Hong Kong Med J 2017 Jun;23(3):251–7 | Epub 9 May 2017
DOI: 10.12809/hkmj164972
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Neoadjuvant chemotherapy increases rates of
breast-conserving surgery in early operable
breast cancer
Vivian CM Man, MB, BS, MRCS1;
Polly SY Cheung, FCSHK, FHKAM (Surgery)2
1 Department of Surgery, Queen Mary Hospital, Pokfulam, Hong Kong
2 Breast Care Centre, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
Corresponding author: Dr Polly SY Cheung (pollyc@pca.hk)
Abstract
Introduction: Neoadjuvant chemotherapy is
commonly used in stage III breast cancer for disease
down-staging. Its use has now been extended to
early breast cancer to increase the rate of breast-conserving
surgery. This study aimed to evaluate the
effectiveness of neoadjuvant chemotherapy in early
operable cancers.
Methods: A retrospective study was carried out
at the Hong Kong Sanatorium & Hospital of 102
patients with stage I to III primary breast cancer. All
patients who underwent neoadjuvant chemotherapy
followed by definitive breast surgery between January
2004 and July 2013 were included. Their pathological
complete response and rate of breast-conserving
surgery were studied. Data were compared using Chi
squared test and Student’s t test.
Results: After neoadjuvant chemotherapy, 23% of
patients achieved a pathological complete response,
of whom 80% had human epidermal growth factor
receptor 2 (HER2)–positive disease or triple-negative
disease. Hormonal receptor negativity
was associated with a higher pathological complete
response rate (P<0.05) that was in turn associated
with a higher likelihood of breast-conserving
surgery (P=0.028). Patients with stage II disease
were more likely to convert from mastectomy to
breast-conserving surgery following neoadjuvant
chemotherapy.
Conclusions: Neoadjuvant chemotherapy is a useful
treatment to downsize tumour in early breast cancer,
thereby increasing the rate of breast-conserving
surgery. It is especially effective in patients with
HER2-positive/oestrogen receptor–negative disease
or triple-negative disease.
New knowledge added by this study
- Neoadjuvant chemotherapy for breast cancer can downsize the tumour with a consequent higher rate of breast-conserving surgery, especially in patients with human epidermal growth factor receptor 2–positive/oestrogen receptor–negative disease or triple-negative disease.
- Neoadjuvant chemotherapy is a useful alternative in early breast cancer for women considering breast-conserving surgery.
Introduction
Breast cancer is the leading cancer affecting women
in Hong Kong, followed by colorectal and lung
malignancy.1 The number of new breast cancer cases
in Hong Kong has tripled since the 1990s and the
lifetime breast cancer risk in women is currently
one in 17.1 Among the 12 345 patients studied in the
cohort of the Hong Kong Breast Cancer Registry
from 2008 to February 2014, 55% were diagnosed
with stage II disease or above and 5% of the cohort
received neoadjuvant chemotherapy.1 This cohort of
patients is estimated to cover approximately 40% of
patients reported by the Hong Kong Cancer Registry
of the Hospital Authority.
Neoadjuvant chemotherapy has played an
increasing role in the management of breast cancer
over the last few decades. It was considered at least as
effective as postoperative chemotherapy in terms of
disease-free survival (DFS) and overall survival (OS)
in the National Surgical Adjuvant Breast and Bowel
Project B-18 trial.2 Neoadjuvant chemotherapy
allows disease down-staging, thus increasing
the probability of successful breast-conserving
therapy.3 4 In addition, tumour response can be monitored ‘in vivo’ and chemotherapeutic regimens
modified accordingly. Studies have also suggested
its role in disease prognostication, especially the
presence of pathological complete response in highly
proliferative tumours.3
The aims of this study were to identify
possible tumour characteristics that may benefit
from neoadjuvant chemotherapy and to evaluate
the effectiveness of neoadjuvant chemotherapy in
increasing the rates of breast-conserving surgery in
early operable breast cancer.
Methods
This was a retrospective study carried out at the
Hong Kong Sanatorium & Hospital and approved
by the hospital research committee in September
2013; the requirement of patient informed consent
was waived because of its retrospective nature. This study was done in accordance with the principles outlined in the Declaration of Helsinki. All patients with breast cancer who underwent
neoadjuvant chemotherapy followed by definitive
breast surgery from January 2004 to July 2013 were
recruited. The choice of definitive breast surgery,
either breast-conserving surgery or mastectomy, was
determined by an experienced breast surgeon (CSY)
and based on the oncological and cosmetic outcome
of each patient. Patients who presented with distant
metastases and those who underwent neoadjuvant
hormonal therapy were excluded. Those who had
stage IV disease were also excluded.
