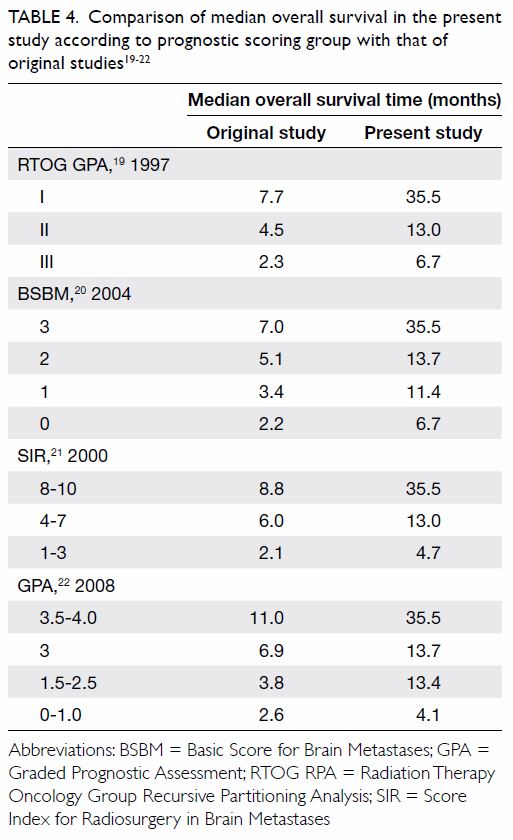
Table 4. Comparison of median overall survival in the present study according to prognostic scoring group with that of original studies19 20 21 22
Discussion
Brain metastasis has previously been considered an
end-of-life event. With the development of new systemic therapies that are
effective in both extracranial and intracranial diseases, together with a
better understanding from clinical trials of the advantages of SRS,
oncologists are more willing to offer SRS to patients with limited brain
metastases.
At the other extreme, studies have compared the
efficacy of WBRT with supportive care in patients with advanced brain
metastases. The latest news from the QUARTZ trial, conducted by the UK
Medical Research Council Group, presented at the American Society of
Clinical Oncology Meeting in 201524
(full paper awaited) was striking for oncologists. They randomly allocated
538 NSCLC patients with brain metastases that were not amenable to surgery
or SRS to either optimal supportive care (OSC) plus WBRT (20 Gy/5
fractions) or OSC alone. There was no significant difference in survival
between the OSC+WBRT group and OSC-alone group, with the median survival
being 65 and 57 days, respectively. Quality of life was also assessed in
this study. The difference between the mean quality-adjusted life-years
was -1.9 days only (OSC+WBRT 43.3 vs OSC-alone 41.4 days) and did not meet
the initial defined criteria of significance. These data revealed that we
are encountering a group of patients with very heterogeneous tumour
behaviour and thus personalised treatment is required.
This retrospective study included patients who
underwent frameless SRS during January 2010 to July 2015, after
commencement of frameless SRS treatment in our centre. Limitations of this
study including small number of patients and information bias are
inevitable. Nonetheless, the outcomes of patients with brain metastases
who underwent frameless SRS in our centre are compatible with those from
other large clinical trials that used frame-based systems in terms of
control rate, median OS and PFS, and toxicities. Approximately 13% of
patients had a complication of steroid dependence that may have been due
to treatment or natural disease progression. Steroid dependence was
associated with poor survival, independent of volume of tumour. Prolonged
use of steroid has been associated with decreased immunity that may
underlie superimposed infection. Therefore, tailing down of steroid dose
as early as possible in accordance with patient symptoms is strongly
recommended.
This study revealed that OS was significantly
associated with previously identified prognostic scoring group such as
BSBM, SIR, and GPA. Among the three, BSBM and GPA are more convenient to
use as only three or four factors are considered respectively, and the
information should be easily available in a clinic (including age, KPS,
control of primary cancer, presence of extracranial metastases, and number
of brain metastases). Data relating to volume of the largest brain lesion
included in SIR may not always be available as the reporting radiologist
may only report lesion diameter. In terms of patient selection, for
patients with GPA of 0-1.0, the median OS was 4.1 months in our study
compared with 2.6 months in the original study, and similar to that of
patients given WBRT alone. It may be more appropriate to prescribe WBRT
alone or best supportive care for this group of patients in lieu of SRS.
