Hong Kong Med J 2017 Oct;23(5):446–53 | Epub 1 Sep 2017
DOI: 10.12809/hkmj176229
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Immunoglobulin G4–related disease in Hong
Kong: clinical features, treatment practices, and
its association with multisystem disease
Philip H Li, MRes (Med), MRCP (UK)1;
KL Ko, MB, BS, MRCP (UK)2;
Carmen TK Ho, FHKCP, FHKAM (Medicine)1;
Leah L Lau, MB, BS3;
Raymond KY Tsang, MS, FRCSEd(ORL)4;
TT Cheung, MS, FRCS (Edin)5;
WK Leung, MD, FRCP (Edin, Lond)2;
CS Lau, MD, FRCP (Edin, Lond, Glasg)1
1 Division of Rheumatology & Clinical Immunology, Department of Medicine, Queen Mary Hospital, Pokfulam, Hong Kong
2 Division of Gastroenterology & Hepatology, Department of Medicine, Queen Mary Hospital, Pokfulam, Hong Kong
3 Department of Ear, Nose & Throat, Queen Mary Hospital, Pokfulam, Hong Kong
4 Division of Otorhinolaryngology – Head and Neck Surgery, Department of Surgery, Queen Mary Hospital, Pokfulam, Hong Kong
5 Division of Hepatobiliary & Pancreatic Surgery and Liver Transplantation, Department of Surgery, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr Philip H Li (philipli@connect.hku.hk)
This paper was presented as a poster at the EULAR Annual European
Congress of Rheumatology held in Madrid, Spain during 14-17 June 2017.
Abstract
Introduction: Immunoglobulin G4–related disease
remains an under-recognised and evolving disease.
Local data are sparse and previous publications have
been limited to individual case reports or case series
only. We conducted this study to review the clinical
features, treatment practices, and factors associated
with multisystem involvement in Hong Kong.
We described the clinical features and treatment
modalities of the largest cohort of immunoglobulin
G4–related disease in our locality thus far.
Methods: We retrospectively evaluated all patients
with immunoglobulin G4–related disease between
January 2003 and December 2015 in Queen Mary
Hospital and combined this with patient data
extracted from previous local publications. We
analysed the clinical features, treatment practices,
and factors associated with the number of organ
systems involved.
Results: A total of 104 patients (55 from Queen
Mary Hospital and 49 from literature review) were
identified. Patients were predominantly older men
(mean [standard deviation] age, 61.9 [12.7] years;
male-to-female ratio=3:1) and 94.4% had elevated
pre-treatment serum immunoglobulin G4 levels.
Hepatobiliary and pancreatic system (40.4%),
salivary gland (33.7%), lymph node (29.8%), and
eye (19.2%) were the most common organ systems
involved. Lymphadenopathy was associated with
glucocorticoid use (odds ratio=2.65; 95% confidence
interval, 1.08-6.54; P=0.034). Pre-treatment
serum immunoglobulin G4 levels correlated with
the number of organ systems involved (β=0.347;
P=0.004) and, specifically, more associated with
patients having salivary gland involvement than
those without (mean, 1109 mg/dL vs 599 mg/dL;
P=0.012).
Conclusion: We identified pre-treatment serum
immunoglobulin G4 to be associated with
multisystem disease, especially with salivary gland
involvement, highlighting its potential for disease
prognostication and monitoring. Increased physician
awareness and multidisciplinary efforts are required
for early diagnosis and optimal management of this
masquerading disease.
New knowledge added by this study
- Hepatobiliary and pancreatic system, salivary gland, lymph node, and eye were the most common organ systems involved in immunoglobulin (Ig) G4–related disease in Hong Kong.
- Pre-treatment serum IgG4 levels were associated with salivary gland involvement and multisystem disease.
- Glucocorticoids were most frequently used, but local experience with other immunomodulatory agents was limited and varied across different centres.
- Serum IgG4 should be used for disease prognostication and monitoring of treatment response.
- Salivary gland involvement should be screened in patients with IgG4-related disease, especially in the presence of higher level of serum IgG4.
- Future studies on treatment strategies within the contexts of different epidemiology and patient characteristics are urgently needed.