Patient records were retrieved from the breast
cancer database at the Hong Kong Sanatorium &
Hospital and out-patient clinic of one of the authors
(CSY) by an independent research assistant who was
blinded to the study hypothesis and outcome. All
recruited patients had their surgery performed by
CSY, who is one of the breast surgery specialists at the
hospital. Patients were followed up perioperatively in
the out-patient clinic of CSY. Patient demographics,
pre-chemotherapy and post-chemotherapy disease
staging, tumour characteristics, positron emission
tomography–computed tomography findings, and
prescribed chemotherapeutic agents were evaluated.
Effectiveness of neoadjuvant chemotherapy
was assessed in two ways: presence of pathological
complete response and the feasibility of breast-conserving
surgery after chemotherapy. Intrinsic
tumour characteristics that influenced treatment
response were analysed. Tumour size, nodal
status, tumour grade, hormonal receptor status,
human epidermal growth factor receptor 2 (HER2)
receptor status, Ki67 level, and chemotherapeutic
agents used were the independent variables in
this study. Statistical analysis was performed with
SPSS (Windows version 20.0; IBM Corp, Armonk
[NY], United States) and a P value of <0.05 was considered
statistically significant. Univariate analysis was
performed with Student’s t test and Chi squared test
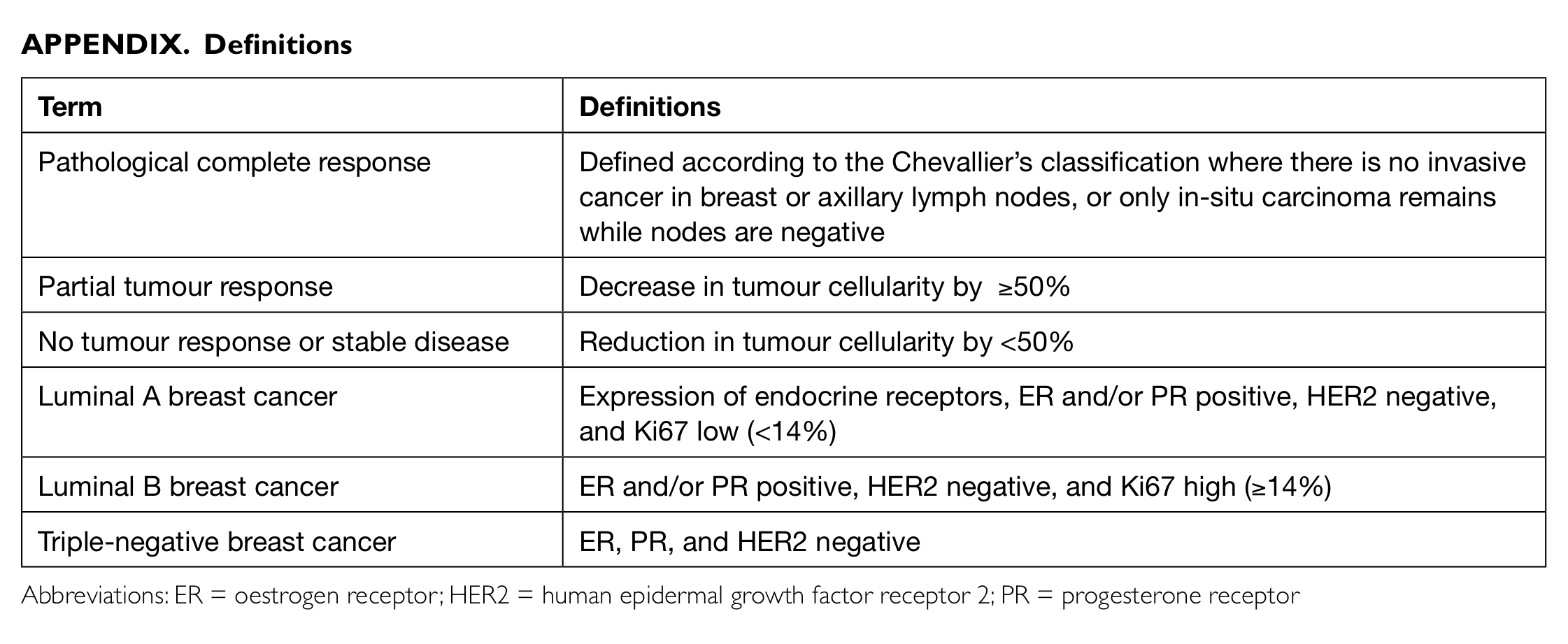
where appropriate. Definitions of various terms used
in this study are listed in the Appendix.