An important observation from the result of our study is that survival of
patients was significantly improved compared with a previous cohort (Table
4). This reflects a significant improvement in systemic treatment
over the last decade. Thus, the use of high technology radiation
techniques such as SRS is increasingly considered by radiation oncologists
to achieve the best outcomes.
Another important aim of this study was to identify
prognostic factors of survival in order to avoid futile treatment in those
patients who will have a poor outcome despite SRS. Due to the small number
of patients in this study, we were not able to identify patients with
superior survival among different primaries, similar to DS-GPA. It is of
note that a large number of patients in our study had primary lung cancer.
In the NSCLC subgroup, patients with an activating EGFR mutation
had significantly better survival than those without mutation, and the
majority of this group had EGFR-TKI lifelong. Of note, EGFR-TKI has been
shown in various studies to have PFS and survival benefit in patients with
EGFR-activating mutation.25
26 27
28 29
30 31
32 In a recent retrospective
multi-institutional study with more than 300 patients, outcomes of
patients with EGFR-activating mutation were analysed following
treatment with upfront SRS followed by EGFR-TKI, upfront WBRT followed by
EGFR-TKI, and upfront EGFR-TKI.33
Patients in the upfront SRS and upfront WBRT group had significantly
superior OS and intracranial PFS compared with those with upfront
EGFR-TKI.33 Therefore, in patients
with oligo-brain metastases harbouring an EGFR-activating
mutation, SRS followed by EGFR-TKI should be considered a standard
treatment, and WBRT reserved until there is frank brain disease
progression to conserve cognitive function. In addition, SRS combined with
efficacious systemic treatment with good brain penetration while omitting
WBRT should also be considered in other primaries, although further
studies are awaited to validate the benefit.
The beneficial effect of WBRT in addition to SRS is
controversial. Recent evidence shows it improves local control but not
survival.34 35 Nonetheless, in view of toxicity of somnolence,
malaise and cognitive impairment with WBRT, many clinicians may prefer
delaying WBRT until there is frank disease progression after SRS. In a
recent meta-analysis, the benefit of additional WBRT was not observed in
patients who were 50 years old or younger in terms of survival or distant
brain control.36 Initial omission
of WBRT in this young age-group had no adverse effect on distant brain
relapse rate. We were unable to replicate improvement in brain control
with WBRT or demonstrate an interaction of age with benefit of concomitant
WBRT, possibly due to the small size and retrospective nature of our
current study. Number of brain metastases was identified as a significant
prognostic factor of brain PFS. Patients with three or more brain
metastases had worse PFS than those with one or two brain metastases (5.8
months vs 11.3 months). Again due to the small number of patients, we were
unable to demonstrate whether concomitant WBRT could improve brain PFS in
patients with three or more brain metastases. Further prospective studies
are warranted to verify whether concomitant WBRT should be considered in
patients with a higher disease load or age over 50 years.
Frameless SRS for oligo-brain metastases is
painless and well tolerated, and should be increasingly considered in
patients with good prognostic scores. Its combination with effective
systemic treatment has significantly improved survival over the past
decade. Nonetheless it is important to individualise treatment for
patients with brain metastases according to their inherited prognostic
risk factors. High precision treatment with SRS with or without WBRT
should be offered to patients with oligo-brain metastases with good
prognostic scores and favourable primary histology. For patients with EGFR-activating
mutation, SRS followed by EGFR-TKI is a superior choice of treatment.
Based on the latest evidence, it may be advisable to give SRS alone and
reserve WBRT as salvage for patients with limited brain metastases who are
50 years or younger. Further, WBRT alone can be offered to patients with
multiple symptomatic brain metastases and unfavourable prognostic scores.
Best supportive care with dexamethasone alone may be considered for
patients with very poor performance status.
Conclusions
Frameless SRS is effective and safe for patients
with oligo-metastases of brain. Identification of patients with brain
metastases who would benefit from SRS is important. Current available
prognostic scoring systems provide a good estimation of survival.
Frameless SRS should be increasingly considered in patients with
favourable prognostic scores.