Introduction
Immunoglobulin (Ig) G4–related disease (IgG4-RD)
is a systemic immune-mediated disease unifying
what were previously considered to be unrelated
individual organ disorders. This characteristic
fibroinflammatory condition continues to be
increasingly recognised but is still an evolving
concept. The disease, IgG4-RD, was first described
in 2003 when extra-pancreatic lesions with IgG4-positive plasmacytic infiltration were identified
in patients with autoimmune pancreatitis (now
known as IgG4-related pancreatitis).1 Involvement
of almost every anatomical site has been reported
since. In addition to IgG4-related hepatobiliary
disease, other examples of previous disease entities
now under the diagnostic umbrella of IgG4-RD
include Riedel’s thyroiditis, Ormond’s disease
(idiopathic retroperitoneal fibrosis), Mikulicz’s
disease (lymphoepithelial sialadenitis), Küttner’s
tumour (chronic sclerosing sialadenitis), and other
‘idiopathic’ pseudotumours.2 Regardless of the organ
involved, patients share similar clinical, serological,
and histopathological features.2 3 According to the ‘comprehensive diagnostic criteria for IgG4-RD’, the diagnosis of IgG4-RD is based on the constellation of clinical, serological and, especially,
histopathological findings.4 The recommended
cut-off value for serum IgG4 level is >135 mg/dL.
The characteristic histopathological findings include
dense lymphoplasmacytic infiltrates, ‘storiform’
or swirling fibrosis, and obliterative phlebitis.
Immunostaining for IgG4 should show >10 IgG4-positive plasma cells per high-power field and an
IgG4-positive–to–IgG-positive ratio (IgG4:IgG)
plasma cell ratio of >0.4.
Despite continued advances in our
understanding of the disease and the various
multinational guidance now available,4 5 few studies have examined factors to predict disease severity
or disease prognostication. The bulk of IgG4-RD–related research originates from Caucasian or
Japanese studies, and local regional data are sparse.
Publications from Hong Kong have been limited
to individual case reports or case series only. In
this study, we performed a retrospective review
of all our IgG4-RD patients between January 2003
and December 2015. To the best of our knowledge,
this is the largest cohort reported in our locality
at the time of writing. By combining our data with
all other available publications from Hong Kong,
we examined the clinical features and treatment
practices of IgG4-RD, as well as its clinical factors
associated with multisystem involvement.
Methods
Retrospective study at Queen Mary Hospital
All available case records of IgG4-RD patients
from Queen Mary Hospital—under the care of the
Hong Kong West Cluster serving a population of
0.53 million—between January 2003 and December
2015 were reviewed. Cases were identified by
the compilation of various databases of multiple
specialist divisions (Rheumatology and Clinical
Immunology, Gastroenterology and Hepatology,
Otorhinolaryngology–Head and Neck Surgery, and
Hepatobiliary and Pancreatic Surgery), in addition
to cluster-wide screening of all patients with
laboratory requests for serum IgG4 within the study
period. Case records were reviewed according to
the ‘comprehensive diagnostic criteria for IgG4-RD’
and all patients with definite, probable, or possible
IgG4-RD were recruited.4 In accordance with these
criteria, patients could also be diagnosed with a
definite diagnosis of IgG4-RD if they fulfilled organ-specific
criteria.6
All data were extracted from patient records,
including sex, age (at onset), organ manifestations,
pre-treatment serum IgG4 and IgG levels,
pathology reports, and treatment modalities.
Organ manifestations were classified into bone,
central nervous system (CNS), hepatobiliary and
pancreatic (HBP) system, lung, lymph node, eye
(including lacrimal gland, extraocular muscles,
and other intraorbital involvement), renal system,
retroperitoneum, salivary glands (parotid and
submandibular glands), and skin/soft tissue
involvement. Treatment modalities were classified
into surgical intervention (including resection and
other mechanical interventions such as biliary or
ureteric stenting), use of glucocorticoids (GCs), or
other specified immunomodulatory therapy. This
study was done in accordance with the principles
outlined in the Declaration of Helsinki. Clinicians
involved in data extraction were unaware of the
studied associations.
Literature review of existing local publications
We searched PubMed, PubMed Central, and
MEDLINE databases without language restrictions
from 1 January 2003 to 31 December 2016 using the
terms ‘Hong Kong’ and ‘immunoglobulin G4’ or ‘IgG4’
or ‘IgG4-related disease’ or ‘IgG4-associated disease’
or ‘IgG4 sclerosing disease’. All patient data available
from local IgG4-RD publications were reviewed
against the ‘comprehensive diagnostic criteria for
IgG4-RD’4 and extracted for analysis. Patients from
publications originating from Queen Mary Hospital,
who were already present in our database, were
excluded. Parallel with the retrospective analysis,
data regarding patients’ age, organ manifestations,
pre-treatment serum IgG4 and IgG levels, pathology
reports, treatment modalities, and medication
regimens were recorded. Clinicians involved in
data extraction were unaware of the studied
associations.
