Hong
Kong Med J 2017;23(6):586–93 | Epub 10 Nov 2017
DOI: 10.12809/hkmj176841
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Moderate iodine deficiency among pregnant women in Hong
Kong: revisit the problem after two decades
WH Tam, MD, FRCOG1; Ruth SM Chan, PhD2;
Michael HM Chan, FRCPA, FHKCPath3; LY Yuen, BSc1;
Liz Li, MSc2; Mandy MM Sea, PhD2; Jean Woo, MD, FRCP2
1 Department of Obstetrics and
Gynaecology, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Medicine and
Therapeutics, The Chinese University of Hong Kong, Shatin, Hong Kong
3 Department of Chemical Pathology,
The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Dr WH Tam (tamwh@cuhk.edu.hk)
Abstract
Introduction: A survey conducted
during 2005 to 2007 by the Centre for Food Safety in Hong Kong suggested
that only 5% of the local population had a sufficient dietary intake of
iodine. The study, however, was limited as biochemical data (ie urinary
iodine concentration) were lacking. Pregnant women are vulnerable to
iodine deficiency because of their increased requirement. Recent studies
have shown that iodine deficiency in early pregnancy is associated with
poorer cognitive development in early childhood. This study reports the
iodine status of women during early gestation at an obstetric unit in
Hong Kong.
Methods: Healthy pregnant women
with no history of hyperemesis gravidarum were enrolled into a study
when they first made a booking in an antenatal clinic of a public
hospital to investigate their iodine status during early pregnancy. All
subjects were asked to collect their morning urine for measurement of
iodine and creatinine levels. Daily dietary intake of iodine was
assessed in a subgroup of participants by structured interview using a
standard food frequency questionnaire.
Results: A total of 600 pregnant
women were enrolled at a median of 7.0 weeks of gestation. The median
urinary iodine concentration and urinary iodine-to-creatinine ratio were
100 µg/L and 98 µg/g, respectively; 429 (71.5%) participants had iodine
insufficiency according to the World Health Organization classification.
Daily dietary intake of iodine was assessed in 146 participants. The
median daily intake of iodine was 69.5 µg and 122 (83.6%) participants
had an intake below the 250 µg recommended during pregnancy by the World
Health Organization.
Conclusions: Local pregnant
women continue to have an inadequate dietary intake of iodine and remain
iodine-deficient.
New knowledge added by this study
- This study confirmed the previous observation that dietary intake of iodine is inadequate in our local population, in particular in women during pregnancy.
- The majority of the pregnant women were taking multivitamin supplements without iodine, suggesting inadequate knowledge about the importance of iodine.
- There is an urgent need to educate the general public, in particular women of childbearing age, about the importance of an adequate dietary intake of iodine and the option of iodine-containing multivitamin supplements before contemplating pregnancy.
- We can consider a policy to monitor the iodine status of the population, especially in vulnerable groups including pregnant and lactating mothers, and their children.
- World Health Organization recommends salt iodisation in countries and areas that are iodine-deficient. As less than 15% of table salt available in Hong Kong is iodised, it may be appropriate to consider this policy.
Introduction
The World Health Organization (WHO) considers
iodine deficiency the single most important preventable cause of brain
damage worldwide. Since 1993, WHO has recommended universal salt
iodisation to eliminate iodine deficiency disorder.1 There is a common belief that the Hong Kong population
residents in coastal regions should have sufficient iodine intake. Twenty
years ago, however, 50% of children and adults were found iodine-deficient
according to the WHO standards.2 At
the same time, studies also reported that one third of local pregnant
women were iodine-deficient based on their urinary iodine concentration
(UIC), from the first through third trimester.3
4 Pregnant and lactating women are
among the most vulnerable groups in the population as iodine plays an
important role in early neuronal migration and maturation in the
developing fetus and infants.
A local expert panel group was established in 2003
to encourage the monitoring of iodine status and rectify the problem.5 The panel concluded that dietary iodine intake of our
population was borderline sufficient, and was also inadequate to meet any
extra requirement at the time of thyroid stress, ie pregnancy, neonatal
period, and in the first few years of life.5
In a local population survey conducted by the Centre for Food Safety
during 2005 to 2007, the median iodine dietary intake was as low as 44
µg/day among 5000 adults, while 60% had an intake of <50 µg/day (the
threshold for normal thyroid functioning).6
Only 5% had an iodine intake within the safe range. This report, however,
also admitted a lack of clinical and biochemical data (ie UIC) that
precluded a conclusion about iodine deficiency. Since this report in 2011,
all mothers are enquired about their dietary intake of iodine and provided
with iodine-containing multivitamin supplements when they book in at a
Maternity and Child Health Centre in Hong Kong.
