Hong Kong Med J 2017 Jun;23(3):246–50 | Epub 27 Jan 2017
DOI: 10.12809/hkmj164880
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Comparison of a commercial interferon-gamma
release assay and tuberculin skin test for the
detection of latent tuberculosis infection in Hong
Kong arthritis patients who are candidates for
biologic agents
H So, MSc, FHKAM (Medicine);
Carol SW Yuen, BNurs, MSc;
Ronald ML Yip, FHKCP, FHKAM (Medicine)
Department of Medicine and Geriatrics, Kwong Wah Hospital, Yaumatei,
Hong Kong
An earlier version of this paper was presented at the ASM of the Hong
Kong Society of Rheumatology held in Hong Kong on 22 November 2015.
Corresponding author: Dr H So (h99097668@hotmail.com)
Abstract
Introduction: It is universally agreed that screening
for latent tuberculosis infection prior to biologic
therapy is necessary, especially in endemic areas
such as Hong Kong. There are still, however,
controversies regarding how best to accomplish this
task. The tuberculin skin test has been the routine
screening tool for latent tuberculosis infection in
Hong Kong for the past decade although accuracy
is far from perfect, especially in patients who have
been vaccinated with Bacillus Calmette–Guérin,
who are immunocompromised, or who have atypical
mycobacterium infection. The new interferon-gamma
release assays have been shown to improve specificity
and probably sensitivity. This study aimed to
evaluate agreement between the interferon-gamma
release assay and the tuberculin skin test in the
diagnosis of latent tuberculosis infection in patients
with arthritic diseases scheduled to receive biologic
agents.
Methods: We reviewed 38 patients with rheumatoid
arthritis, psoriatic arthritis, or spondyloarthritis at a
local hospital in Hong Kong from August 2013 to April
2014. They were all considered candidates for biologic
agents. The patients underwent both the interferon-gamma
release assay (ASACIR.TB; A.TB) and the
tuberculin skin test simultaneously. Concurrent
medications were documented. Patients who tested
positive for either test (ie A.TB+ or TST+) were
prescribed treatment for latent tuberculosis if they
were to be given biologic agents. All patients were
followed up regularly for 1 year and the development
of active tuberculosis infection was evaluated.
Results: Based on an induration of 10 mm in diameter
as the cut-off value, 13 (34.2%) of 38 patients had a
positive tuberculin skin test. Of the 38 patients, 11
(28.9%) also had a positive interferon-gamma release
assay. The agreement between interferon-gamma
release assay and tuberculin skin test was 73.7%
(kappa=0.39). Six patients were TST+/A.TB– and four
were TST–/A.TB+. When positive tuberculin skin
test was defined as an induration of 5-mm diameter,
the agreement between the two tests improved with
a kappa value of 0.47. In that case, half of the patients
had a positive tuberculin skin test; among them,
nine were TST+/A.TB–. Only one was TST–/A.TB+. Subgroup analysis showed that the agreement
between both tests improved further (kappa=0.69) in
patients not taking a concurrent systemic steroid. For
patients prescribed systemic steroid, the agreement
was only slight with a kappa value of 0.066. Finally,
none of the 38 patients, of whom 32 had an exposure
to biologic agents, developed active tuberculosis
during the 1-year follow-up period.
Conclusion: In a tuberculosis-endemic population,
although 10-mm diameter induration is the usual
cut-off for a positive tuberculin skin test, the level
of agreement between the interferon-gamma release
assay and tuberculin skin test improved from fair to
moderate when the cut-off was lowered to 5 mm.
A dual testing strategy of tuberculin skin test and
interferon-gamma release assays appeared to be
effective and should be pursued especially in patients
who are on systemic steroid therapy. Nonetheless, the
issue of potential overtreatment is yet to be evaluated.
New knowledge added by this study
- In Hong Kong, a tuberculosis-endemic area, the level of agreement between tuberculin skin test (TST) and interferon-gamma release assay (IGRA) for detecting latent tuberculosis infection was only fair in arthritis patients scheduled to receive biologic therapy.
- Although 10 mm is the cut-off for positive TST according to the local guideline, the level of agreement between the two tests improved when a 5-mm cut-off was used.
- Dual testing strategy with TST and IGRA appeared to be effective and should be employed, especially in patients who are prescribed systemic steroid therapy.
Introduction
The advent of biologic agents has revolutionised
the treatment of patients with rheumatoid arthritis
(RA), psoriatic arthritis (PSA), and spondyloarthritis
(SPA). The outcome is now greatly improved. This,
however, comes at the price of a clear heightened risk
of active tuberculosis (TB) as a progression of latent
TB infection (LTBI).1 Therefore, it is universally
agreed that screening for LTBI prior to biologic
therapy is necessary, especially in endemic areas
such as Hong Kong.2 Unfortunately, there remains
controversy regarding how best to accomplish this
task.