Statistical analysis
Potential factors associated with multisystem
disease, reflected by the number of involved organ
systems, were investigated. Univariate analysis was
performed first using the independent samples
t-test to compare categorical variables (such as sex)
and linear regression was used to compare between
continuous variables (such as age). Variables with a P
value of ≤0.1 from univariate analysis were included
in a multivariate linear regression to determine which
were independently associated with the number
of involved organ systems. The two-sided Fisher’s
exact test was used to evaluate the association
between treatment modalities and presence of organ
manifestations. A P value of <0.05 was considered
statistically significant. Statistical Package of the
Social Sciences (Windows version 20.0; IBM Corp,
Armonk [NY], US) was used for all analyses. The
Venn diagram was created using jvenn.7
Results
Demographics, clinical features, and
treatment modalities
Between January 2003 and December 2015, a
total of 55 patients with IgG4-RD were identified
at Queen Mary Hospital. Patients were under
the care of a variety of medical and surgical
subspecialties, including Rheumatology and Clinical
Immunology, Gastroenterology and Hepatology,
Otorhinolaryngology–Head and Neck Surgery, and
Hepatobiliary and Pancreatic Surgery, in addition to
multidisciplinary care between other departments
and disciplines. Baseline demographics, clinical
features, and treatment modalities are summarised
in Table 1.8 9 10 11 12 13 14 15 16 17 18 19 20 21 Pre-treatment serum IgG4 level,
total IgG level, and IgG4:IgG ratio were available
for 48, 43, and 43 patients, respectively. A total
of 46/48 (95.8%) patients had a serum IgG4 level
of >135 mg/dL, and 39/43 (90.7%) patients had a
IgG4:IgG ratio of >8%. Of the 55 patients, 40 (72.7%)
had histopathological confirmation, of which 32
samples had immunohistochemical staining with
anti-IgG4 monoclonal antibodies. All treatment
modalities were primarily used for the treatment
of IgG4-RD except for one patient who was treated
with COPP chemotherapy (cyclophosphamide,
vincristine, procarbazine, and prednisone) because
of concomitant lymphoma. Of the patients, 19
(34.5%) underwent surgical treatment, including
sialoadenectomy (n=7), pancreaticoduodenectomy
(n=6), orbitotomy (n=2), cholecystectomy (n=2),
and excision of musculoskeletal lesions (n=2).
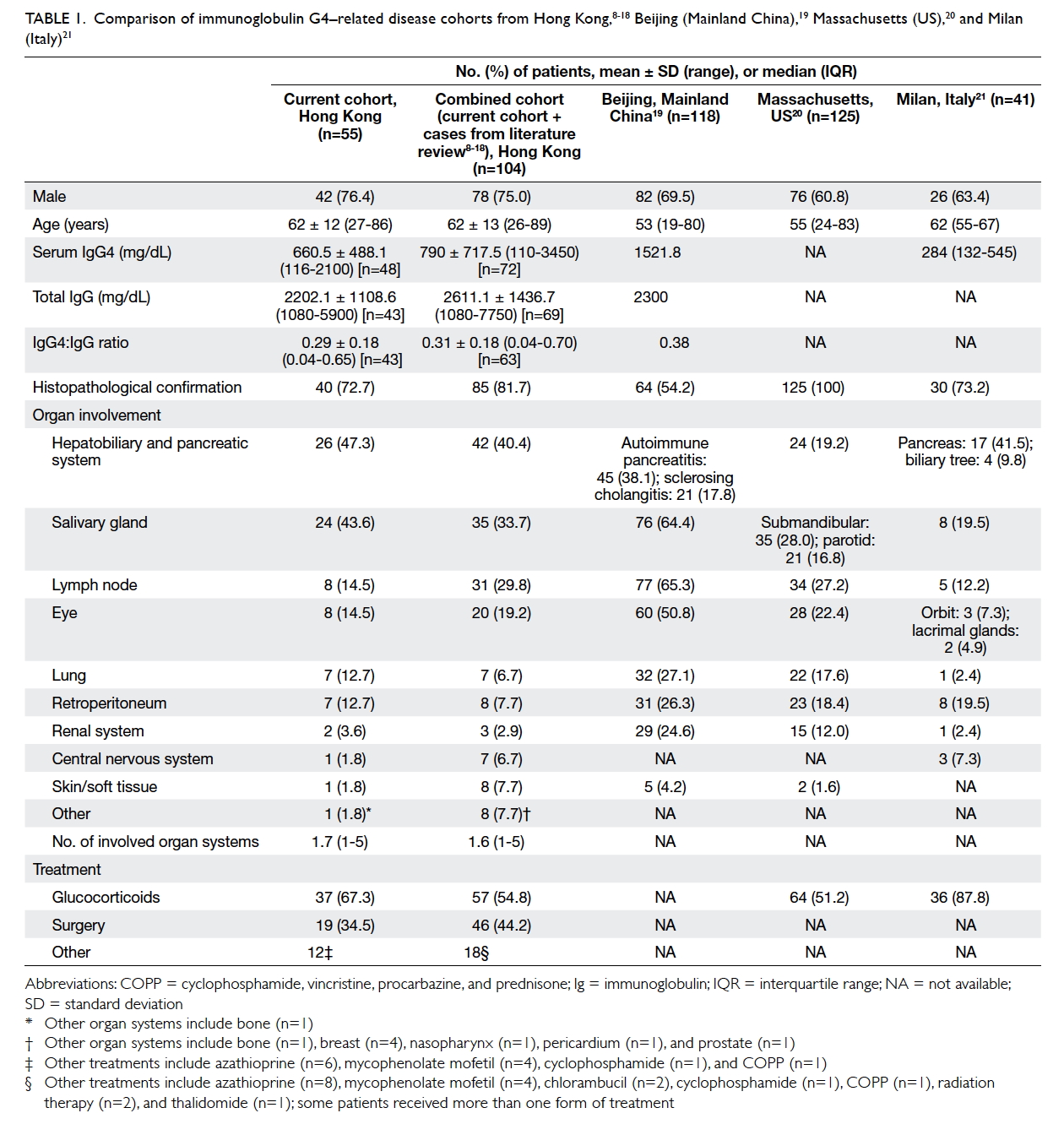
Table 1. Comparison of immunoglobulin G4–related disease cohorts from Hong Kong,8 9 10 11 12 13 14 15 16 17 18 Beijing (Mainland China),19 Massachusetts (US),20 and Milan (Italy)21
We identified an additional 49 IgG4-RD
patients from 11 published case reports and case
series from Hong Kong.8 9 10 11 12 13 14 15 16 17 18 Only those reporting
pre-treatment serum IgG4 and IgG levels were
included in our study. Treatment modalities were
not reported in four patients. A summary of patient
