Hong Kong Med J 2017 Jun;23(3):272–81 | Epub 5 May 2017
DOI: 10.12809/hkmj165061
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Common urological problems in children:
inguinoscrotal pathologies
Ivy HY Chan, FRCSEd(Paed), FHKAM (Surgery);
Kenneth KY Wong, PhD, FHKAM (Surgery)
Division of Paediatric Surgery, Department of Surgery, The University of
Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr Kenneth Wong (kkywong@hku.hk)
Abstract
Urological problems in children are often
encountered in general clinical practice. This review
forms the second paper of our series on common
urological problems in children about inguinoscrotal
pathologies. We aimed to provide concise
information for doctors who are unfamiliar with this
topic.
Introduction
Our previous review paper described and discussed
disorders of the prepuce.1 In this paper, we focus on
common inguinoscrotal pathologies in children.
Inguinal hernia/hydrocoele, undescended testis,
acute scrotum, and varicocoele will be discussed.
These have a broad disease spectrum, but may have
similar clinical presentation and may be discovered
by parents, during routine paediatric assessment
or incidentally in the clinic. Despite advances in
medical technology, proper history taking, physical
examination, and understanding of these conditions
remain crucial for management and specialist
referral.
Inguinal hernia/hydrocoele
These two conditions account for most of the
pathologies in the inguinoscrotal region in children.
For paediatric inguinal hernia, the cumulative
incidence was reported to be 6.62% in boys up to 15
years old in a nationwide study in Taiwan: one in 15
boys would develop inguinal hernia before the age
of 15 years.2
Both inguinal hernia and hydrocoele occur
because of non-closure of the processus vaginalis
(patent processus vaginalis). The processus vaginalis
forms an extension of the peritoneum during the
time of testicular descent into the scrotum in the
fetus. It normally undergoes fusion or closure when
testicular descent is complete. Failure to close
may result in either inguinal hernia or hydrocoele,
depending on the size of the defect. If it remains
patent or unfused in girls, it becomes the canal of
Nuck and may also result in inguinal hernia (Fig 1).
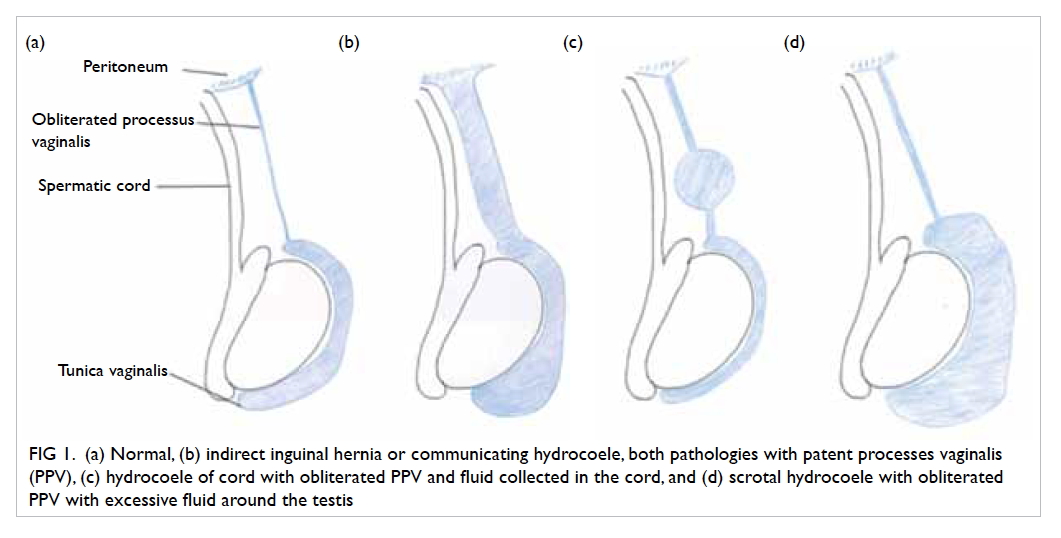
Figure 1. (a) Normal, (b) indirect inguinal hernia or communicating hydrocoele, both pathologies with patent processes vaginalis (PPV), (c) hydrocoele of cord with obliterated PPV and fluid collected in the cord, and (d) scrotal hydrocoele with obliterated PPV with excessive fluid around the testis
The incidence of inguinal hernia is highest
in children under 1 year of age and thereafter
decreases.2 In premature babies, the incidence is
even higher with babies born less than 32 weeks of
gestation having a reported incidence of 9.34%.3
Right-sided hernias are more common (ratios
of right, left, and bilateral hernia are approximately
59%, 29%, and 12%, respectively).4 Boys are affected
around 10 times more often than girls2; 99% of
inguinal hernias in children are of the indirect type.4
Hydrocoele is an abnormal collection
of fluid along the processus vaginalis, and can
be communicating or non-communicating. In
communicating hydrocoele, there is a patent
processus vaginalis. Non-communicating hydrocoele
includes scrotal hydrocoele and hydrocoele of cord,
and here the processus vaginalis is obliterated
with the collection of fluid in the tunica vaginalis.
Hydrocoele can present in infants or older children
and its management differs. For hydrocoele that
presents before 1 year of age, there is a 62.7% to
89% chance of spontaneous complete resolution or
significant improvement,5 6 7 with the mean time of
resolution being about 6 months. Thus, it is worth
allowing a period of time to observe a hydrocoele
provided other conditions like inguinal hernia or
testicular pathology have been excluded.
Clinical features
Parents usually notice the inguinoscrotal pathology
in their child during bathing or changing nappies. A
painless bulge over the inguinal region or even down
to the scrotum may be seen in boys, or a bulge over
the vulva in girls with inguinal hernia. The bulge will
increase in size when the child cries and decrease
when lying down. A proper clinical examination may
not be easy in the clinic setting for children and the
hernia may not be apparent. A well-taken clinical
history is very important in making the correct
diagnosis. An ‘intermittent inguinal swelling’ may
indicate an inguinal hernia. A static painless scrotal
swelling may indicate a scrotal/encysted hydrocoele.