Nevertheless, the problem remains unresolved as two
recent cohort studies in the UK and Australia consistently reported that
low maternal first-trimester UIC was associated with poorer childhood
cognitive development at the age of 8 to 9 years.7
8 The investigators of the Avon
Longitudinal Study of Parents and Children in the UK also reported a
dose-effect association; child neurocognitive scores were lower when
maternal urinary iodine-to-creatinine ratio (UICr) was <50 µg/g.7
As no salt iodisation programme has been
implemented since the first study was carried out, and with the changes in
social circumstances and dietary trends over the last two decades, it was
deemed necessary and important to reassess the iodine status of pregnant
and lactating women in Hong Kong to guide future policy. The objective of
this paper was to report the iodine status of women during early gestation
at an obstetric unit in Hong Kong.
Methods
Between July 2014 and November 2015, healthy
pregnant women were enrolled at the antenatal clinic of Prince of Wales
Hospital into a study of gestational age-specific thyroid function test
reference intervals. Women without a history of thyroid dysfunction,
hyperemesis gravidarum, autoimmune disease, or any other major medical
conditions were eligible. Consecutive cases were recruited at their first
attendance for antenatal booking. We estimated that 250 blood samples were
required for each block of gestational age range (<6, 6-10, >10-14,
>14-18, >18-24, >24-32, >32-38, and >38 weeks) to generate
a nomogram; 600 subjects were required to provide 2000 samples, each had
an average of four blood samples across the gestation, with an estimated
dropout rate of 15%. All subjects underwent early ultrasound scan for
dating with gestational age adjusted according to the ultrasound date if
it differed to that calculated from the last menstrual period. The study
was approved by the Chinese University of Hong Kong Clinical Research
Ethics Committee (CRE-2013.500), and written informed consent was obtained
from all women.
As part of the study design, UIC was measured at
the time of recruitment to determine the participant’s iodine status.
Women were asked to collect a morning urine sample into an acid-washed
trace element urine bottle within 1 week of recruitment for the assay. In
this study, UIC was measured by an inductively coupled plasma mass
spectrometer (ICP-MS 7700; Agilent Technologies, US). The intra- and
inter-assay coefficients of variation for UIC were 3.3% and 7.4% at 11.9
µg/L, and 2.7% and 4.9 % at 49.6 µg/L, respectively. Urinary creatinine
concentration was also measured by IDMS-traceable Jaffe kinetic reaction
(cobas 8000; Roche Diagnostics Inc, US) to calculate the UICr.
All participants were interviewed by a research
nurse who asked about the duration, frequency, and brand of any
multivitamin supplements taken to quantify daily iodine supplementation.
Due to the limited funding, dietary intake of iodine-containing foods was
only assessed in a conveniently sampled subgroup of the population by
another research assistant using a food frequency questionnaire (FFQ; Appendix).