demographics, clinical features, and treatment
modalities is shown in Table 2.8 9 10 11 12 13 14 15 16 17 18
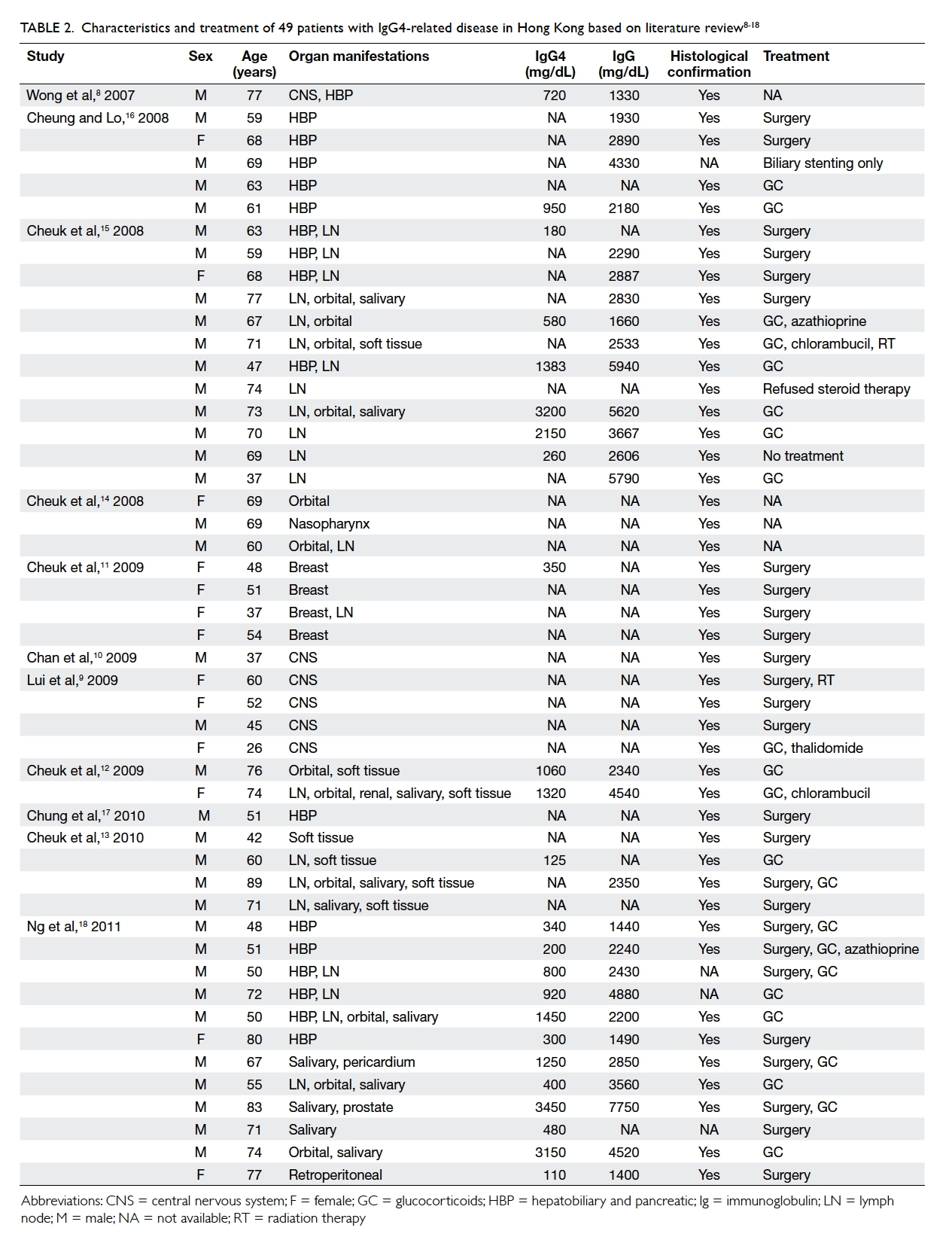
Table 2. Characteristics and treatment of 49 patients with immunoglobulin G4–related disease in Hong Kong based on literature review8 9 10 11 12 13 14 15 16 17 18
As a result, a total of 104 patients were identified
from Queen Mary Hospital and literature review. For
patients with pre-treatment results available, 68/72
(94.4%) patients had a serum IgG4 level of >135
mg/dL, and 58/63 (92.1%) patients had an IgG4:IgG
ratio of >8%. A summary of the demographics,
clinical features, and treatment modalities in
comparison to other cohorts is shown in Table 1.8 9 10 11 12 13 14 15 16 17 18 19 20 21
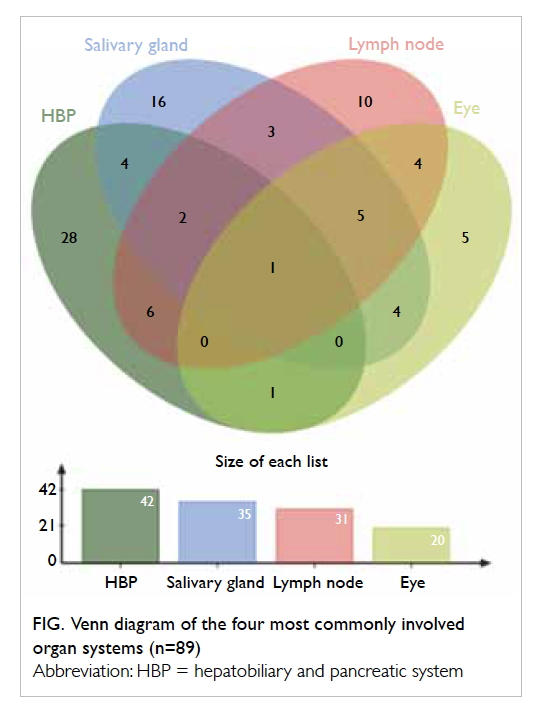
The most common organ systems involved were HBP
system (40.4%), salivary gland (33.7%), lymph node
(29.8%), and eye (19.2%). A Venn diagram of these
most common involved systems from the combined
cohort is shown in the Figure.
Treatment practices: associations between
organ manifestations and treatment
modalities
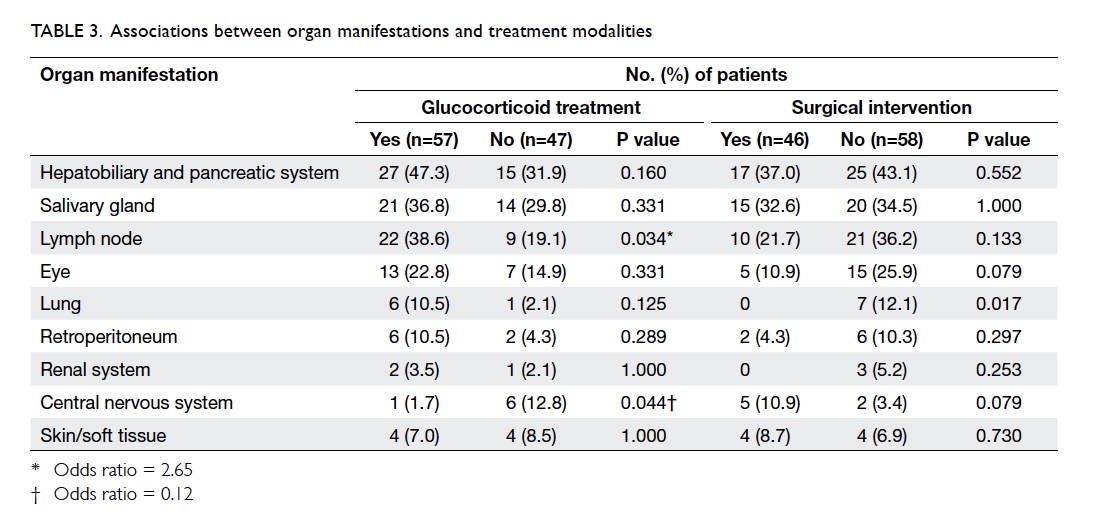
The associations between various organ
manifestations and treatment modalities are shown
in Table 3. Lymphadenopathy was associated with GC
use (odds ratio [OR]=2.65; 95% confidence interval
[CI], 1.08-6.54; P=0.034). Involvement of CNS was
negatively associated with GC use (OR=0.12; 95%
CI, 0.01-1.05; P=0.044).