For children who cooperate, the upper and
lower extent of the inguinoscrotal mass should be
carefully examined. The upper extent of an inguinal
hernia should start at the internal ring, that is, the
midpoint between the pubic tubercle and anterior
superior iliac spine. For scrotal/encysted hydrocoele,
clinicians should be able to get above the lesions. A
transillumination test is a very helpful in adults but is
not reliable in infants/small children. Because of the
thin bowel wall the transillumination test can also
be positive in infants/small children with inguinal
hernia.
Pain and inconsolable crying in a child with
a tender irreducible inguinal bulge may indicate
an incarcerated inguinal hernia. A younger child is
more likely to be affected. The mean age of hernia
incarceration was shown to be 1.5 years in a previous
study.4 The incidence of incarceration is 3 times
higher in premature babies with inguinal hernia.
Very often, parents may not be able to describe
the clinical features properly and no pathology can
be demonstrated during a clinic visit. We suggest
that parents take a clinical photo using mobile
phones at home, which can be shown to the doctor
during the subsequent clinic visit.
Investigations
Diagnosis of an inguinal hernia depends largely on
clinical history and physical examination. Different
imaging techniques can sometimes be helpful in
making the diagnosis. Contrast herniography is only
of historical interest because of its invasiveness.
Ultrasonography (USG) of the inguinal canal to
detect occult inguinal hernia has been described and
its use varies in different countries. Studies show
that the preoperative USG can decrease the future
risk of developing metachronous inguinal hernia.8
A positive finding on USG strongly correlates with
positive operative findings.9 10 Nonetheless as the accuracy of USG is largely operator-dependent, we
feel that while a positive USG finding is strongly
suggestive of a clinical hernia, a negative finding
should be interpreted with care. Diagnosis of
inguinal hernia will very much depend on clinical
examination. Of note, USG of the inguinal canal is
not a routine procedure when making a diagnosis
of inguinal hernia. It may serve as an adjunct when
there is doubt about incarcerated hernia versus
hydrocoele of cord or concern about underlying
testicular pathology for hydrocoele.
Indications and timing of surgery
Once the diagnosis of inguinal hernia is made,
operation is indicated regardless of age due to
the risk of incarceration, with a reported rate of
approximately 4.19% to 8.2%.2 11 As young children and infants have a higher risk of incarceration, it
may be wise to arrange earlier operation. Surgery,
however, should still be arranged as early as possible
in an otherwise healthy child.
For the management of premature babies with
inguinal hernia, there are many factors that should
be taken into consideration such as postoperative
apnoea, respiratory distress, co-morbidities (eg
chronic lung disease in premature babies), and risk
of incarceration. There is always debate about the
optimal timing of hernia repair in premature babies,
that is, surgery just before discharge from neonatal
intensive care unit or when the child is older. Although
a long waiting time is associated with higher risk of
incarceration in infants and premature babies, and
emergency repair in patients with incarceration
is associated with a higher likelihood of testicular
atrophy,12 13 14 surgery is less technically demanding
in older babies. Fewer perioperative morbidities are
also observed when performing surgery later. Early
inguinal hernia repair is associated with prolonged
hospital stay and prolonged intubation.15 A study
from Hong Kong reported one (1.3%) incarceration
in 79 premature patients with a mean body weight
at operation of 4360 g.16 This incarceration rate is
relatively lower when compared with other studies—9% to 21% by Lautz et al13 from the United States, and 5.2% to 10.1% by Zamakhshary et al12 from
Canada. It is likely to be due to the shorter travelling
time between a patient’s home and hospital in Hong
Kong. Based on our experience, it is reasonable in
Hong Kong to wait for surgery until premature babies
have achieved a reasonable body weight. Parents
should be taught how to observe the symptoms and
signs of incarceration before discharge. If the patient
lives unreasonably far from the hospital and parents
are not able to observe the symptoms and signs of
incarceration, earlier surgery should be offered.
From our perspective, we would offer repair when
the patient’s body weight reaches 2.5 kg, provided
there are no other indications for earlier or delayed
repair.
As mentioned earlier, there is a high chance
of spontaneous resolution of infantile hydrocoele
during the first year of life. Patients should therefore
be observed and monitored in the first 1 to 2 years
of life.5 6 Parents should also be taught about the symptoms and signs of inguinal hernia during
this observation period as an inguinal hernia may
present as a communicating hydrocoele on first
sight. Patients with persistent hydrocoele, giant or
symptomatic hydrocoele, hydrocoele associated
with inguinal hernia or other conditions (eg presence
of ventriculoperitoneal shunt), or who require
peritoneal dialysis should be offered surgery.
Open versus laparoscopic surgery
Open high ligation of patent processus vaginalis was
the mainstay of treatment for both inguinal hernia and
hydrocoele in children until the advent of laparoscopic
surgery. Different laparoscopic techniques are largely
categorised into intracorporeal or extracorporeal
ligation of the patent processus vaginalis.17 18 19
Benefits of laparoscopic surgery include the possible
visualisation of the contralateral deep ring and better
cosmetic outcome. Yet it may incur a higher set-up
cost or longer operating time.
Two recent systematic reviews/meta-analyses
showed very similar results when comparing open and
laparoscopic inguinal hernia repair in children.17 20 Both studies showed no significant difference in
recurrence rate. Esposito et al17 reported a 1.4%
recurrence rate following laparoscopic repair and
1.6% following open repair. For operating time, they
noted a significantly shorter time for laparoscopic
repair of bilateral hernia when compared with
an open approach. There was no difference for
unilateral hernia repair. Feng et al20 reported that a
laparoscopic extraperitoneal method had a much
shorter operating time in both unilateral and bilateral
hernia repair. Lower pain scores were also reported
in two randomised controlled trials for laparoscopic
repair.21 22
Feng et al20 further showed more testicular
complications in open repair. Another retrospective
review showed similar results.23 Of note, a higher
recurrence rate of up to 3.4% for laparoscopic
repair was observed in the early era of laparoscopic
surgery.18 There is no consensus, however, on
whether a laparoscopic or open approach is superior.
In contrast to laparoscopic inguinal hernia
repair, laparoscopic repair of hydrocoele has not
received the same popularity, with open high ligation
still being the mainstay of treatment. Indeed, only a
handful of studies can be found in the literature.