The tuberculin skin test (TST) has been
the routine screening tool in Hong Kong for
the past decade.3 Its accuracy, however, is far
from perfect, especially in patients who have
been vaccinated with Bacillus Calmette–Guérin
(BCG), are immunocompromised, or have been
infected with atypical mycobacterium.4 Recently,
interferon-gamma release assays (IGRAs) that
measure interferon-gamma secretion in response to
Mycobacterium tuberculosis–specific antigens have
become available to detect LTBI. They have been
shown to offer improved specificity and probably
sensitivity.5 6 Other shortcomings of the TST, such as the need for return visits and reader variability, are
also overcome. One of the IGRAs, the ASACIR.TB
(A.TB; Haikou VTI Biological Institute, Hainan,
China), has shown encouraging results in a large-scale
clinical trial conducted in China and might be
more appropriate in Chinese populations.7
On the other hand, IGRAs are not flawless.
The rate of indeterminate results has been reported
to be as high as 40%.8 The immunocompromised
state of arthritic patients will also induce a depressed
response to a T-cell reaction leading to an inaccurate
IGRA result. There are recent data to argue that an
IGRA alone is insufficient to identify all patients at
risk.9 10 Furthermore, various studies have suggested very different concordance figures between the
IGRA and the TST, likely as a result of heterogeneity
(eg differing background TB prevalence, variable
immunosuppressive therapies, or underlying BCG
status).11
This study aimed to evaluate the
agreement between the IGRA and the TST in the
diagnosis of LTBI in patients with arthritic diseases
scheduled to receive biologic agents in Hong Kong.
Methods
Patients
We reviewed 38 patients with RA, PSA, or SPA at
a local hospital in Hong Kong from August 2013 to
April 2014. They were diagnosed according to the
2010 classification criteria for RA of the American
College of Rheumatology/European League Against
Rheumatism, the Classification Criteria for Psoriatic
Arthritis, and the Assessment of SpondyloArthritis
international Society classification criteria,
respectively. Patients were included if they
were considered candidates for biologic agents.
Concurrent medications were documented.
Candidates were excluded if they had active TB
infection, a history of incomplete TB treatment, or
no measured induration. Patients underwent both
the IGRA and the TST simultaneously. Those who
tested positive for either test and who were due to
be prescribed biologic agents were given latent TB
treatment with isoniazid or rifampicin for 9 months.
All patients were followed up regularly for 1 year
and the development of active TB infection was
evaluated. This study conforms to the provisions
of the Declaration of Helsinki and the guidelines of
the local ethical committee. Informed consent was
considered not necessary due to the retrospective
nature of the study.
Tuberculin skin test
The TST was performed by rheumatologists. A
0.1 mL of 2-TU PPD (tuberculin units of purified
protein derivative) was injected intradermally into
the volar aspect of the forearm. The indurations were
measured in millimetres after 48 hours of inoculation
by rheumatologists who were blinded to the IGRA
results. According to the local guideline, induration
of ≥10 mm was considered a positive result of LTBI.3
Interferon-gamma release assay
We performed the A.TB IGRA test (Haikou VTI
Biological Institute) in all study patients. This assay
employs Haikou VTI’s patented technology (US
patent number 7754219) that enables intracellular
delivery of the full-length protein CFP-10 and the
antigen ESAT-6 to stimulate antigen-specific T-cells
through the major histocompatibility complex class
1 pathway.12 13 The assay was performed according to the user manual. In brief, negative control phosphate
buffered saline (N), positive control concanavalin A (P), and
the TB stimulators CFP-10 and ESAT-6 (T) were
mixed with fresh heparinised whole blood and
incubated for approximately 24 hours at 37.8°C. The
plasma was collected and stored at 48°C for up to 2
weeks. The interferon-gamma level in the plasma was
then determined by enzyme-linked immunosorbent
assay. If N was <0.5 IU/mL and (T-N)/(P-N) ≥0.6, or
if N was ≥0.5 IU/mL and (T-N)/(P-N) ≥0.85, the test
was considered to be positive (A.TB+), otherwise the
result was negative (A.TB–).
Statistical analysis
Descriptive statistics were presented as frequencies
and means ± standard deviations as appropriate. The
concordance between TST and IGRA was evaluated
by the Cohen’s weighted k statistic. A kappa value of
>0.6 represents substantial agreement, 0.41 to 0.60
moderate agreement, 0.21 to 0.40 fair agreement, and
<0.21 slight agreement. The concordance was subanalysed
in patients with and without prednisolone.