Associations of serum immunoglobulin G4
with multisystem disease and specific organ
manifestations
Age, sex, pre-treatment serum IgG4, total IgG, and
IgG4:IgG ratio were used in univariate analysis. Both
age (P=0.021) and pre-treatment serum IgG4 levels
(P=0.020) significantly correlated with the number
of involved organ systems in univariate analysis.
Other variables did not reach statistical significance
(data not shown). Only pre-treatment serum IgG4
levels remained statistically significant in subsequent
multivariate analysis (β=0.347; P=0.004). For specific
organ manifestations, pre-treatment serum IgG4
level was more associated with patients having
salivary gland involvement than those without (mean,
1109 mg/dL vs 599 mg/dL; P=0.012). No associations
of serum IgG4 with other organ manifestations were
found (P>0.1; data not shown).
Discussion
In this study, we describe the clinical features and
treatment practices of the largest cohort of IgG4-RD
in our locality. After combination of our patients
with all other published cases of IgG4-RD from
Hong Kong, we analysed 104 cases comprising
predominantly older men (mean age, 62 ± 13 years;
male-to-female ratio=3:1), which is consistent with
other reports.19 20 21 22 Over 95% of patients had serum
IgG4 level of >135 mg/dL and an IgG4:IgG ratio of
>8%. Although these cut-offs are often quoted in the
diagnostic criteria for IgG4-RD,4 23 it is important to note that elevated serum IgG4 levels can be seen in
a variety of other conditions such as malignancies,
infections, or autoimmune disorders. Serum IgG4
level and IgG4:IgG ratio alone have poor specificity
and low positive predictive value. The specificity and
positive predictive value of serum IgG4 and IgG4:IgG
ratio have been reported to be approximately only
0.6 and 0.3, respectively.24 Of note, 4/72 (5.6%) of our
patients with biopsy-proven IgG4-RD had normal
serum IgG4 levels (ie false negatives). The gold
standard for diagnosis of IgG4-RD in most cases
therefore remains biopsy with histopathological
confirmation. Over 70% of patients in Queen Mary
Hospital had positive histopathological confirmation,
in comparison to 54.2% in a large Mainland Chinese
cohort.19 Although this proportion increased to
81.7% in the combined analysis, this could be an overestimation
of real clinical practice with potential
publication and selection bias from literature review.
Similar to other reported populations, HBP system,
salivary gland, lymph node, and eye were the most
common organ systems involved in both Queen
Mary Hospital and the combined analysis. Although
involvement of HBP system seemed more prevalent
in the Queen Mary Hospital and Mainland China
cohorts, the rate of HBP involvement is similar to
other studies dedicated to IgG4-related hepatobiliary
disease (approximately 40%-60%).25
We also examined the treatment practices
employed in our locality. Treatment of IgG4-RD
is typically individualised because of substantial
disease heterogeneity—even subclinical disease
can lead to irreversible organ damage and not all
manifestations require immediate treatment. For
example, a watchful ‘wait and see’ approach may
be an appropriate option for mild disease or after
surgical debulking. However, there is currently
no high-quality evidence-based guidance for the
management of IgG4-RD and practices often vary
significantly across different countries. In the
combined analysis, GCs were the most popular
treatment option and over half of the patients
had received GCs either alone or in combination
with other treatment modalities. In the combined
analysis, use of GCs was significantly associated
with lymphadenopathy, which may reflect their
preferential use, especially in patients with systemic
involvement. This is consistent with the general
consensus and recommendations made by most
experts because of their good initial efficacy.26
The opposite was seen with an inverse association
between CNS involvement and GC use. This was
expected because most of these patients with CNS
involvement had localised disease and the diagnosis
of IgG4-RD was not readily established prior to
surgical resection. Only one case was diagnosed by
open brain biopsy and subsequently treated with
GCs and thalidomide.9
Local experience with other immunomodulatory
agents was limited and choices for
steroid-sparing agents varied between different
centres. Conventional agents such as azathioprine,
cyclophosphamide, methotrexate, mycophenolate,
and tacrolimus have all demonstrated similar
efficacy, although head-to-head comparisons are not
available.27 B-cell depletion with rituximab has also
gained much popularity in recent years and proven
to be effective as induction and maintenance therapy,
even without concomitant GCs.27 28 Nonetheless, we were unaware of any published experience with its
use for IgG4-RD in Hong Kong at the time of writing.