Results
Patient’s characteristics
From January 2004 to July 2013, 2156 patients
underwent breast cancer surgery at Hong Kong
Sanatorium & Hospital by an experienced breast
surgeon (CSY). Stage II or III disease was diagnosed
in 48% and 105 (5%) of all patients underwent
neoadjuvant chemotherapy. Three patients were
excluded due to significant missing data. A total of
102 were ultimately recruited.
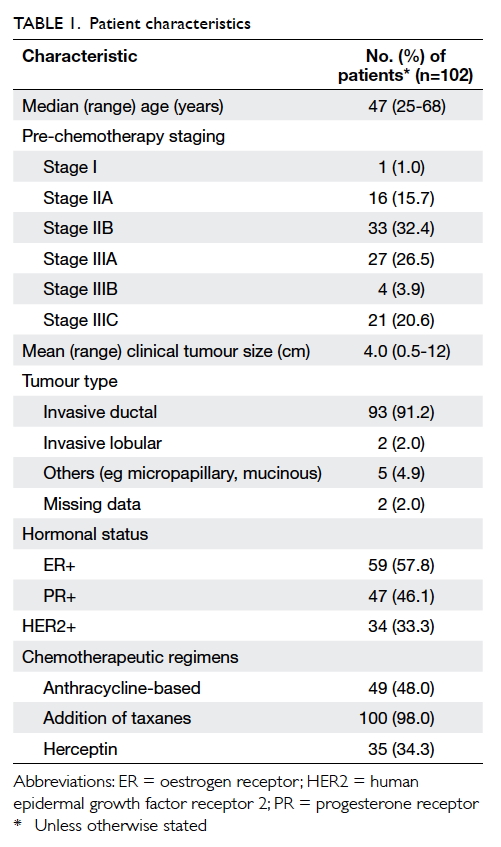
Characteristics of patients are summarised in
Table 1. Almost all recruited patients had stage II or
III disease before commencement of neoadjuvant
chemotherapy. Invasive ductal carcinoma constituted
more than 90% of all diagnosed breast cancers.
One quarter of the recruited patients had triple-negative
disease and one third had HER2-positive
disease. In our study, 48 patients received sequential
anthracycline-taxane-based chemotherapy and 52
received taxane-based chemotherapy only. One
patient received four cycles of anthracycline-based
chemotherapy only and another patient received
gemcitabine and vinorelbine. There were 35 patients
prescribed herceptin as part of their neoadjuvant
chemotherapy.
Tumour size
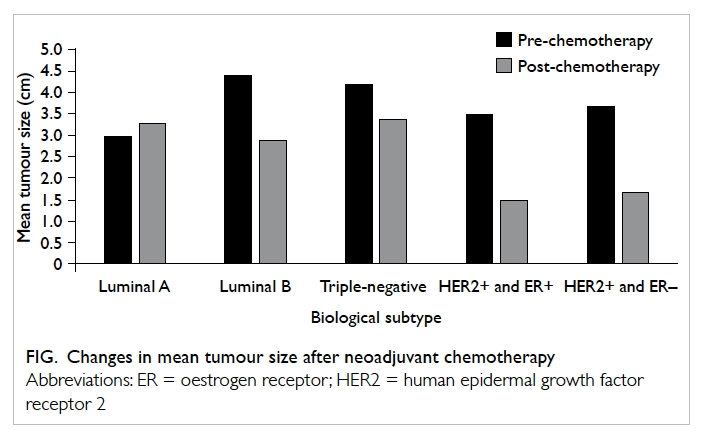
After commencement of neoadjuvant chemotherapy,
the mean tumour size reduced by more than half,
from 4 cm to <2 cm. The HER2-positive group
showed a relatively greater tumour size reduction to
almost 75% (Fig). On the contrary, the mean tumour
size in luminal A breast cancers remained relatively
static despite neoadjuvant chemotherapy.