One reason may be the belief that the deep ring
is already closed. A recent study noted that 97%
of patients with a clinically non-communicating
hydrocoele had a patent processus vaginalis during
laparoscopy.24 Among the small number of reports,
Saka et al19 showed no significant difference in terms
of outcome between open and laparoscopic repair of
hydrocoele.
Contralateral exploration
Metachronous contralateral inguinal hernia
(MCH)—presence of contralateral inguinal hernia—may present in some patients after successful repair
of inguinal hernia. An initial presentation with left-sided
hernia,25 26 27 and prematurity are risk factors for
MCH.
Before the era of laparoscopy, USG of the groin
or routine contralateral exploration were suggested
by some surgeons to reduce the risk of MCH and the
need for second operation/anaesthesia. Miltenburg et al28 reviewed the use of laparoscopic evaluation and concluded that it could successfully decrease the
incidence of MCH.
Today, routine examination during
laparoscopic hernia repair has shown the rate of
contralateral patent contralateral processus vaginalis
(CPPV) to be 30% to 39.7%.25 29 Yet, a meta-analysis revealed that only 6% of all patients returned with
MCH after unilateral inguinal hernia repair.26 Thus,
the presence of CPPV does not necessarily equate to
subsequent clinical inguinal hernia. Kokorowski et al29 concluded that patent processus vaginalis might be over-treated. Repair of the patent processus
vaginalis will definitely decrease the risk of MCH, the
costs, and the risk of future hernia incarceration. On
the other hand, it also carries an operative risk of vas
injury, haematoma, and infection of the contralateral
side. In view of the uncertainties about the overall
balance of risks and benefits of routine prophylactic
repair of CPPV, parents should be counselled on the
benefits of avoiding potential future development of
clinical hernia and the risks of the procedure.
Undescended testis (cryptorchidism)
Cryptorchidism has an estimated incidence of 22.8
per 10 000 live births.30 It remains an important
condition because of its potential sequelae of subfertility,
testicular malignancy, and accompanying
inguinal hernia.
Embryology and pathophysiology
Testicular descent is a complex process. Male
differentiation starts at around 7 to 8 weeks of
gestation under the influence of the SRY gene.
Testicular descent involves two phases, the
transabdominal phase and the inguinoscrotal
phase. The gonad descends from the area near the
urogenital ridge to the level of the deep ring in the
transabdominal phase. This phase ends at around
15 weeks of gestation. The inguinoscrotal phase
is a more complex process in which the peritoneal
membrane bulges out and elongates to form the
processus vaginalis through which the testes
then descend. This phase usually starts at around
25 weeks and ends at 35 weeks of gestation. After
descent, the testes will anchor to the connective
tissue within the scrotum.
Both phases of testicular descent are controlled
by hormones. Any derangement in the pathway can
result in cryptorchidism. Conditions known to be
associated with cryptorchidism include prematurity,
Klinefelter syndrome, and abdominal wall defects
like omphalocoele. There is no single cause that
can be identified to account for all the scenarios of
cryptorchidism.
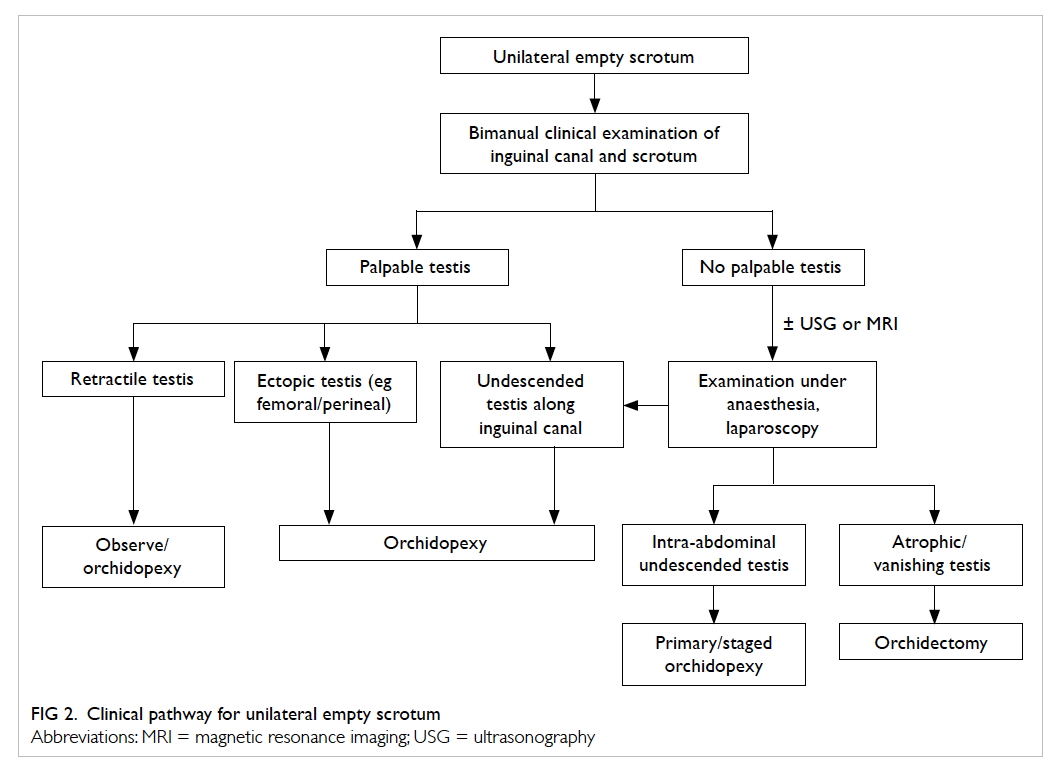
Clinical evaluation
Cryptorchidism is mostly diagnosed by history
and proper clinical examination (Fig 2). As most newborns will undergo a clinical examination before
discharge from hospital, the position of the testes
should be ascertained. During clinical examination,
the infant should be placed supine with legs
abducted in a relaxed and warm environment. A
cold environment may induce a cremasteric reflex
that may move the testes up. Bimanual examination
(two-handed technique) should be performed. The
doctor’s left hand should be placed at the deep ring
and gently sweep along the inguinal canal down to
the scrotum. The right hand should try to detect the
testes at the scrotum or the lowest possible position.