The advent of this ground-breaking treatment will
likely require multidisciplinary expertise as well
as further research, especially in the context of the
high prevalence of chronic hepatitis B infection and
subsequent risk of viral reactivation in Hong Kong.
Finally, we also recommend the utility of pre-treatment
serum IgG4 in disease prognostication
and treatment monitoring. Pre-treatment serum
IgG4 levels (but not IgG4:IgG ratios) significantly
correlated with the number of organ systems
involved. The correlation with age did not remain
statistically significant after multivariate regression.
Interestingly, Wallace et al20 also described elevated
serum IgG4 levels associated with both age and
number of organs involved, but analysis with
multivariate regression was not reported in their
study. Specifically, serum IgG4 levels also correlated
with salivary gland involvement, but not with
other individual organ systems. The reason for
this particular correlation remains uncertain, but
highlights the importance of screening for salivary
gland involvement in all IgG4-RD patients, especially
in the presence of higher serum IgG4 levels.
The main limitations of this study included its
retrospective nature and restrictions of literature
review. Limited clinical data were available and
we were unable to determine or standardise for
the disease duration of each patient. There were
substantial missing data for some key variables,
reducing the effective number of patients suitable
for analysis. The nature of a literature review also
harbours risk of publication or selection bias,
although it was reassuring that findings from the
combined analysis were similar to those obtained
from Queen Mary Hospital. Some indefinite findings,
such as lower prevalence of renal involvement,
highlight the necessity of future prospective and
multicentre studies. Specifically, we advocate the
need for more uniform international diagnostic
criteria and the establishment of a region-wide
registry with longitudinal data collection.
In conclusion, we found that pre-treatment
serum IgG4 significantly correlated with the number
of organ systems involved, highlighting its potential
for disease prognostication and guiding treatment.
We also describe the clinical characteristics,
treatment practices, and factors associated with
multisystem disease in IgG4-RD in Hong Kong.
There is vast disease and patient heterogeneity,
making local research and expertise exchange
imperative. Increased physician awareness and a
multidisciplinary approach will be required for
early diagnosis and optimal management of this
masquerading disease. Further studies, especially
focusing on treatment strategies within the contexts
of different epidemiology and patient characteristics,
are urgently needed.
Acknowledgements
Dr Rex Au-Yeung (Department of Pathology, Queen
Mary Hospital) reviewed the histology available from
patients diagnosed with IgG4-RD under the care of
the Hong Kong West Cluster. The authors thank the
Shun Tak District Min Yuen Tong (MYT) of Hong
Kong for their generous donations to support the
work on clinical immunology and rheumatology;
MYT had no role in the design of the study;
collection, analysis, or interpretation of the data;
writing, review, or approval of the manuscript; or the
decision to submit the manuscript for publication.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Kamisawa T, Funata N, Hayashi Y, et al. A new
clinicopathological entity of IgG4-related autoimmune
disease. J Gastroenterol 2003;38:982-4. Crossref
2. Stone JH, Khosroshahi A, Deshpande V, et al.
Recommendations for the nomenclature of IgG4-related
disease and its individual organ system manifestations.