Education level and occupation were recorded for this subgroup. The FFQ
was based on the literature and previously validated FFQs that have been
used in the local population.6 9 10
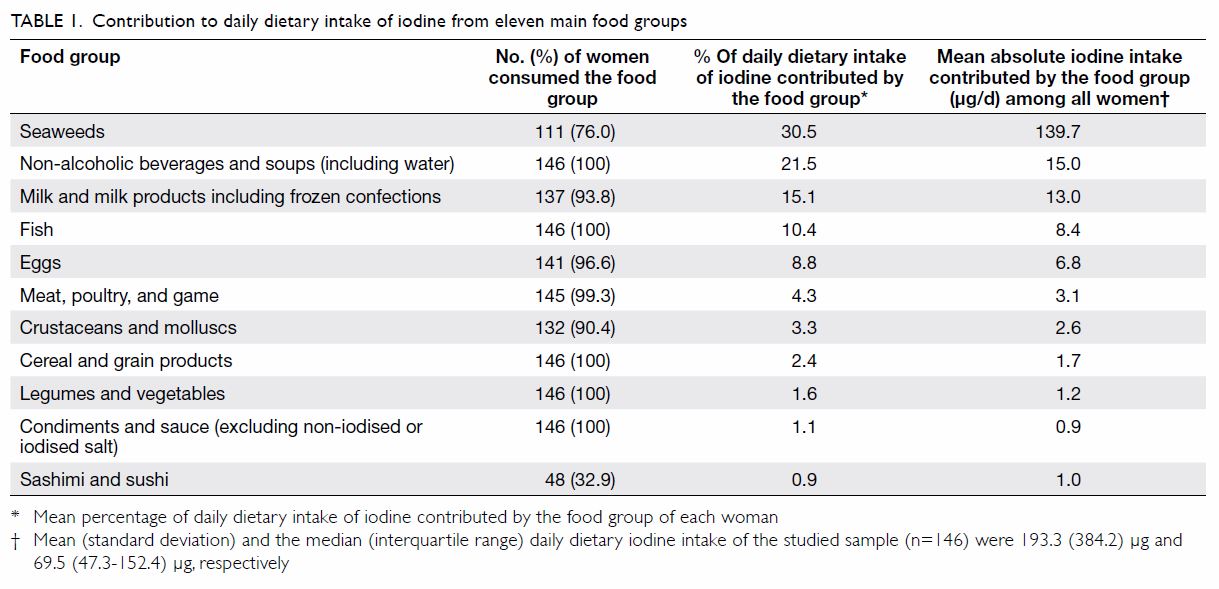
Eleven main food groups that contribute to iodine intake in the local
population were incorporated into the FFQ (Table 1). Portions were reported based on standard
reference sizes, eg cups, grams, centilitres. To facilitate understanding
and recall, food photo albums and eating utensils of standard portions
were presented when completing the FFQ. Use of iodised salt was also
documented. Daily dietary intake of iodine was calculated using Food
Processor Nutrition Analysis and Fitness software, version 8.0 (ESHA
Research, Salem, US). The iodine content of local foods was also
programmed into the software, extracted from food composition tables from
Hong Kong SAR and Mainland China.11
We used the WHO definition to categorise insufficient, adequate, above
requirements, and excessive by UIC of <150, 150-249, 250-499, and ≥500
µg/L, respectively12; UICr of
<150 µg/g was also used to define insufficiency according to Bath et
al.7 An average daily iodine intake
of 250 µg/day was considered adequate for pregnancy.1 Data are expressed as mean ± standard deviation (SD),
median and interquartile range (IQR), or counts with proportion.
Between-group differences were compared using the Student’s t test
and χ2/Fisher’s exact tests, as appropriate. Multivariate
logistic regression analyses were used to obtain adjusted odds ratios with
95% confidence intervals, with the forced entry of covariates, namely
maternal age, parity, education level, occupation, body mass index, and
gestational age at recruitment, to assess factors associated with low UIC
(<150 µg/L), low UICr (<150 µg/g) and iodine-containing
supplementation. Multivariate linear regression analyses were used to
assess the association with UIC and UICr. Statistical analysis was
performed using SPSS (Windows version 20.0; IBM Corp, Armonk [NY], US). A
P value of <0.05 was used to indicate significance for two-tailed
statistical test results. The gestational age-specific thyroid function
test reference intervals are not included in this article but will be
published elsewhere.
Results
A total of 600 healthy pregnant women were enrolled
during the study period at a median gestational age of 7.0 (IQR, 5.9-8.6)
weeks. The mean (± SD) age and body mass index at recruitment were 31.3 ±
3.9 years and 21.9 ± 3.1 kg/m2, respectively. Of the subjects,
382 (63.7%) women were nulliparous and two (0.3%) were twin pregnancies.