Acknowledgements
I would like to thank Dr SF Leung and Dr Kennis
Ngar of Department of Clinical Oncology, Prince of Wales Hospital, Hong
Kong for their professional opinion and support in this study.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Tsao MN, Lloyd NS, Wong RK, et al.
Radiotherapeutic management of brain metastases: a systematic review and
meta-analysis. Cancer Treat Rev 2005;31:256-73. Crossref
2. Nieder C, Spanne O, Mehta MP, Grosu AL,
Geinitz H. Presentation, patterns of care, and survival in patients with
brain metastases: what has changed in the last 20 years? Cancer
2011;117:2505-12. Crossref
3. Leksell L. The stereotaxic method and
radiosurgery of the brain. Acta Chir Scand 1951;102:316-9.
4. Lo SS. Stereotactic radiosurgery.
Available from: http://emedicine.medscape.com/article/1423298-overview#a2.
Accessed 19 Mar 2015.
5. Minniti G, Scaringi C, Clarke E,
Valeriani M, Osti M, Enrici RM. Frameless linac-based stereotactic
radiosurgery (SRS) for brain metastases: analysis of patient repositioning
using a mask fixation system and clinical outcomes. Radiat Oncol
2011;6:158. Crossref
6. Ramakrishna N, Rosca F, Friesen S,
Tezcanli E, Zygmanszki P, Hacker F. A clinical comparison of patient setup
and intra-fraction motion using frame-based radiosurgery versus a
frameless image-guided radiosurgery system for intracranial lesions.
Radiother Oncol 2010;95:109-15. Crossref
7. ASTRO Policies for Stereotactic
radiosurgery (SRS). Available from: https://www.astro.org/uploadedFiles/
Main_Site/Practice_Management/Reimbursement/ASTROSRSModelPolicy.pdf.
Accessed 2014.
8. Shaw E, Scott C, Souhami L, et al.
Single dose radiosurgical treatment of recurrent previously irradiated
primary brain tumors and brain metastases: final report of RTOG protocol
90-05. Int J Radiat Oncol Biol Phys 2000;47:291-8. Crossref
9. Sneed PK, Lamborn KR, Forstner JM, et
al. Radiosurgery for brain metastases: is whole brain radiotherapy
necessary? Int J Radiat Oncol Biol Phys 1999;43:549-58. Crossref
10. Pirzkall A, Debus J, Lohr F, et al.
Radiosurgery alone or in combination with whole-brain radiotherapy for
brain metastases. J Clin Oncol 1998;16:3563-9. Crossref
11. Andrews DW, Scott CB, Sperduto PW, et
al. Whole brain radiation therapy with or without stereotactic
radiosurgery boost for patients with one to three brain metastases: Phase
III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. Crossref
12. Cho KH, Hall WA, Gerbi BJ, Higgins PD,
Bohen M, Clark HB. Patient selection criteria for the treatment of brain
metastases with stereotactic radiosurgery. J Neurooncol 1998;40:73-86. Crossref
13. Varlotto JM, Flickinger JC, Niranjan
A, Bhatnagar AK, Kondziolka D, Lunsford LD. Analysis of tumor control and
toxicity in patients who have survived at least one year after
radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys
2003;57:452-64. Crossref
14. Bhatnagar AK, Flickinger JC,
Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more
intracranial metastases. Int J Radiat Oncol Biol Phys 2006;64:898-903. Crossref
15. Nieder C, Grosu AL, Gaspar LE.
Stereotactic radiosurgery (SRS) for brain metastases: a systematic review.
Radiat Oncol 2014;9:155. Crossref
16. Pham NL, Reddy PV, Murphy JD, et al.
Frameless, real-time, surface imaging-guided radiosurgery: update on
clinical outcomes for brain metastases. Transl Cancer Res 2014;3:351-7.