Size, mobility, and consistency of the testes should be
assessed. If there are no palpable testes, a potential
location of ectopic testes should be sought such as the
femoral canal or perineum. The suprapubic region
should be examined as well. Both sides should be
examined carefully with the same technique. Other
concurrent urological anomalies (eg hypospadias)
or problems other than undescended testes (eg
disorder of sexual differentiation) should be checked.
These may warrant further in-depth investigations
or indicate a need for urgent karyotyping in the
newborn period.
A retractile testis should be distinguished from
genuine undescended testis. The clinician should be
able to bring the retractile testis down to the scrotum
and the testis should stay in the scrotum for a while.
It may retract with the cremasteric reflex. Patients
with retractile testes should have normal testicular
volume and fertility. If there is doubt about the
diagnosis, regular assessment or scrotal orchidopexy
can be offered.
Ascending testis is a condition in which the
testis was previously within the scrotum but later
ascends. Presence is usually associated with a history of retractile testis. The postulation is that the
spermatic cord does not elongate with age. These
patients usually present late. A history of previously
noted testicular position should be sought. It has
been shown that ascending testis shares a similar
histopathology to congenital undescended testis.31
Orchidopexy is also recommended.
Investigations
Surgical approach for the undescended testis
differs depending on the location of the testis. The
most important factor is whether or not the testis
is palpable. Clinicians, however, may not be able
to locate the testis during a clinical examination
if the child struggles. If the testis is impalpable,
conditions such as intra-abdominal testis, atrophic
testis, and ectopic testis should also be considered.
Ultrasonography can be used to detect the location
and size of the testis.32 Nijs et al33 noted a high sensitivity of USG scan for inguinal testis but very
low sensitivity for intra-abdominal testis. They
suggested laparoscopic exploration when the testis
cannot be detected on physical examination and
USG.33 34
Use of gadolinium-enhanced magnetic
resonance angiogram was described in 1998 as
another investigation for undescended testes
with high sensitivity and specificity.35 In another
study published in 2000 that compared magnetic
resonance imaging (MRI) with laparoscopy in non-palpable
testes, the false-positive rate of MRI was
32% and true-positive rate for laparoscopy was
100%.36 As MRI is also limited by its costs, availability
and potential need for general anaesthesia in a small
child, many clinicians prefer laparoscopy as the
method of choice for impalpable testis. Nonetheless
there may still be a role for MRI in patients with
suspected persistent Müllerian structures or a
disorder of sexual differentiation.37
Nowadays, early operation on undescended
testis is advocated to reduce the risk of malignancy
and potentially preserve fertility. Early referral
to a surgical specialist may be more important
than investigations. The guideline from American
Urological Association (AUA) published in 2014
advised that health care providers should not
perform USG or other imaging modalities in the
evaluation of boys with cryptorchidism prior to
referral, as these studies rarely assist in decision making
by the general practitioner.38
Treatment
As testicular descent will continue after birth,
watchful waiting should be the initial management
of undescended testes. However, it is advised that all
patients with undescended testis discovered at birth
be re-examined at 6 months of age and referral made
to a specialist if the condition persists.38
A study performed in 1974, which compared
the histology of those with undescended testes
before and after 2 years of age,39 showed loss of
spermatogonia in patients with undescended testis
after 2 years of age. Another study also showed that
the uncorrected position of undescended testes was
associated with ongoing germ cell damage and later
fertility problems and risk of malignancy.40 Thus,
orchidopexy is advised before 18 months of age.
The surgical plan for undescended testes
will be dictated by the position and size of the
testis. For palpable testis in the inguinal canal,
inguinal orchidopexy should be performed. If no
palpable testis can be identified during clinical
examination, adjunct imaging studies in an attempt
to locate the testis can be ordered before surgery.
Laparoscopy, however, remains the gold standard
in diagnosing clinically impalpable testis. In most
cases the testis is located along the line of normal
testicular descent, intra-abdominally or just at
the deep ring of the inguinal canal. Rarely, the
testis can also be atrophic, ectopic, or even absent.
Three approaches have been described for intra-abdominal
testes: primary orchidopexy, one-stage
Fowler-Stephens orchidopexy, or two-stage Fowler-Stephens orchidopexy. Short testicular vessels are
postulated to be the reason why the testis cannot
be brought to the scrotum. The Fowler-Stephens
procedure involves the division of testicular vessels
and repositioning of the testis to the scrotum, either
immediately during the procedure (one-stage) or 6
months later (two-stage). The vascular supply will
depend on the cremasteric vessels and collateral
vessels to the vas after this procedure. Testicular
atrophy and ascent (incidence of both approximately
8%) are potential complications of this procedure.41
On the subject of alternative therapy, the AUA
guideline advises against using hormone therapy
to induce testicular descent as evidence shows low
response rates and a lack of evidence for long-term
efficacy.38
Prognosis/long-term outcome
Patients should be counselled about the increased
risk of testicular malignancy and subfertility.40
A histological study of the undescended testes in
human fetuses, neonates, and infants showed that
the absolute number of germ cells was decreased.42
Mengel et al39 noted a significant decrease in the
content of spermatogonia and a lack of tubular
growth at the beginning of the third year of life. Lee
and Coughlin40 compared paternity rates, the semen
and hormone profiles of patients with previously
unilateral undescended testes, bilateral undescended
testes and normal control subjects; the paternity
rates of these three groups of subjects were 89.7%,
65.3% and 93.2%, respectively. Historical studies
have indicated improved fertility in patients who had
orchidopexy at an earlier age.43
Undescended testis is a known risk factor
for testicular germ cell tumour. In a meta-analysis,
Dieckmann and Pichlmeier44 showed a relative risk
of 4.8 compared with the normal population. The age
at which orchidopexy is performed is also important.