The median (IQR) UIC and UICr were 100 (58-165) µg/L and 98 (67-150) µg/g,
respectively (Table 2). According to the WHO definition, 429
(71.5%) participants were regarded as iodine deficient (Fig
a). Moreover, 450 (75.0%) participants had UICr of <150 µg/g (Fig b).7
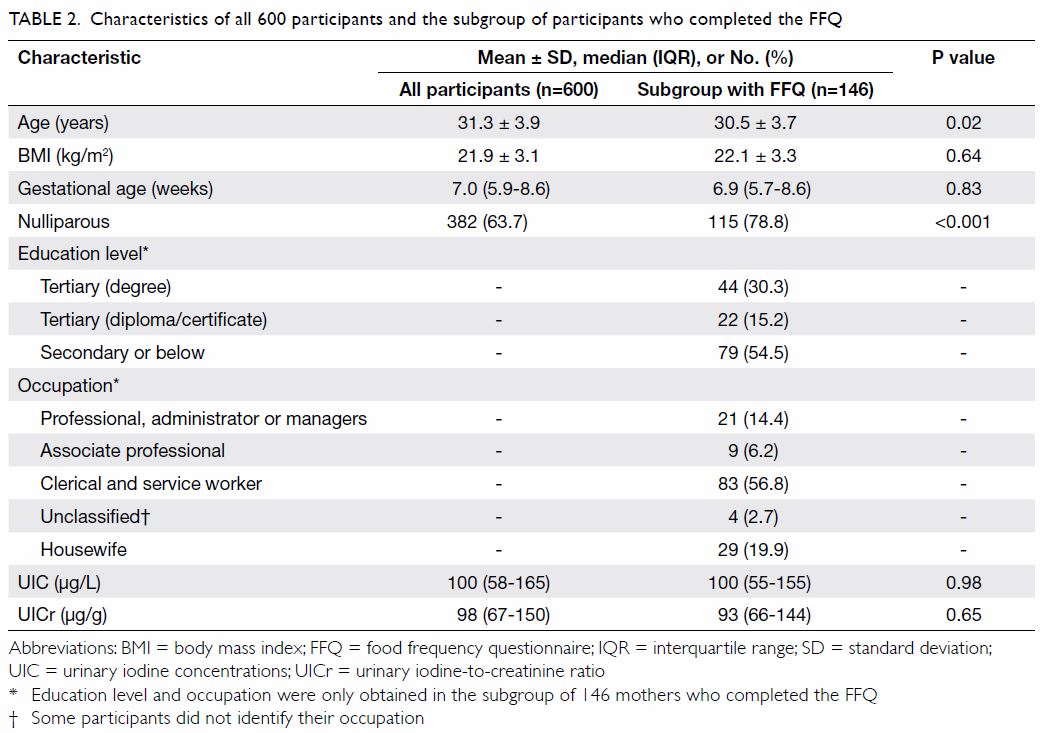
Table 2. Characteristics of all 600 participants and the subgroup of participants who completed the FFQ
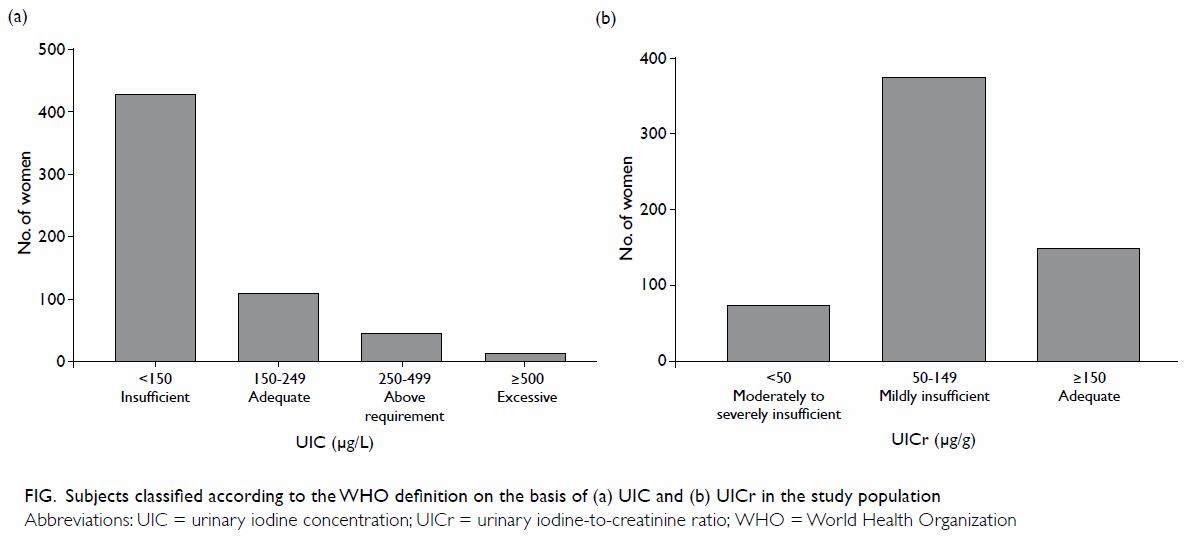
Figure. Subjects classified according to the WHO definition on the basis of (a) UIC, and (b) UICr in the study population
Daily dietary intake of iodine was assessed in 146
participants. They were slightly younger in age and had a higher rate of
nulliparity; their UIC and UICr were no different to all participants (Table 2). The median (IQR) daily dietary intake of
iodine was 69.5 (47.3-152.4) µg. The greatest source of iodine among the
studied sample was from seaweeds that were consumed by 76% of women (Table
1). The next most common source of iodine was non-alcoholic
beverages such as soy milk, soup, soft drinks, and water (21.5% of the
daily iodine intake). Of all the 146 women surveyed, only four (2.7%)
regularly used iodised salt in their diet. Several food sources were
commonly eaten by all women including fish, cereal and grain products,
vegetables and legumes, and condiments. Nonetheless these combined food
sources only contributed to 15.5% of daily dietary iodine intake. There
were 44 (30.1%), 92 (63.0%) and 122 (83.6%) participants who had a daily
iodine intake below 50, 100, and 250 µg respectively.