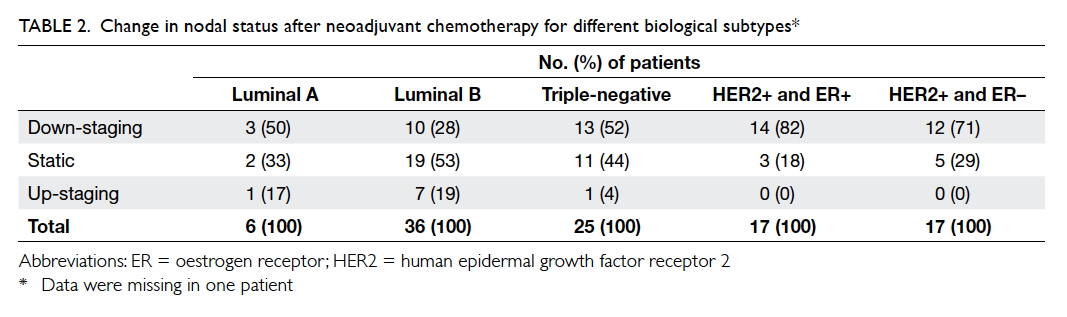
Nodal status
More than 80% of the studied population presented
with N1 disease or above. After neoadjuvant
chemotherapy, the proportion of patients with N0
disease increased from 15% to 43%. Just over half
(51%) of the studied group achieved a reduction in
nodal staging following neoadjuvant chemotherapy
(Table 2). Similarly, patients with HER2-positive
disease or triple-negative disease showed a more
significant nodal down-staging after chemotherapy
(P=0.007).
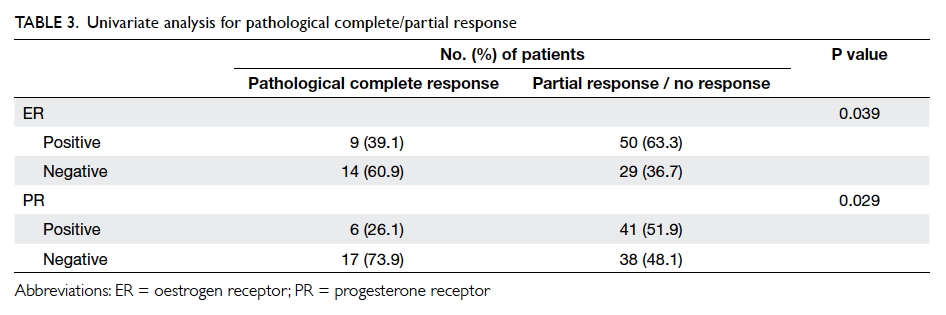
Pathological complete response
Effectiveness of neoadjuvant chemotherapy was
determined by the presence of pathological complete
response. Pathological complete response was
achieved by 23% (n=23) of patients and 60% had a
partial tumour response. Among these 23 patients,
18 (78%) had triple-negative disease or HER2-positive
disease; oestrogen receptor (ER) status was negative in 14
patients and progesterone receptor (PR) status was
negative in 17 patients. Four patients with triple-negative
disease or HER2-positive disease had nodal
down-staging from N2 or N3. Breast cancers with
negative ER status (P=0.039) or negative PR status
(P=0.029) had a higher chance of pathological
complete response in univariate analysis (Table 3). Other factors including the Ki67 value, tumour grade, and the prescribed chemotherapeutic regimen
did not appear to influence the rate of pathological
complete response.
Does pathological complete response predict
likelihood of breast-conserving surgery?
Pathological complete response was achieved by
23 patients, of whom 15 (65%) underwent breast-conserving
surgery; whereas only 39% of those with
partial or no response had breast conservation.
Univariate analysis revealed that patients who
had pathological complete response following
neoadjuvant chemotherapy had a higher chance
of successful breast-conserving surgery (P=0.028).
Mastectomy was required in eight patients
despite a pathological complete response due to
pre-chemotherapy large tumour size, extensive
carcinoma in situ, or central location of the tumour.
Among those with pathological complete
response, 11 (79%) of 14 stage II patients and four
(50%) of eight stage III patients eventually had
breast-conserving surgery. Patients with stage II
disease showed a trend for more breast-conserving
surgery after neoadjuvant therapy although this was
not statistically significant (P=0.15).