Arthritis Rheum 2012;64:3061-7. Crossref
3. Carruthers MN, Stone JH, Khosroshahi A. The latest
on IgG4-RD: a rapidly emerging disease. Curr Opin
Rheumatol 2012;24:60-9. Crossref
4. Umehara H, Okazaki K, Masaki Y, et al. Comprehensive
diagnostic criteria for IgG4-related disease (IgG4-RD),
2011. Mod Rheumatol 2012;22:21-30. Crossref
5. Deshpande V, Zen Y, Chan JK, et al. Consensus statement
on the pathology of IgG4-related disease. Mod Pathol
2012;25:1181-92. Crossref
6. Umehara H, Okazaki K, Nakamura T, et al. Current
approach to the diagnosis of IgG4-related disease—combination of comprehensive diagnostic and organ-specific criteria. Mod Rheumatol 2017;27:381-91. Crossref
7. Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn:
An interactive Venn diagram viewer. BMC Bioinformatics
2014;15:293. Crossref
8. Wong S, Lam WY, Wong WK, Lee KC. Hypophysitis
presented as inflammatory pseudotumor in
immunoglobulin G4-related systemic disease. Hum Pathol
2007;38:1720-3. Crossref
9. Lui PC, Fan YS, Wong SS, et al. Inflammatory pseudotumors
of the central nervous system. Hum Pathol 2009;40:1611-7. Crossref
10. Chan SK, Cheuk W, Chan KT, Chan JK. IgG4-related
sclerosing pachymeningitis: a previously unrecognized
form of central nervous system involvement in IgG4-related sclerosing disease. Am J Surg Pathol 2009;33:1249-52. Crossref
11. Cheuk W, Chan AC, Lam WL, et al. IgG4-related sclerosing
mastitis: description of a new member of the IgG4-related
sclerosing diseases. Am J Surg Pathol 2009;33:1058-64. Crossref
12. Cheuk W, Lee KC, Chong LY, Yuen ST, Chan JK. IgG4-related sclerosing disease: a potential new etiology
of cutaneous pseudolymphoma. Am J Surg Pathol
2009;33:1713-9. Crossref
13. Cheuk W, Tam FK, Chan AN, et al. Idiopathic cervical
fibrosis—a new member of IgG4-related sclerosing
diseases: report of 4 cases, 1 complicated by composite
lymphoma. Am J Surg Pathol 2010;34:1678-85. Crossref
14. Cheuk W, Yuen HK, Chan AC, et al. Ocular adnexal
lymphoma associated with IgG4+ chronic sclerosing
dacryoadenitis: a previously undescribed complication
of IgG4-related sclerosing disease. Am J Surg Pathol
2008;32:1159-67. Crossref
15. Cheuk W, Yuen HK, Chu SY, Chiu EK, Lam LK, Chan JK.
Lymphadenopathy of IgG4-related sclerosing disease. Am
J Surg Pathol 2008;32:671-81. Crossref
16. Cheung MT, Lo IL. IgG4-related sclerosing
lymphoplasmacytic pancreatitis and cholangitis mimicking
carcinoma of pancreas and Klatskin tumour. ANZ J Surg
2008;78:252-6. Crossref
17. Chung DT, Tang CN, Lai EC, Yang GP, Li MK.
Immunoglobulin G4-associated sclerosing cholangitis
mimicking cholangiocarcinoma. Hong Kong Med J
2010;16:149-52.
18. Ng TL, Leong IS, Tang WL, et al. Immunoglobulin G4-related sclerosing disease: experience with this novel entity
in a local hospital. Hong Kong Med J 2011;17:280-5.
19. Lin W, Lu S, Chen H, et al. Clinical characteristics of
immunoglobulin G4-related disease: a prospective
study of 118 Chinese patients. Rheumatology (Oxford)
2015;54:1982-90. Crossref
20. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related
disease: clinical and laboratory features in one hundred
twenty-five patients. Arthritis Rheumatol 2015;67:2466-75. Crossref
21. Campochiaro C, Ramirez GA, Bozzolo EP, et al. IgG4-related disease in Italy: clinical features and outcomes of a large cohort of patients. Scand J Rheumatol 2016;45:135-45. Crossref
22. Martinez-Valle F, Fernández-Codina A, Pinal-Fernández I, Orozco-Gálvez O, Vilardell-Tarrés M. IgG4-related
disease: evidence from six recent cohorts. Autoimmun Rev
2017;16:168-72. Crossref
23. Masaki Y, Kurose N, Yamamoto M, et al. Cutoff values
of serum IgG4 and histopathological IgG4+ plasma cells
for diagnosis of patients with IgG4-related disease. Int J
Rheumatol 2012;2012:580814. Crossref
24. Carruthers MN, Khosroshahi A, Augustin T, Deshpande
V, Stone JH. The diagnostic utility of serum IgG4
concentrations in IgG4-related disease. Ann Rheum Dis
2015;74:14-8. Crossref
25. Culver EL, Chapman RW. IgG4-related hepatobiliary
disease: an overview. Nat Rev Gastroenterol Hepatol
2016;13:601-12. Crossref
26. Khosroshahi A, Wallace ZS, Crowe JL, et al. International
consensus guidance statement on the management and
treatment of IgG4-related disease. Arthritis Rheumatol
2015;67:1688-99. Crossref
27. Hart PA, Topazian MD, Witzig TE, et al. Treatment
of relapsing autoimmune pancreatitis with
immunomodulators and rituximab: the Mayo Clinic
experience. Gut 2013;62:1607-15. Crossref
28. Carruthers MN, Topazian MD, Khosroshahi A, et al.
Rituximab for IgG4-related disease: a prospective, open-label
trial. Ann Rheum Dis 2015;74:1171-7. Crossref