Nutritional supplementation was reported by 414
(69.0%) of all participants at the time of enrolment but only 163 (39.4%)
of these supplements contained iodine; the daily iodine supplement was
between 140 and 290 µg if taken according to the prescription. The
subgroup of participants who had taken iodine-containing supplements for 2
weeks or more had an adequate iodine status both by their median UIC and
UICr (Table 3). In 122 subjects who completed the FFQ and
had urinary iodine level measured within 1 week of each other, total daily
iodine intake (iodine supplementation included) had a weak correlation
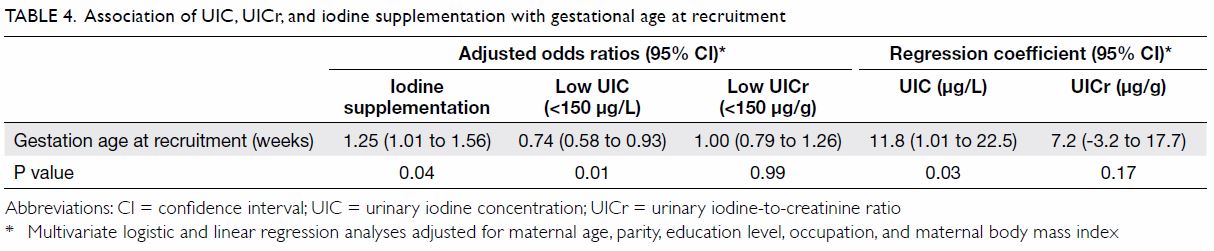
with UICr (r=0.20, P=0.03). In multivariate regression analyses, no
covariates except gestational age at recruitment were associated with low
UIC or UICr, or iodine supplementation; women recruited at a later
gestational age had significantly higher UIC and were more likely to have
started taking an iodine-containing supplement (Table 4).
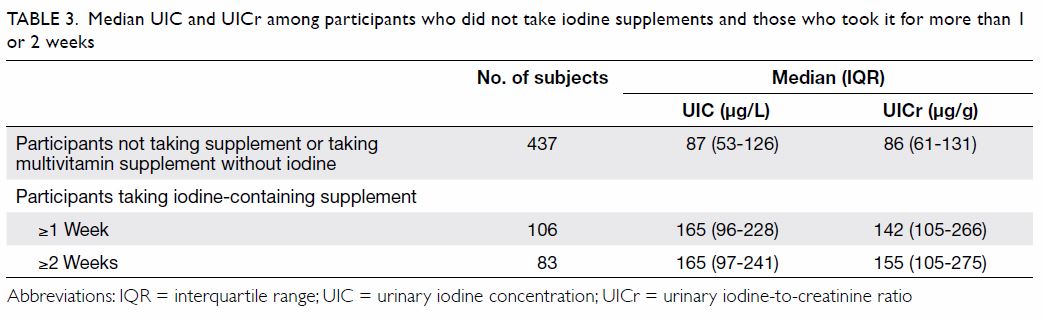
Table 3. Median UIC and UICr among participants who did not take iodine supplements and those who took it for more than 1 or 2 weeks
Discussion
The findings from this study suggest that local
pregnant women might still be iodine-deficient when the WHO definition is
applied. In the subgroup of participants who completed the FFQ, more than
80% did not have an adequate daily iodine intake as recommended by the WHO
and other authorities.13 14 This is in keeping with the survey conducted by the
Centre for Food Safety 10 years ago. A recent local study of 95 lactating
mothers showed that the mean daily dietary intake of iodine was 62.6 µg
with only 2% having a sufficient iodine intake.15
The result also showed that only 48% of breast milk samples from 39 women
with an infant younger than 6 months had an adequate level of iodine (85
µg per day) as recommended by the Chinese Dietary Reference Intake.15 The revised guideline from the Department of Health
in 2016 suggested that mothers consider taking iodine-containing prenatal
supplements during pregnancy and breastfeeding in view of the difficulties
in obtaining sufficient iodine from food alone.16
The policy appears to be in agreement with our findings that mothers
taking iodine-containing supplements were iodine sufficient according to
their median UIC. Nonetheless universal iodine supplementation remains
controversial. The American Thyroid Association, Endocrine Society, and
New Zealand Ministry of Health recommend that women who are planning a
pregnancy or who are currently pregnant or lactating should receive 150 µg
per day of iodine supplementation in the form of potassium
iodide-containing supplements.17 18 On the contrary, Cochrane
systematic review did not support routine iodine supplementation in women
before, during or after pregnancy, while WHO does not recommend iodine
supplementation in regions where the median UIC indicates iodine
sufficiency, or where a salt iodisation programme is in place.1 19 All eight
iodine-containing supplements that were reportedly taken by participants
in this study also contained iron, and frequently resulted in varying
degrees of constipation. Most women did not start taking supplements prior
to their first antenatal visit. Even in those who did, 60% of the
supplements contained no iodine, similar to that reported in the US.20 21 Our
results also show that mothers were more likely to have commenced iodine
supplementation and have an iodine adequate status at a later gestation;