Feasibility of breast-conserving surgery
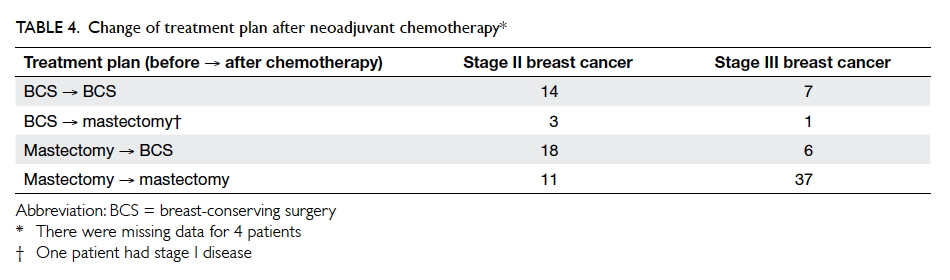
The change of treatment plan after neoadjuvant
chemotherapy is shown in Table 4. Before the
commencement of neoadjuvant chemotherapy,
one quarter of patients (n=26) were scheduled for
breast-conserving surgery and three quarters for
mastectomy (n=72). After chemotherapy, one third
of those scheduled for mastectomy (24 patients)
changed to breast-conserving surgery. The number
of breast-conserving surgeries increased from 26 to
45, with an increase of 19% of all patients.
After neoadjuvant chemotherapy, 24
patients with planned mastectomy underwent
breast-conserving surgery and 48 continued with
mastectomy. On the other hand, five patients with
planned breast-conserving surgery underwent
mastectomy after neoadjuvant chemotherapy as a
result of disease progression or patient’s preference
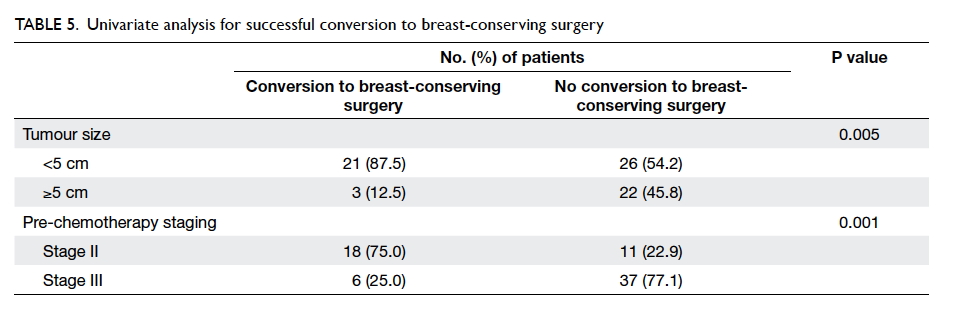
(Table 4). Among the 24 patients with successful
conversion from mastectomy to breast-conserving
surgery, 21 had tumour size of <5 cm and 18 had
stage II disease. Pre-chemotherapy disease staging
(P=0.001) and tumour size (P=0.005) were important
factors that determined successful conversion to
breast-conserving treatment in univariate analysis
(Table 5). The breast-conserving surgery to
mastectomy ratio in patients with stage II disease
was 32:14 patients, ie 2.3 to 1. On the contrary, 13
patients with stage III disease underwent breast-conserving
surgery and 38 underwent mastectomy,
ie a ratio of 1:3 for stage III disease. Among those
who underwent breast-conserving surgery, 93%
had tumour size of <5 cm. The corresponding
proportion in those who underwent mastectomy
was 60%. Tumours with size of <5 cm were more
likely to be amenable to successful breast-conserving
surgery. Other factors including the Ki67 index,
tumour grade, and the prescribed chemotherapeutic
regimen did not appear to influence the rate of
breast-conserving surgery.
Discussion
Neoadjuvant chemotherapy was introduced in the
1980s as standard treatment for locally advanced
breast cancers, defined as stage III disease (and
a subset of stage IIB disease).5 6 In the last decade, the use of neoadjuvant chemotherapy has been
extended to patients with early operable primary
breast cancers with promising results. The aim of this
study was to evaluate the response of early operable
breast cancers to neoadjuvant chemotherapy and the
predictors of good responders.
Efficacy of neoadjuvant chemotherapy
and adjuvant chemotherapy has been carefully
evaluated in a number of publications. A prospective
randomised trial of the Austrian Breast and
Colorectal Cancer Study Group (ABCSG-07)
recruited 423 breast cancer patients with stage II
to III disease and randomised them to neoadjuvant
CMF (cyclophosphamide, methotrexate, fluorouracil)
or adjuvant CMF.7 The adjuvant CMF group
showed superior results in recurrence-free survival,
although the OS was similar. Nonetheless, this
‘old’ chemotherapeutic regimen has now mostly
been replaced by anthracycline-taxane-based
chemotherapy.