Results
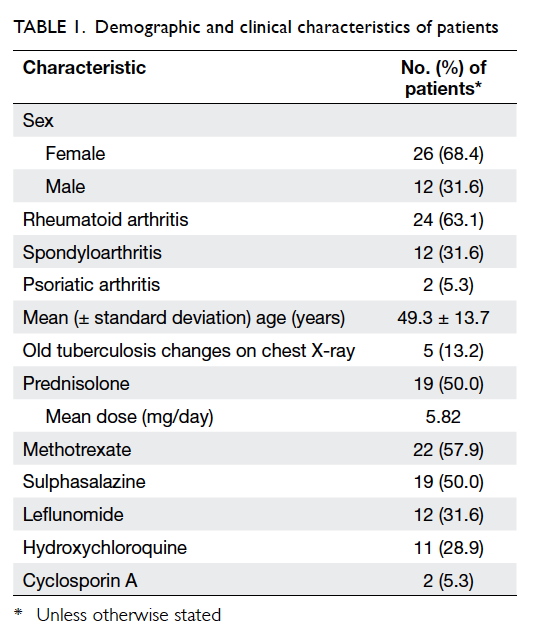
The demographic and clinical characteristics of 38
patients are summarised in Table 1. All patients were
residents of Hong Kong. Half of the patients were
prescribed systemic steroid therapy that comprised
prednisolone at a dose of 2.5 mg daily to 15 mg daily.
All except three patients with SPA were on various
conventional disease-modifying antirheumatic
drugs.
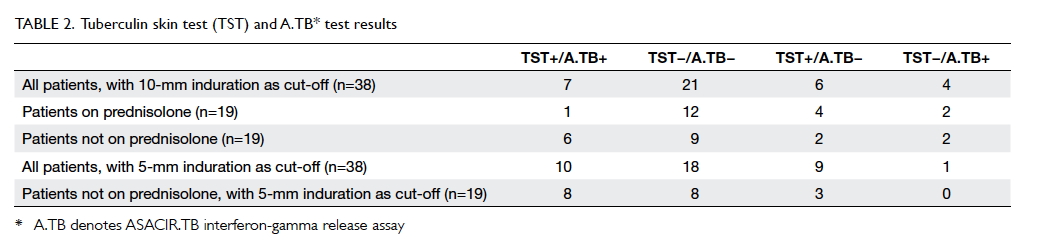
The results of the concomitant TST and IGRA
are shown in Table 2. Of the 38 patients, based on an
induration of 10-mm diameter as the cut-off value,
13 (34.2%) had a positive TST, 11 (28.9%) had a
positive IGRA. The agreement between A.TB IGRA
test and TST was 73.7%. Six patients were TST+/A.TB–
and four were TST–/A.TB+. Subgroup analysis
showed that four of the six divergent TST+/A.TB–
results were in patients on systemic steroid, and only
three patients with systemic steroid were A.TB+
versus eight patients without. When positive TST
was defined as an induration of 5-mm diameter, half
of the patients had a positive TST, among them nine
were TST+/A.TB–. Only one was TST–/A.TB+. In
patients prescribed a systemic steroid, with 5-mm
induration as positivity, TST missed no patients who
had positive IGRAs.
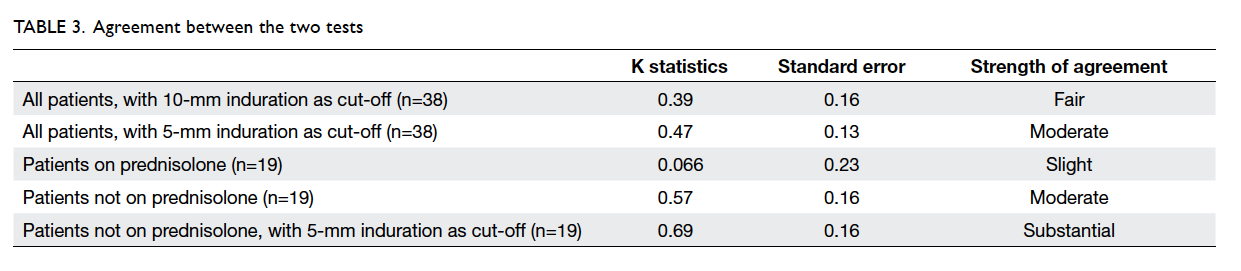
Analysis of the agreement between the two
tests, assessed by kappa statistic, showed only fair
strength in our study, with a kappa value of 0.39
(Table 3). When a 5-mm induration was taken as a
positive TST, however, the agreement between the
two tests improved to moderate with a kappa value of
0.47. Subgroup analysis revealed that the agreement
between both tests improved further (kappa=0.57)
in patients not taking a concurrent systemic steroid.