this suggests mothers might acquire the knowledge as pregnancy progresses.
Although the UIC is designated for assessment of a
population, not individual iodine status, our results suggest that three
quarters of the participants may have been iodine insufficient during
early pregnancy according to their UIC and UICr. Similarly, iodine
deficiency in pregnancy has re-emerged in several developed countries.22 Subjects enrolled into the Avon study in the UK were
mildly-to-moderately iodine deficient with a median UIC of 91 µg/L.7 A more recent study among schoolgirls in the UK also
reported a similarly low UIC suggesting that mild-to-moderate iodine
deficiency remains a problem.23
Such deficiency was probably caused by a poor availability of iodised
salt, few UK recommendations for increased iodine intake in pregnancy, and
insufficient use of iodine-containing prenatal supplements. The
International Council for Control of Iodine Deficiency Disorders global
network now places the UK on the list of mildly deficient nations.24 The Department of Health in the UK has also added
iodine to its National Diet and Nutrition Survey that checks the nutrient
intake of adults and children in the UK.25
In Australia, mandatory iodine fortification of salt used in bread was
introduced in 2009 in order to tackle mild iodine deficiency in the
population; iodine status of women has improved since then but only those
taking iodine-containing supplements have UIC indicative of sufficiency.26 According to data from the
National Health and Nutrition Examination Survey 2005-2010, more than 55%
of pregnant women had UIC that suggested inadequate iodine intake.27 Mainland China has led the way in sustaining the
elimination of iodine deficiency through iodised salt since the early
1990s.28 Nonetheless dietary
iodine intake remains insufficient among pregnant women in Shanghai,
Zhejiang, and other coastal cities where iodine is considered sufficient
for the general population.29 30 A national survey from the
Mainland reported that the median UIC levels of pregnant and lactating
women were 123-224 µg/L and 109-224 µg/L, respectively; median UIC was
higher among those in inland cities, because of a higher iodine level in
the salt and greater household coverage.31
In contrast, the progress in tackling iodine insufficiency in Hong Kong
has been far from satisfactory.
Due to the limitations in study design, this study
can only provide a snapshot overview of the iodine status of local
pregnant women during early pregnancy from a single obstetric unit. Given
that the main objective of the study was to ensure our study group had
sufficient iodine intake in order to establish a thyroid function test
reference range for the local population, our results cannot draw any
conclusions about iodine status during the second and third trimesters.
Moreover, dietary iodine intake could only be assessed in a subgroup of
the population due to limited funding. The WHO has considered iodine
deficiency the single most important preventable cause of brain damage
worldwide. Recent study also highlighted the impact of iodine deficiency
in the first 1000 days of life especially among breast-fed infants.32 As the Hong Kong SAR Government is actively promoting
breastfeeding, it is also important to ensure adequate iodine in lactating
mothers and breast-fed infants. It is time to systematically revisit the
iodine status of our local women at pre-conception, and during pregnancy
and lactation.