With the emergence of newer chemotherapeutic
agents, the National Surgical Adjuvant Breast
and Bowel Project B-18 published the
largest prospective study with the use of AC
(doxorubicin and cyclophosphamide).2 Neoadjuvant
chemotherapy was at least as effective as adjuvant
chemotherapy after a 9-year follow-up. A similar
study by the European Organization for Research
and Treatment of Cancer published an
update after 10 years of follow-up.8 There was no
difference in OS or relapses between patients with
preoperative and postoperative chemotherapy. Those
with neoadjuvant chemotherapy had more breast-conserving
treatment. Further subgroup analysis
showed a comparable loco-regional recurrence
rate between patients initially allocated to receive
breast-conserving treatment and those who did after
tumour downsizing.8
Meta-analysis of 14 randomised controlled
trials that included patients with mostly stage II or
III disease showed similar results.9 The loco-regional
recurrence rate was also comparable between the two
groups. There was a statistically significant decrease
in mastectomy rate that favoured neoadjuvant
chemotherapy.
In our study, patients with stage II to III
disease were further stratified in the subgroup
analysis. Stage II disease was considered early
operable breast cancer while patients with stage
III disease represented those with locally advanced
disease. This stratification was in line with the MD
Anderson Cancer Centre Classification of locally
advanced disease.5 Patients with early operable
breast cancer showed comparatively greater benefits
following neoadjuvant chemotherapy in terms of the
rate of pathological complete response and breast-conserving
surgery.
Pathological complete response has been
one of the commonly used study endpoints in
publications. It has been suggested to correlate
with a better long-term outcome. Meta-analysis by
Mieog et al9 found improved OS in patients with
pathological complete response. The definition
of pathological complete response varies from
institution to institution, however. In our study,
we adopted the definition recognised by the MD
Anderson Cancer Centre and in the ABCSG study,10
in which there should be no invasive residual
disease in breast or nodes although non-invasive
breast residuals are allowed. Studies have shown
no difference in DFS or OS between patients with
ypT0ypN0 and ypTisypN0 tumours.3 11
Of note, the rate of pathological complete
response appears to be different among various
intrinsic types of breast cancer.12 In 2005, Rouzier
et al13 stratified breast cancer patients into four
molecular classes using the genetic profile from a
fine-needle aspiration specimen. Patients with basal-like
and c-erbB2+ breast cancers had the highest rate
of pathological complete response. Age younger than
50 years and ER-negative status were independent
variables with a higher likelihood of pathological
complete response. In our study, core biopsies with
immunohistochemical staining and proliferation
index were used to classify patients into luminal
A, luminal B, triple-negative, or HER2-positive
subgroups and also showed consistent findings.
Carey et al14 described the phenomenon of
triple-negative paradox in 2007. Basal-like and
HER2+/ER- subtypes were more chemosensitive
than their luminal counterparts. They were more
likely to have pathological complete response
but those with residual disease also had a higher
likelihood of relapse and worse outcome. The study
by the German Breast Group in 2012 highlighted
the impact of pathological complete response on
prognosis in different intrinsic subtypes of breast
cancer.10 Patients with ypT0N0 tumours had the
best DFS (P<0.001) and a trend of better OS. More
importantly, pathological complete response was
predictive of DFS and OS in highly aggressive
tumours only such as those with negative ER or
PR status. Patients with HER2-positive or triple-negative
tumours did better if they achieved
pathological complete response after neoadjuvant
chemotherapy. Residual disease in breast and nodes,
on the contrary, was associated with worse distant
DFS.15
Last but not the least, recent publications have
described possible changes in receptor status before
and after neoadjuvant chemotherapy although the
significance remains controversial.16 In our study,
change in ER status was evident in 10% of the study
group and that of HER2 in 50%.
The current study has several limitations.