Pettersson et al45 compared the risk of testicular
malignancy between patients who received early
or late orchidopexy and showed the relative risk
of testicular cancer among those who underwent
orchidopexy before reaching 13 years of age was 2.23
(95% confidence interval [CI], 1.58-3.06); for those
treated at 13 years of age or older, the relative risk
was 5.40 (95% CI, 3.20-8.53). It is postulated that the
higher surrounding temperature in undescended
testis could arrest germ cell maturation and become
carcinoma in situ.46
Acute scrotum
Although different disease conditions can present
clinically with acute scrotum, testicular torsion
should be the top differential diagnosis for all
patients, as this condition needs precise clinical
appreciation and urgent management. The time
from presentation to operation is critical and will
determine if the affected testis can be salvaged. A
high index of suspicion with immediate referral is
crucial.
A nationwide epidemiological study in Korea
showed the incidence of testicular torsion was 2.9
per 100 000 person-years of males younger than
25 years, and with a bimodal age distribution with
peak incidence in infancy and adolescents.47 The
salvage rates differ, ranging from 75.7% to 29%.48 49 The diagnosis of torsion depends mostly on
clinical evaluation. Diffuse testicular tenderness,
hydrocoele, high-lying testis with transverse lie, and
absent cremasteric reflex are clinical signs of torsion.
Scrotal exploration should be the management of
choice in clinically suspect cases. As exploration may
be negative, many clinicians have tried adjunctive
methods such as the TWIST (Testicular Workup
for Ischemia and Suspected Torsion) clinical scoring
system,49 Doppler USG, nuclear scintigraphy, MRI,
or evaluation of testicular oxygen saturation with
near-infrared spectroscopy.50 For Doppler USG,
accuracy depends on the skill of the radiologist.
Normal intra-testicular perfusion does not exclude
the possibility of torsion and there remains a false-negative
rate in this modality.51 52 As a missed
diagnosis of testicular torsion is one of the most
common medicolegal claims in adolescent patients
in the United States,53 we must stress again the prime
importance of clinical evaluation and decision.
Nuclear scintigraphy and MRI are not popular for
the same reason. Near-infrared spectroscopy is a
novel technology that measures testicular oxygen
saturation but its accuracy has not been fully
validated in a large population. Scrotal exploration
involves open examination of the affected testes
and a decision on whether to keep or remove the
affected testes and also fix the contralateral side. A
bell-clapper anomaly may be appreciated during
examination of the testes (Fig 3).
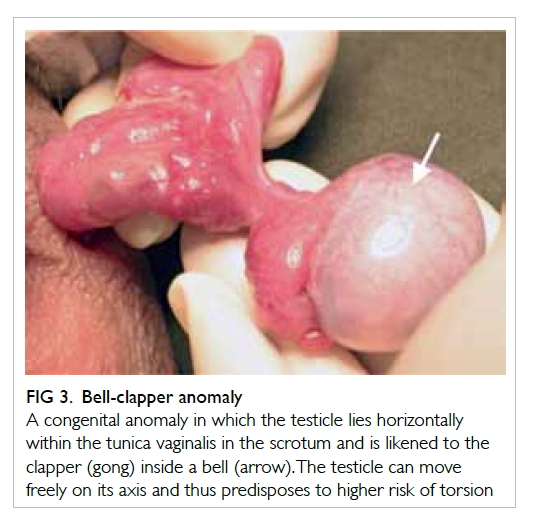
Figure 3. Bell-clapper anomaly
A congenital anomaly in which the testicle lies horizontally within the tunica vaginalis in the scrotum and is likened to the clapper (gong) inside a bell (arrow). The testicle can move freely on its axis and thus predisposes to higher risk of torsion
Even after successful testicular salvage,
testicular atrophy has been reported in almost 50%
of patients. Those patients who presented with pain
for more than 1 day were more likely to develop
testicular atrophy. No testes survived in a patient
who presented with pain for more than 3 days.54
Other causes of acute scrotum include torsion
of the testicular appendage (hydatid of Morgagni),
epididymo-orchitis, idiopathic scrotal oedema,
incarcerated hernia, or Henoch-Schönlein purpura.
Torsion of the testicular appendage presents in a
very similar manner to torsion of the testes (Fig 4).
The patient also complains of intense scrotal pain.
A firm-to-hard pea-sized lesion may be palpable
at the head of the epididymis. The classic ‘blue dot
sign’ may also be appreciated. If testicular torsion
can confidently be excluded, treatment can be by
observation or simple excision of the testicular
appendage. Epididymo-orchitis is a form of urinary
tract infection. In small children or early adolescence,
it may be a presentation of congenital anatomical
urinary tract anomalies.55
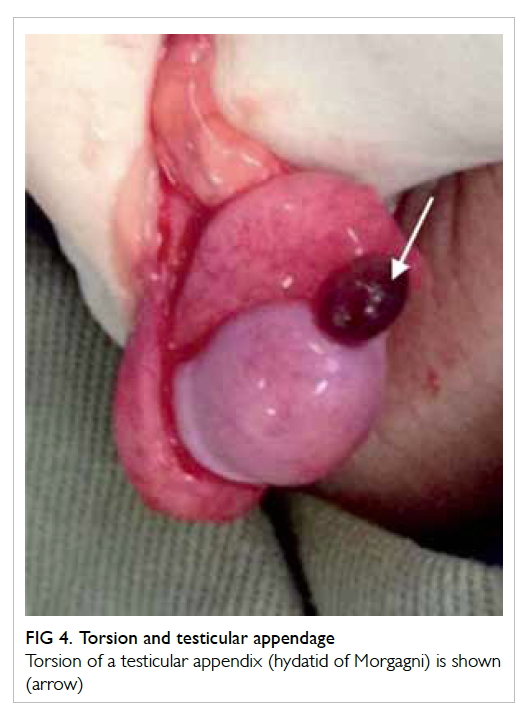
Figure 4. Torsion and testicular appendage
Torsion of a testicular appendix (hydatid of Morgagni) is shown (arrow)
Overall we advise general practitioners to seek
urgent referral to a specialist without any delay if
a patient presents with acute scrotum. Operative
exploration should not be delayed by investigations
whenever there is a suspicion of testicular torsion.