For patients taking a systemic steroid, the agreement
was only slight (kappa=0.066). Again, the agreement
of the TST and IGRA was substantial (kappa=0.69)
in patients not on systemic steroid therapy when a
5-mm induration was regarded as positive.
At the end of the study, 32 of the initial 38
patients had received biologic agents. None of them
developed active TB during the 1-year follow-up
period.
Discussion
In clinical practice there is no gold standard test for
diagnosing LTBI. Both IGRA and TST have strengths
and weaknesses. In a meta-analysis performed on an
unselected population, the specificity of IGRA was
99% in a non-BCG–vaccinated population and 96%
in a BCG-vaccinated population.14 The specificity
of the TST was 97% in a non-BCG–vaccinated
population but dropped to 59% in a BCG-vaccinated
population.14 In addition to BCG history, comparison
between the two tests must take into account the
underlying disease, the immunosuppression status,
and the background TB burden of the population
being screened.
In this study, we found only a fair agreement
(kappa=0.39) between the results of TST and A.TB IGRA in arthritis patients from a TB-endemic
area. Some studies have also reported a discrepancy
between the two tests in countries with intermediate
TB burden.15 16 Nonetheless, the reported agreement between TST and the IGRA was good (kappa of 0.72 in United Kingdom17 and 0.87 in Denmark18). Consequently, the incidence of TB is a crucial determinant of
agreement between the two tests.
In this study the concordance between the
TST and the IGRA was also affected by immune
status. There was only slight agreement in patients
taking concurrent prednisolone, but the agreement
improved when these patients were excluded
from the analysis. Some previous studies of
immunosuppressed RA patients have shown similarly
poor concordance between the two tests regardless of
TB burden.19 20 There was also discordance between the two tests in LTBI diagnosis among individuals
infected with the human immunodeficiency virus.21
The patients on prednisolone in our study had
a lower rate of positivity for both tests. It seems
intuitive to assume that an immunosuppressed state
will induce a depressed response to a T-cell reaction.
In the literature, a systematic review showed that
both positive IGRA and positive TST results were
significantly influenced by immunosuppressive
therapy.22
The Hong Kong guideline for the TST cut-off
value for LTBI diagnosis before anti-tumour necrosis
factor treatment is an induration of >10 mm.3 In the
current study, we showed that the TST cut-off value
that achieved better agreement between IGRA and
TST results was 5 mm. If we cannot rely on IGRA to
diagnose LTBI, it may be more appropriate to lower
that TST cut-off to 5 mm. We also showed that our
approach to LTBI screening with both TST and
IGRA was successful in preventing the development
of active TB in patients who would receive biologic
therapy. This dual testing strategy might be especially
applicable to patients on systemic steroid, as they are
at higher risk of developing active TB and the two
test results are more discordant. The consequent
improved sensitivity will invariably lower the
specificity and cause a potential overtreatment. In
these high-risk settings, however, it is reasonable to
favour sensitivity in screening for LTBI.
Only five patients could give a definite history
of BCG vaccination. For other patients, such
information was uncertain. While this might reflect
the local clinical situation, it is one of the limitations
of the present study. Despite the mechanistic
similarity, because of the different interpretation
methods employed for the test results and the lack of
comparative trials of the performance of A.TB IGRA
and other IGRAs, the conclusions drawn from the
current study may not be applicable to patients who
are given a different IGRA. Further studies using
individual IGRAs may be warranted to address the
same question.
Conclusion
In arthritis patients in a TB-endemic population,
the level of agreement between TST and A.TB
IGRA for detecting LTBI was only fair. Although
10 mm is the usual cut-off for TST, the level of
agreement between the two tests improved from
fair to moderate when a 5-mm cut-off was used. A
dual testing strategy with TST and IGRA appeared
to be effective and should be pursued, especially
in patients who are prescribed a systemic steroid.
The issue of potential overtreatment is yet to be
evaluated.
Declaration
All authors have disclosed no conflicts of interst.
References
1. Furst DE. The risk of infections with biologic therapies for
rheumatoid arthritis. Semin Arthritis Rheum 2010;39:327-46. Crossref
2. World Health Organization. Guidelines on the
management of latent tuberculosis infection. Geneva,
Switzerland: World Health Organization; 2015.