First, this was a retrospective study and the
database in the earlier period was incomplete with
missing information. There were three patients
with significant missing information who were
excluded from this small study. Second, there may
be selection bias as patients chosen for neoadjuvant
chemotherapy were subject to surgeon assessment
and patient preference. This study represents the
experience of neoadjuvant chemotherapy by one
experienced breast surgery specialist in one private
hospital in Hong Kong. As such, the findings may
not apply to other breast cancer patients in public
hospitals or in other countries. Third, long-term
survival data are not included in the present study,
and significance of pathological complete response
is not known. Lastly, the number of cases in this
study was small, therefore further subgroup analysis
in patients with pathological complete response
or successful conversion to breast-conserving
surgery was not possible. It does not allow further
multivariate analysis for controlling potential
confounding factors. Future study in this area with
a larger sample size may be useful to guide patient
selection for systemic treatment of breast cancer in a
neoadjuvant setting.
Conclusions
Neoadjuvant chemotherapy has expanded
indications from treatment of locally advanced
breast cancers to render it operable, to downsizing
early operable breast cancers enabling breast-conserving
surgery. The current study has shown
an increased rate of breast-conserving surgery with
neoadjuvant chemotherapy, especially in the early
operable group. Negative hormonal status was an
independent variable that determined pathological
complete response.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Hong Kong Breast Cancer Registry Report No. 8. Hong
Kong Breast Cancer Foundation. Available from: http://www.hkbcf.org. Accessed Feb 2016.
2. Fisher B, Brown A, Mamounas E, et al. Effect of preoperative
chemotherapy on local-regional disease in women with
operable breast cancer: findings from National Surgical
Adjuvant Breast and Bowel Project B-18. J Clin Oncol
1997;15:2483-93. Crossref
3. Gampenrieder S, Rinnerthaler G, Greil R. Neoadjuvant
chemotherapy and targeted therapy in breast cancer: past,
present, and future. J Oncol 2013;2013:732047. Crossref
4. Thompson AM, Moulder-Thompson SL. Neoadjuvant
treatment of breast cancer. Ann Oncol 2012;23 Suppl
10:x231-6. Crossref
5. Giordano SH. Update on locally advanced breast cancer.
Oncologist 2003;8:521-30. Crossref
6. Alassas M, Chu Q, Burton G, Ampil F, Mizell J, Li BD.
Neoadjuvant chemotherapy in stage III breast cancer. Am
Surg 2005;71:487-92.
7. Taucher S, Steger GG, Jakesz R, et al. The potential risk
of neoadjuvant chemotherapy in breast cancer patients—results from a prospective randomized trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-07).
Breast Cancer Res Treat 2008;112:309-16. Crossref
8. van Nes JG, Putter H, Julien JP, et al. Preoperative
chemotherapy is safe in early breast cancer, even after 10
years of follow-up; clinical and translational results from the
EORTC trial 10902. Breast Cancer Res Treat 2009;115:101-13. Crossref
9. Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant
chemotherapy for operable breast cancer. Br J Surg
2007;94:1189-200. Crossref
10. von Minckwitz G, Untch M, Blohmer JU, et al. Definition
and impact of pathologic complete response on prognosis
after neoadjuvant chemotherapy in various intrinsic breast
cancer subtypes. J Clin Oncol 2012;30:1796-804. Crossref
11. Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal
carcinoma in situ in patients with complete eradication of
invasive breast cancer after neoadjuvant chemotherapy
does not adversely affect patient outcome. J Clin Oncol
2007;25:2650-5. Crossref
12. Bhargava R, Beriwai S, Dabbs DJ, et al.
Immunohistochemical surrogate markers of breast cancer
molecular classes predicts response to neoadjuvant
chemotherapy: a single institutional experience with 359
cases. Cancer 2010;116:1431-9. Crossref
13. Rouzier R, Perou CM, Symmans WF, et al. Breast cancer
molecular subtypes respond differently to preoperative
chemotherapy. Clin Cancer Res 2005;11:5678-85. Crossref
14. Carey LA, Dees EC, Sawyer L, et al. The triple negative
paradox: primary tumor chemosensitivity of breast cancer
subtypes. Clin Cancer Res 2007;13:2329-34. Crossref
15. Corben AD, Abi-Raad R, Popa I, et al. Pathologic response
and long-term follow-up in breast cancer patients treated
with neoadjuvant chemotherapy: a comparison between
classifications and their practical application. Arch Pathol
Lab Med 2013;137:1074-82. Crossref
16. Pedrini JL, Francalacci Savaris R, Casales Schorr M,
Cambruzi E, Grudzinski M, Zettler CG. The effect
of neoadjuvant chemotherapy on hormone receptor
status, HER2/neu and prolactin in breast cancer. Tumori
2011;97:704-10.