Conclusions
Results of our study are in line with those from
the survey by the Centre for Food Safety, suggesting that our local
pregnant women are borderline iodine-deficient and have an inadequate
dietary iodine intake during early pregnancy. There is a need to educate
the public and to advise women of childbearing age to maintain sufficient
dietary iodine before contemplating pregnancy. A policy of salt iodisation
and regular monitoring of iodine status with UIC in our population should
be considered.
Acknowledgements
This study was supported by the Hospital Authority
of Hong Kong on a project to derive a gestational age-specific thyroid
function reference interval for the local pregnant population. Ms Sharon
Lai-kwai Chan, research nurse, recruited all subjects, and collected and
organised the clinical data. Ms Macy Kwan conducted face-to-face
interviews for the dietary questionnaire for the subgroup of subjects.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. World Health Organization; United
Nations Children's Fund; International Council for the Control of Iodine
Deficiency Disorders. Assessment of iodine deficiency disorders and
monitoring their elimination: A guide for programme managers. 3rd ed.
Available from:
http://apps.who.int/iris/bitstream/10665/43781/1/9789241595827_eng.pdf.
Accessed 22 Mar 2017.
2. Kung AW, Chan LW, Low LC, Robinson JD.
Existence of iodine deficiency in Hong Kong—a coastal city in southern
China. Eur J Clin Nutr 1996;50:569-72.
3. Kung AW, Lao TT, Low LC, Pang RW,
Robinson JD. Iodine insufficiency and neonatal hyperthyrotropinaemia in
Hong Kong. Clin Endocrinol (Oxf) 1997;46:315-9. Crossref
4. Kung AW, Lao TT, Chau MT, Tam SC, Low
LC. Goitrogenesis during pregnancy and neonatal hypothyroxinaemia in a
borderline iodine sufficient area. Clin Endocrinol (Oxf) 2000;53:725-31. Crossref
5. But B, Chan CW, Chan F, et al. Consensus
statement on iodine deficiency disorders in Hong Kong. Hong Kong Med J
2003;9:446-53.
6. Centre for Food Safety, Hong Kong SAR
Government. Risk Assessment Studies Report No. 45: Dietary iodine intake
in Hong Kong adults. Jul 2011. Available from:
http://www.cfs.gov.hk/english/programme/programme_rafs/programme_rafs_n_01_12_Dietary_Iodine_Intake_HK.html.
Accessed 20 Apr 2015.
7. Bath SC, Steer CD, Golding J, Emmett P,
Rayman MP. Effect of inadequate iodine status in UK pregnant women on
cognitive outcomes in their children: results from the Avon Longitudinal
Study of Parents and Children (ALSPAC). Lancet 2013;382:331-7. Crossref
8. Hynes KL, Otahal P, Hay I, Burgess JR.
Mild iodine deficiency during pregnancy is associated with reduced
educational outcomes in the offspring: 9-year follow-up of the gestational
iodine cohort. J Clin Endocrinol Metab 2013;98:1954-62. Crossref
9. Combet E, Lean ME. Validation of a short
food frequency questionnaire specific for iodine in U.K. females of
childbearing age. J Hum Nutr Diet 2014;27:599-605. Crossref
10. Condo D, Makrides M, Skeaff S, Zhou
SJ. Development and validation of an iodine-specific FFQ to estimate
iodine intake in Australian pregnant women. Br J Nutr
2015;113:944-52. Crossref
11. 楊月欣. 食物營養成分速查 [in Chinese]. 人民日報出版社;
2006.
12. World Health Organization. Urinary
iodine concentrations for determining iodine status in populations. 2013.
Available from: http://www.who.int/vmnis/indicators/urinaryiodine/en/.
Accessed 22 Apr 2015.
13. Institute of Medicine (US) Panel on
Micronutrients. Dietary reference intakes for vitamin A, vitamin K,
arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum,
nickel, silicon, vanadium, and zinc. Washington, DC: National Academies
Press; 2001. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK222310/. Accessed 22 Mar 2017.
14. American Thyroid Association. Iodine
deficiency. 4 Jun 2012. Available from:
http://www.thyroid.org/iodine-deficiency/. Accessed 25 Mar 2015.
15. PolyU discovers inadequate calcium,
iron and iodine intakes of Hong Kong lactating women. 26 Jul 2016.
Available from:
https://www.polyu.edu.hk/web/en/media/media_releases/index_id_6237.html.