Varicocoele
Varicocoele is an abnormal venous dilatation and/or tortuosity of the pampiniform plexus in the
cord. It may present as ‘bags of worms’ clinically
and is identified in 15% of healthy men and up to
35% of men with primary infertility.56 It appears in
adolescent boys, with 7.8% in boys aged 11 to 14
years and 14.1% aged 15 to 19 years.57
Potential clinical problems
Patients may not have any symptoms and can present
late with subfertility. Increased temperature around
the testis is believed to affect spermatogenesis and
endocrine function of the testis.
The World Health Organization stated that
varicocoele was clearly associated with impaired
testicular function and infertility.58 They investigated
34 subfertility centres with 9034 men and identified
varicocoele in 25.4% of men with abnormal semen
compared with 11.7% of men with normal semen.
Comparison of semen analysis in young men
(17-19 years old) with and without varicocoele revealed
significant differences in total and progressive sperm
motility and vitality, which were lower in boys with
varicocoele, and number of normal sperm forms.59
It was concluded that spermatogenesis could be
affected even at this young age.
Clinical evaluation
Clinical (palpable) varicocoele is detected and
graded based on physical examination: a grade 1
varicocoele is one that is only palpable during the
Valsalva manoeuvre; a grade 2 varicocoele is easily
palpable with or without Valsalva but is not visible;
grade 3 refers to a large varicocoele that is easily
palpable and detected on visual inspection of the
scrotum. Size of both testes should be examined and
measured with an orchidometer. Size discrepancy or
testicular atrophy may indicate the need for further
surgical management. Apart from examination
of the genitalia, abdominal examination is also
needed. Any abdominal mass/tumour that causes
compression or obstruction of the renal veins
or gonadal vessels can present with varicocoele.
Ultrasonography of the abdomen and scrotum may
be needed if an abdominal mass is suspected. Semen
analysis may not be practical or necessary in these
child or adolescent patients.
Indications for surgical management
Catch-up growth of the testis after varicocoele
operation has been demonstrated.60 61 Nonetheless the same has also been demonstrated in varicocoele
patients managed conservatively.62 Guidelines or
recommendations have been suggested by different
professional bodies including the AUA, American
Society for Reproductive Medicine, European
Association of Urology, and European Society of
Paediatric Urology (ESPU). These guidelines are
mainly directed to adult patients and there is no
consensus on the indications for surgery. A systematic
review tried to collaborate the opinions from all the
four professional bodies.63 Some agreed indications
include varicocoele with reduced ipsilateral testicular
volume, bilateral varicocoeles, and varicocoele with
pathological semen quality. Symptomatic varicocoele
was suggested as an indication by ESPU but not the
other three associations.
Surgical options and complications
Different open surgical methods have been
described: subinguinal, inguinal (Palomo), and
microsurgical subinguinal approach. All involve
ligation of testicular vessels, with or without the
testicular artery. Laparoscopic high ligation of the
vessels has become popular in this era. Lymphatic or
artery-sparing techniques are preferred by some as
they decrease the postoperative hydrocoele rate.64 65 Radiological embolisation of the testicular vein is
another option.
Recurrence rates vary for different surgical
procedures but are reported to be ranging from 0%
to 35%. The rate of recurrence following various
surgical methods have been quoted as: laparoscopic
(1.2%), non-magnified inguinal (1.3%), open
retroperitoneal (9.3%), microsurgical subinguinal
(0.9%-2.5%), retrograde sclerotherapy (3.6%-8.6%),
and antegrade sclerotherapy (9%).66
Conclusion
Inguinoscrotal pathologies encompass a large
disease spectrum in children. They may have very
similar clinical presentations. Careful history taking
and accurate clinical examination are crucial in
achieving a correct diagnosis resulting in proper and
timely management. There are some points to note:
- A properly performed clinical examination is important in establishing the diagnosis of hydrocoele, inguinal hernia, and undescended testes.
- Clinically evident paediatric inguinal hernia should be offered surgical repair when the patient’s general condition permits general anaesthesia, no matter the age.
- Infantile hydrocoele should be managed with an expectant approach. Parents should be taught to observe for associated inguinal hernia.
- Undescended testis is associated with clinical problems of subfertility, increased risk of testicular torsion, and increased risk of testicular malignancy. Early orchidopexy (<18 months of age) should be advised.
- Testicular torsion is a surgical emergency. Urgent referral to a specialist should be made. Insistent investigations (USG) may confuse the clinical diagnosis and delay surgical management.
- Varicocoele is associated with testicular atrophy and subfertility. Intra-abdominal mass should be excluded. Catch-up growth of the affected testis has been noted in some studies after operation.