3. Mok CC. Consensus statements on the indications and
monitoring of anti-tumor necrosis factor (TNF) therapy
for rheumatic diseases in Hong Kong. Hong Kong Bull
Rheum Dis 2005;5:19-25.
4. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin
test. Clin Infect Dis 1993;17:968-75. Crossref
5. Matulis G, Jüni P, Villiger PM, Gadola SD. Detection of
latent tuberculosis in immunosuppressed patients with
autoimmune diseases: performance of a Mycobacterium
tuberculosis antigen-specific interferon gamma assay. Ann
Rheum Dis 2008;67:84-90. Crossref
6. Ponce de Leon D, Acevedo-Vasquez E, Alvizuri S, et al.
Comparison of an interferon-gamma assay with tuberculin
skin testing for detection of tuberculosis (TB) infection
in patients with rheumatoid arthritis in a TB-endemic
population. J Rheumatol 2008;35:776-81.
7. Song Q, Guo H, Zhong H, et al. Evaluation of a new
interferon-gamma release assay and comparison to
tuberculin skin test during a tuberculosis outbreak. Int J
Infect Dis 2012;16:e522-6. Crossref
8. Ferrara G, Losi M, Meacci M, et al. Routine hospital use
of a new commercial whole blood interferon-gamma assay
for the diagnosis of tuberculosis infection. Am J Respir Crit
Care Med 2005;172:631-5. Crossref
9. Kleinert S, Tony HP, Krueger K, et al. Screening for latent
tuberculosis infection: performance of tuberculin skin
test and interferon-gamma release assays under real-life
conditions. Ann Rheum Dis 2012;71:1791-5. Crossref
10. Mariette X, Baron G, Tubach F, et al. Influence of replacing
tuberculin skin test with ex vivo interferon gamma
release assays on decision to administer prophylactic
antituberculosis antibiotics before anti-TNF therapy. Ann
Rheum Dis 2012;71:1783-90. Crossref
11. Winthrop KL, Weinblatt ME, Daley CL. You can’t always
get what you want, but if you try sometimes (with two
tests—TST and IGRA—for tuberculosis) you get what you
need. Ann Rheum Dis 2012;71:1757-60. Crossref
12. Cao H, Agrawal D, Kushner N, Touzjian N, Essex M,
Lu Y. Delivery of exogenous protein antigens to major
histocompatibility complex class I pathway in cytosol. J
Infect Dis 2002;185:244-51. Crossref
13. McEvers K, Elrefaei M, Norris P, et al. Modified anthrax
fusion proteins deliver HIV antigens through MHC class I
and II pathways. Vaccine 2005;23:4128-35. Crossref
14. Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent tuberculosis
infection: an update. Ann Intern Med 2008;149:177-84. Crossref
15. Yilmaz N, Zehra Aydin S, Inanc N, Karakurt S, Direskeneli
H, Yavuz S. Comparison of QuantiFERON-TB Gold test
and tuberculin skin test for the identification of latent
Mycobacterium tuberculosis infection in lupus patients.
Lupus 2012;21:491-5. Crossref
16. Lee JH, Sohn HS, Chun JH, et al. Poor agreement between
QuantiFERON-TB Gold test and tuberculin skin test
results for the diagnosis of latent tuberculosis infection in
rheumatoid arthritis patients and healthy controls. Korean
J Intern Med 2014;29:76-84. Crossref
17. Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis
of Mycobacterium tuberculosis infection in a school
tuberculosis outbreak. Lancet 2003;361:1168-73. Crossref
18. Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P.
Comparison of tuberculin skin test and new specific blood
test in tuberculosis contacts. Am J Respir Crit Care Med
2004;170:65-9. Crossref
19. Huang YW, Shen GH, Lee JJ, Yang WT. Latent tuberculosis
infection among close contacts of multidrug-resistant
tuberculosis patients in central Taiwan. Int J Tuberc Lung
Dis 2010;14:1430-5.
20. Shalabi NM, Houssen ME. Discrepancy between the
tuberculin skin test and the levels of serum interferon-gamma
in the diagnosis of tubercular infection in contacts.
Clin Biochem 2009;42:1596-601. Crossref
21. Mandalakas AM, Hesseling AC, Chegou NN, et al. High
level of discordant IGRA results in HIV-infected adults
and children. Int J Tuberc Lung Dis 2008;12:417-23.
22. Shahidi N, Fu YT, Qian H, Bressler B. Performance
of interferon-gamma release assays in patients with
inflammatory bowel disease: a systematic review and
meta-analysis. Inflamm Bowel Dis 2012;18:2034-42. Crossref