17. Breneman JC, Steinmetz R, Smith A,
Lamba M, Warnick RE. Frameless image-guided intracranial stereotactic
radiosurgery: clinical outcomes for brain metastases. Int J Radiat Oncol
Biol Phys 2009;74:702-6. Crossref
18. Muacevic A, Kufeld M, Wowra B, Kreth
FW, Tonn JC. Feasibility, safety, and outcome of frameless image-guided
robotic radiosurgery for brain metastases. J Neurooncol 2010;97:267-74. Crossref
19. Gaspar L, Scott C, Rotman M, et al.
Recursive partitioning analysis (RPA) of prognostic factors in three
Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J
Radiat Oncol Biol Phys 1997;37:745-51. Crossref
20. Lorenzoni J, Devriendt D, Massager N,
et al. Radiosurgery for treatment of brain metastases: estimation of
patient eligibility using three stratification systems. Int J Radiat Oncol
Biol Phys 2004;60:218-24. Crossref
21. Weltman E, Salvajoli JV, Brandt RA, et
al. Radiosurgery for brain metastases: a score index for predicting
prognosis. Int J Radiat Oncol Biol Phys 2000;46:1155-61. Crossref
22. Sperduto PW, Berkey B, Gaspar LE,
Mehta M, Curran W. A new prognostic index and comparison to three other
indices for patients with brain metastases: an analysis of 1,960 patients
in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510-4. Crossref
23. Sperduto PW, Kased N, Roberge D, et
al. Summary report on the graded prognostic assessment: an accurate and
facile diagnosis-specific tool to estimate survival for patients with
brain metastases. J Clin Oncol 2012;30:419-25. Crossref
24. Mulvenna PM, Nankivell MG, Barton R,
et al. Whole brain radiotherapy for brain metastases from non-small lung
cancer: Quality of life (QoL) and overall survival (OS) results from the
UK Medical Research Council QUARTZ randomised clinical trial (ISRCTN
3826061). J Clin Oncol 2015;33(Suppl);abstract8005.
25. Mok TS, Wu YL, Thongprasert S, et al.
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J
Med 2009;361:947-57. Crossref
26. Han JY, Park K, Kim SW, et al.
First-SIGNAL: first-line single-agent iressa versus gemcitabine and
cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin
Oncol 2012;30:1122-8. Crossref
27. Mitsudomi T, Morita S, Yatabe Y, et
al. Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal growth
factor receptor (WJTOG3405): an open label, randomised phase 3 trial.
Lancet Oncol 2010;11:121-8. Crossref
28. Maemondo M, Inoue A, Kobayashi K, et
al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated
EGFR. N Engl J Med 2010;362:2380-8. Crossref
29. Jänne PA, Wang X, Socinski MA, et al.
Randomized phase II trial of erlotinib alone or with carboplatin and
paclitaxel in patients who were never or light former smokers with
advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol
2012;30:2063-9. Crossref
30. Rosell R, Carcereny E, Gervais R, et
al. Erlotinib versus standard chemotherapy as first-line treatment for
European patients with advanced EGFR mutation–positive
non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised
phase 3 trial. Lancet Oncol 2012;13:239-46. Crossref
31. Zhou C, Wu YL, Chen G, et al.
Erlotinib versus chemotherapy as first-line treatment for patients with
advanced EGFR mutation–positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet
Oncol 2011;12:735-42. Crossref
32. Yang JC, Wu YL, Schuler M, et al.
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation–positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
analysis of overall survival data from two randomised, phase 3 trials.
Lancet Oncol 2015;16:141-51. Crossref
33. Magnuson WJ, Lester-Coll NH, Wu AJ, et
al. Management of brain metastases in tyrosine kinase inhibitor-naïve
epidermal growth factor receptor-mutant non-small-cell lung cancer: a
retrospective multi-institutional analysis. J Clin Oncol 2017;35:1070-7. Crossref
34. Mehta MP, Tsao MN, Whelan TJ, et al.
The American Society for Therapeutic Radiology and Oncology (ASTRO)
evidence-based review of the role of radiosurgery for brain metastases.
Int J Radiat Oncol Biol Phys 2005;63:37-46. Crossref
35. Patil CG, Pricola K, Sarmiento JM,
Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone
versus WBRT and radiosurgery for the treatment of brain metastases.
Cochrane Database Syst Rev 2012;(9):CD006121. Crossref
36. Sahgal A, Aoyama H, Kocher M, et al.
Phase 3 trials of stereotactic radiosurgery with or without whole-brain
radiation therapy for 1 to 4 brain metastases: individual patient data
meta-analysis. Int J Radiat Oncol Biol Phys 2015;91:710-7. Crossref