References
1. Chan IH, Wong KK. Common urological problems in
children: prepuce, phimosis, and buried penis. Hong Kong
Med J 2016;22:263-9. Crossref
2. Chang SJ, Chen JY, Hsu CK, Chuang FC, Yang SS. The
incidence of inguinal hernia and associated risk factors of
incarceration in pediatric inguinal hernia: a nation-wide
longitudinal population-based study. Hernia 2016;20:559-63. Crossref
3. Kumar VH, Clive J, Rosenkrantz TS, Bourque MD, Hussain
N. Inguinal hernia in preterm infants (≤32-week
gestation). Pediatr Surg Int 2002;18:147-52. Crossref
4. Ein SH, Njere I, Ein A. Six thousand three hundred sixty-one
pediatric inguinal hernias: a 35-year review. J Pediatr
Surg 2006;41:980-6. Crossref
5. Naji H, Ingolfsson I, Isacson D, Svensson JF. Decision
making in the management of hydroceles in infants and
children. Eur J Pediatr 2012;171:807-10. Crossref
6. Koski ME, Makari JH, Adams MC, et al. Infant
communicating hydroceles—do they need immediate
repair or might some clinically resolve? J Pediatr Surg
2010;45:590-3. Crossref
7. Christensen T, Cartwright PC, Devries C, Snow BW. New
onset of hydroceles in boys over 1 year of age. Int J Urol
2006;13:1425-7. Crossref
8. Toki A, Watanabe Y, Sasaki K, et al. Ultrasonographic
diagnosis for potential contralateral inguinal hernia in
children. J Pediatr Surg 2003;38:224-6. Crossref
9. Chen KC, Chu CC, Chou TY, Wu CJ. Ultrasonography for
inguinal hernias in boys. J Pediatr Surg 1998;33:1784-7. Crossref
10. Kaneda H, Furuya T, Sugito K, et al. Preoperative
ultrasonographic evaluation of the contralateral patent
processus vaginalis at the level of the internal inguinal ring
is useful for predicting contralateral inguinal hernias in
children: a prospective analysis. Hernia 2015;19:595-8. Crossref
11. Gholoum S, Baird R, Laberge JM, Puligandla PS.
Incarceration rates in pediatric inguinal hernia: do not
trust the coding. J Pediatr Surg 2010;45:1007-11. Crossref
12. Zamakhshary M, To T, Guan J, Langer JC. Risk of
incarceration of inguinal hernia among infants and young
children awaiting elective surgery. CMAJ 2008;179:1001-5. Crossref
13. Lautz TB, Raval MV, Reynolds M. Does timing matter?
A national perspective on the risk of incarceration in
premature neonates with inguinal hernia. J Pediatr
2011;158:573-7. Crossref
14. Pini Prato A, Rossi V, Mosconi M, et al. Inguinal hernia
in neonates and ex-preterm: complications, timing and
need for routine contralateral exploration. Pediatr Surg Int
2015;31:131-6. Crossref
15. Lee SL, Gleason JM, Sydorak RM. A critical review of
premature infants with inguinal hernias: optimal timing
of repair, incarceration risk, and postoperative apnea. J
Pediatr Surg 2011;46:217-20. Crossref
16. Chan IH, Lau CT, Chung PH, et al. Laparoscopic inguinal
hernia repair in premature neonates: is it safe? Pediatr Surg
Int 2013;29:327-30. Crossref
17. Esposito C, St Peter SD, Escolino M, et al. Laparoscopic
versus open inguinal hernia repair in pediatric patients:
a systematic review. J Laparoendosc Adv Surg Tech A
2014;24:811-8. Crossref
18. Schier F, Montupet P, Esposito C. Laparoscopic inguinal
herniorrhaphy in children: a three-center experience with
933 repairs. J Pediatr Surg 2002;37:395-7. Crossref
19. Saka R, Okuyama H, Sasaki T, Nose S, Yoneyama C, Tsukada
R. Laparoscopic treatment of pediatric hydrocele and the
evaluation of the internal inguinal ring. J Laparoendosc
Adv Surg Tech A 2014;24:664-8. Crossref
20. Feng S, Zhao L, Liao Z, Chen X. Open versus laparoscopic
inguinal herniotomy in children: a systematic review and
meta-analysis focusing on postoperative complications.
Surg Laparosc Endosc Percutan Tech 2015;25:275-80. Crossref
21. Chan KL, Hui WC, Tam PK. Prospective randomized
single-center, single-blind comparison of laparoscopic
vs open repair of pediatric inguinal hernia. Surg Endosc
2005;19:927-32. Crossref
22. Celebi S, Uysal AI, Inal FY, Yildiz A. A single-blinded,
randomized comparison of laparoscopic versus open
bilateral hernia repair in boys. J Laparoendosc Adv Surg
Tech A 2014;24:117-21. Crossref
23. Steven M, Carson P, Bell S, Ward R, McHoney M. Simple
purse string laparoscopic versus open hernia repair. J
Laparoendosc Adv Surg Tech A 2016;26:144-7. Crossref
24. Wang Z, Xu L, Chen Z, Yao C, Su Z. Modified single-port
minilaparoscopic extraperitoneal repair for pediatric
hydrocele: a single-center experience with 279 surgeries.
World J Urol 2014;32:1613-8. Crossref
25. Toufique Ehsan M, Ng AT, Chung PH, Chan KL, Wong
KK, Tam PK. Laparoscopic hernioplasties in children: the
implication on contralateral groin exploration for unilateral
inguinal hernias. Pediatr Surg Int 2009;25:759-62. Crossref
26. Wenk K, Sick B, Sasse T, Moehrlen U, Meuli M, Vuille-dit-Bille RN. Incidence of metachronous contralateral
inguinal hernias in children following unilateral repair—A meta-analysis of prospective studies. J Pediatr Surg
2015;50:2147-54. Crossref
27. Hoshino M, Sugito K, Kawashima H, et al. Prediction of
contralateral inguinal hernias in children: a prospective
study of 357 unilateral inguinal hernias. Hernia
2014;18:333-7. Crossref
28. Miltenburg DM, Nuchtern JG, Jaksic T, Kozinetiz C, Brandt
ML. Laparoscopic evaluation of the pediatric inguinal
hernia—a meta-analysis. J Pediatr Surg 1998;33:874-9. Crossref
29. Kokorowski PJ, Wang HH, Routh JC, Hubert KC, Nelson
CP. Evaluation of the contralateral inguinal ring in clinically
unilateral inguinal hernia: a systematic review and meta-analysis.
Hernia 2014;18:311-24. Crossref
30. Agopian AJ, Langlois PH, Ramakrishnan A, Canfield MA.
Epidemiologic features of male genital malformations and
subtypes in Texas. Am J Med Genet A 2014;164A:943-9. Crossref
31. Rusnack SL, Wu HY, Huff DS, et al. The ascending testis
and the testis undescended since birth share the same
histopathology. J Urol 2002;168:2590-1. Crossref
32. Kullendorff CM, Hederström E, Forsberg L. Preoperative
ultrasonography of the undescended testis. Scand J Urol
Nephrol 1985;19:13-5. Crossref
33. Nijs SM, Eijsbouts SW, Madern GC, Leyman PM, Lequin
MH, Hazebroek FW. Nonpalpable testes: is there a
relationship between ultrasonographic and operative
findings? Pediatr Radiol 2007;37:374-9. Crossref
34. Shoukry M, Pojak K, Choudhry MS. Cryptorchidism
and the value of ultrasonography. Ann R Coll Surg Engl
2015;97:56-8. Crossref
35. Lam WW, Tam PK, Ai VH, et al. Gadolinium-infusion
magnetic resonance angiogram: a new, noninvasive, and
accurate method of preoperative localization of impalpable
undescended testes. J Pediatr Surg 1998;33:123-6. Crossref
36. Siemer S, Humke U, Uder M, Hildebrandt U, Karadiakos
N, Ziegler M. Diagnosis of nonpalpable testes in
childhood: comparison of magnetic resonance imaging
and laparoscopy in a prospective study. Eur J Pediatr Surg
2000;10:114-8. Crossref
37. Tasian GE, Copp HL, Baskin LS. Diagnostic imaging in
cryptorchidism: utility, indications, and effectiveness. J
Pediatr Surg 2011;46:2406-13. Crossref
38. Kolon TF, Herndon CD, Baker LA, et al. Evaluation and
treatment of cryptorchidism: AUA guideline. J Urol
2014;192:337-45. Crossref
39. Mengel W, Hienz HA, Sippe WG 2nd, Hecker WC. Studies
on cryptorchidism: a comparison of histological findings
in the germinative epithelium before and after the second
year of life. J Pediatr Surg 1974;9:445-50. Crossref
40. Lee PA, Coughlin MT. Fertility after bilateral
cryptorchidism. Evaluation by paternity, hormone, and
semen data. Horm Res 2001;55:28-32.