Accessed 25 Jun 2017.
16. Family Health Service, Department of
Health, Hong Kong SAR Government. Healthy eating during pregnancy and
breastfeeding. Revised Nov 2016. Available from:
http://www.fhs.gov.hk/english/health_info/woman/20036.html. Accessed 21
Jun 2017.
17. Alexander EK, Pearce EN, Brent GA, et
al. 2017 Guidelines of the American Thyroid Association for the Diagnosis
and Management of Thyroid Disease During Pregnancy and the Postpartum.
Thyroid 2017;27:315-89. Crossref
18. Ministry for Primary Industries;
Ministry of Health, New Zealand. Mandatory iodine fortification in New
Zealand: Supplement to the Australian Institute of Health and Welfare 2016
report—Monitoring the health impacts of mandatory folic acid and iodine
fortification. MPI Technical—Paper No: 2016/32. Available from:
https://www.mpi.govt.nz/dmsdocument/12786-mandatory-iodine-fortification-in-new-zealand-supplement-to-the-australian-institute-of-health-and-welfare-2016-report-monitoring-the-health-impacts-of-mandatory-folic-acid-and-iodine-fortification.
Accessed 22 Mar 2017.
19. Harding KB, Peña-Rosas JP, Webster AC,
et al. Iodine supplementation for women during the preconception,
pregnancy and postpartum period. Cochrane Database Syst Rev
2017;(3):CD011761. Crossref
20. Gregory CO, Serdula MK, Sullivan KM.
Use of supplements with and without iodine in women of childbearing age in
the United States. Thyroid 2009;19:1019-20. Crossref
21. Leung AM, Pearce EN, Braverman LE.
Iodine content of prenatal multivitamins in the United States. N Engl J
Med 2009;360:939-40. Crossref
22. Li M, Eastman CJ. The changing
epidemiology of iodine deficiency. Nat Rev Endocrinol 2012;8:434-40. Crossref
23. Vanderpump MP, Lazarus JH, Smyth PP,
et al. Iodine status of UK schoolgirls: a cross-sectional survey. Lancet
2011;377:2007-12. Crossref
24. Iodine global network. Global
Scorecard 2014: Number of iodine deficient countries more than halved in
past decade. IDD Newsletter 2015;Feb:5-7. Available from:
http://www.ign.org/cm_data/IDD_feb15_mail.pdf. Accessed 25 Mar 2015.
25. Whitton C, Nicholson SK, Roberts C, et
al. National Diet and Nutrition Survey: UK food consumption and nutrient
intakes from the first year of the rolling programme and comparisons with
previous surveys. Br J Nutr 2011;106:1899-914. Crossref
26. Charlton KE, Yeatman H, Brock E, et
al. Improvement in iodine status of pregnant Australian women 3 years
after introduction of a mandatory iodine fortification programme. Prev Med
2013;57:26-30. Crossref
27. Caldwell KL, Pan Y, Mortensen ME,
Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the
National Children's Study and in U.S. women (15-44 years), National Health
and Nutrition Examination Survey 2005-2010. Thyroid 2013;23:927-37. Crossref
28. Sun D, Codling K, Chang S, et al.
Eliminating iodine deficiency in China: achievements, challenges and
global implications. Nutrients 2017;9.pii:E361. Crossref
29. Zou S, Wu F, Guo C, et al. Iodine
nutrition and the prevalence of thyroid disease after salt iodization: a
cross-sectional survey in Shanghai, a coastal area in China. PLoS One
2012;7:e40718. Crossref
30. Wang Z, Zhu W, Mo Z, et al. An
increase in consuming adequately iodized salt may not be enough to rectify
iodine deficiency in pregnancy in an iodine-sufficient area of China. Int
J Environ Res Public Health 2017;14:piiE206.
31. National Food Safety and Risk
Assessment Expert Committee. Salt iodization and risk assessment of iodine
status in Chinese population [in Chinese]. Technical report No. 2010-002.
May 2010. Available from:
http://www.nhfpc.gov.cn/cmsresources/mohwsjdj/cmsrsdocument/doc9250.pdf.
Accessed 21 Jun 2017.
32. Stinca S, Andersson M, Herter-Aeberli
I, et al. Moderate-to-severe iodine deficiency in the “first 1000 days”
causes more thyroid hypofunction in infants than in pregnant or lactating
women. J Nutr 2017;147:589-95. Crossref