41. Alagaratnam S, Nathaniel C, Cuckow P, et al. Testicular
outcome following laparoscopic second stage Fowler-Stephens orchidopexy. J Pediatr Urol 2014;10:186-92. Crossref
42. Cortes D, Thorup JM, Beck BL. Quantitative histology of
germ cells in the undescended testes of human fetuses,
neonates and infants. J Urol 1995;154:1188-92. Crossref
43. Hanerhoff BL, Welliver C. Does early orchidopexy improve
fertility? Transl Androl Urol 2014;3:370-6.
44. Dieckmann KP, Pichlmeier U. Clinical epidemiology of
testicular germ cell tumors. World J Urol 2004;22:2-14. Crossref
45. Pettersson A, Richiardi L, Nordenskjold A, Kaijser M,
Akre O. Age at surgery for undescended testis and risk of
testicular cancer. N Engl J Med 2007;356:1835-41. Crossref
46. Hutson JM, Li R, Southwell BR, Petersen BL, Thorup J,
Cortes D. Germ cell development in the postnatal testis:
the key to prevent malignancy in cryptorchidism? Front
Endocrinol (Lausanne) 2013;3:176. Crossref
47. Lee SM, Huh JS, Baek M, et al. A nationwide epidemiological
study of testicular torsion in Korea. J Korean Med Sci
2014;29:1684-7. Crossref
48. Yu Y, Zhang F, An Q, Wang L, Li C, Xu Z. Scrotal
exploration for testicular torsion and testicular appendage
torsion: emergency and reality. Iran J Pediatr 2015;25:e248. Crossref
49. Sheth KR, Keays M, Grimsby GM, et al. Diagnosing
testicular torsion before urological consultation
and imaging: validation of the TWIST score. J Urol
2016;195:1870-6. Crossref
50. Burgu B, Aydogdu O, Huang R, Soygur T, Yaman O, Baker
L. Pilot feasibility study of transscrotal near infrared
spectroscopy in the evaluation of adult acute scrotum. J
Urol 2013;190:124-9. Crossref
51. Kalfa N, Veyrac C, Lopez M, et al. Multicenter assessment
of ultrasound of the spermatic cord in children with acute
scrotum. J Urol 2007;177:297-301. Crossref
52. Lam WW, Yap TL, Jacobsen AS, Teo HJ. Colour Doppler
ultrasonography replacing surgical exploration for acute
scrotum: myth or reality? Pediatr Radiol 2005;35:597-600. Crossref
53. Selbst SM, Friedman MJ, Singh SB. Epidemiology and
etiology of malpractice lawsuits involving children in US
emergency departments and urgent care centers. Pediatr
Emerg Care 2005;21:165-9.
54. Lian BS, Ong CC, Chiang LW, Rai R, Nah SA. Factors
predicting testicular atrophy after testicular salvage
following torsion. Eur J Pediatr Surg 2016;26:17-21. Crossref
55. Redshaw JD, Tran TL, Wallis MC, deVries CR. Epididymitis:
a 21-year retrospective review of presentations to an
outpatient urology clinic. J Urol 2014;192:1203-7. Crossref
56. Alsaikhan B, Alrabeeah K, Delouya G, Zini A. Epidemiology
of varicocele. Asian J Androl 2016;18:179-81. Crossref
57. Akbay E, Cayan S, Doruk E, Duce MN, Bozlu M. The
prevalence of varicocele and varicocele-related testicular
atrophy in Turkish children and adolescents. BJU Int
2000;86:490-3. Crossref
58. World Health Organization. The influence of varicocele on
parameters of fertility in a large group of men presenting to
infertility clinics. Fertil Steril 1992;57:1289-93. Crossref
59. Paduch DA, Niedzielski J. Semen analysis in young men
with varicocele: preliminary study. J Urol 1996;156(2 Pt 2):788-90. Crossref
60. Paduch DA, Niedzielski J. Repair versus observation
in adolescent varicocele: a prospective study. J Urol
1997;158(3 Pt 2):1128-32. Crossref
61. Kass EJ, Belman AB. Reversal of testicular growth failure
by varicocele ligation. J Urol 1987;137:475-6.
62. Preston MA, Carnat T, Flood T, Gaboury I, Leonard MP.
Conservative management of adolescent varicoceles: a
retrospective review. Urology 2008;72:77-80. Crossref
63. Roque M, Esteves SC. A systematic review of clinical
practice guidelines and best practice statements for the
diagnosis and management of varicocele in children and
adolescents. Asian J Androl 2016;18:262-8. Crossref
64. Esposito C, Valla JS, Najmaldin A, et al. Incidence and
management of hydrocele following varicocele surgery in
children. J Urol 2004;171:1271-3. Crossref
65. Chiarenza SF, Giurin I, Costa L, et al. Blue patent
lymphography prevents hydrocele after laparoscopic
varicocelectomy: 10 years of experience. J Laparoendosc
Adv Surg Tech A 2012;22:930-3. Crossref
66. Rotker K, Sigman M. Recurrent varicocele. Asian J Androl
2016;18:229-33. Crossref