Hong Kong Med J 2017 Apr;23(2):134–9 | Epub 2 Dec 2016
DOI: 10.12809/hkmj164879
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Ten-year review of survival and management of
malignant glioma in Hong Kong
Danny TM Chan, FRCS, FHKAM (Surgery);
Sonia YP Hsieh, MB, BS, MSc;
Claire KY Lau, MSc;
Michael KM Kam, FRCR, FHKAM (Radiology);
Herbert HF Loong, MB, BS, MRCP (UK);
WK Tsang, FRCR, FHKAM (Radiology);
Darren MC Poon, FRCR, FHKAM (Radiology);
WS Poon, FRCS, FHKAM (Surgery)
CUHK Otto Wong Brain Tumour Centre, 1/F, Sir Yue-kong Pao Centre for
Cancer, Prince of Wales Hospital, Shatin, Hong Kong
Corresponding author: Dr Danny TM Chan (tmdanny@surgery.cuhk.edu.hk)
Abstract
Introduction: Surgical resection used to be the
mainstay of treatment for glioma. In the last decade,
however, opinion has changed about the goal of
surgical resection in treating glioma. Ample evidence
shows that maximum safe resection in glioblastoma
improves survival. Neurosurgeons have therefore
revised their objective of surgery from diagnostic
biopsy or limited debulking to maximum safe
resection. Given these changes in the management
of glioma, we compared the survival of local Chinese
patients with glioblastoma multiforme over a period
of 10 years.
Methods: We retrospectively reviewed the data of
the brain tumour registry of the CUHK Otto Wong
Brain Tumour Centre in Hong Kong. Data of patients
with glioblastoma multiforme were reviewed for two
periods, during 1 January 2003 to 31 December 2005
and 1 January 2010 to 31 December 2012. Overall
survival during these two periods of time was
assessed by Kaplan-Meier survival estimates. Risk
factors including age, type and extent of resection,
use of chemotherapy, and methylation status of
O6-methylguanine-DNA methyltransferase were
also assessed.
Results: There were 26 patients with glioblastoma
multiforme with a mean age of 52.2 years during
2003 to 2005, and 42 patients with a mean age of 55.1
years during 2010 to 2012. The mean overall survival
during these two periods was 7.4 months and 12.7
months, respectively (P<0.001). The proportion
of patients who underwent surgical resection was
similar: 69.2% in 2003 to 2005 versus 78.6% in 2010
to 2012 (P=0.404). There was a higher proportion of
patients in whom surgery achieved total removal in
2010 to 2012 than in 2003 to 2005 (35.7% and 7.7%,
respectively; P=0.015). During 2010 to 2012, patients
who were given concomitant chemoradiotherapy
showed definitively longer survival than those who
were not (17.9 months vs 4.5 months; P=0.001). The
proportion of patients who survived 2 years after
surgery increased from 11.5% in 2003 to 2005 to
21.4% in 2010 to 2012.
Conclusions: Hong Kong has made substantial
improvements in the management of glioblastoma
multiforme over the last decade with corresponding
improved survival outcomes. The combination of
an aggressive surgical strategy and concomitant
chemoradiotherapy are probably the driving force
for the improvement.
New knowledge added by this study
- Maximum safe resection of glioblastoma multiforme (GBM) is feasible and has improved survival of patients over the last decade.
- Concomitant chemoradiotherapy has been shown to improve overall survival of patients with GBM.
- A combined multidisciplinary approach with surgery, radiotherapy, and chemotherapy should be adopted for treatment of GBM.
Introduction
Glioblastoma multiforme (GBM) is a malignant
primary brain tumour with an incidence of 1 to 2
per 100 000 population in Hong Kong.1 The survival
of patients with GBM remains dismal, mainly
due to its inevitable progression and recurrence.
Little progress was made until the last decade.
The establishment of concomitant chemoradiotherapy (CCRT) with temozolomide
(TMZ) and the discovery of O6-methylguanine-DNA
methyltransferase (MGMT) promoter methylation
in association with significantly better outcome were
the two major and inspiring breakthroughs.2 3 Before
these developments, the treatment for GBM was
homogeneous but desperate, and comprised surgery
and irradiation only.
In 2001, TMZ was first used in the treatment
of recurrent high-grade glioma in Hong Kong. Its
favourable anti-tumour activity and acceptable
safety profile were proven in a local study.4 In 2005,
TMZ was the first chemotherapy to show objective
survival benefit as a primary treatment when used
together with radiotherapy as part of CCRT in
GBM.2 Since then, CCRT for GBM has become the
norm in Hong Kong.
In the last decade, opinion has changed about
the goal of surgical resection in treating glioma.
Ample evidence has shown that maximum safe
resection in GBM improves survival. Neurosurgeons
have therefore revised their objective of surgery
from a diagnostic biopsy or limited debulking to a
maximum safe resection. Knowing the infiltrative
nature of the tumour, surgeons have a demanding
job of balancing maximum resection and safe
surgery. Awake craniotomy and mapping technique
are two essential surgical techniques that enable safe
resection.5 The goal of maximum resection can be
achieved with a fluorescence-guided surgery with
5-aminolevulinic acid (5-ALA).6
Given these changes to the management of
GBM, we therefore analysed the changes in overall
survival of GBM over the past 10 years in Hong
Kong.
Methods
Data were retrieved from the Chinese University of
Hong Kong Otto Wong Brain Tumour Centre brain
tumour registry. The registry has been collecting
data from all histology-proven glioma patients in
the institute since January 2003. Patients aged 18
years or above with histologically proven glioma
diagnosed in the institute were included in the
registry. Patients with histologically confirmed
World Health Organization grade IV GBM during
January 2003 to December 2005 and January 2010 to
December 2012 were recruited and grouped. Patients
treated between 2006 and 2009 were excluded
because the surgical policy was evolving and the
availability of chemotherapy was variable during
the period. Therefore this would be a heterogeneous
group of patients with various treatments due to
availability or affordability. Patients with an unstable
neurological condition or who were considered a
poor medical risk after surgery resulting in Karnofsky
Performance Scale score of below 70 were excluded, as
were those who received initial chemotherapeutics
other than TMZ, ie procarbazine, lomustine,
vincristine, or bevacizumab. Data on type of surgery,
extent of resection, tumour histology, irradiation
and chemotherapy parameters were collected as
well as information about patient’s age and gender.
The registry defined the death date according to the
electronic patient record in the Hospital Authority
Clinical Management System. For patients who
defaulted from clinical follow-up, telephone follow-up
ascertained death date. The study end date was
30 June 2015.
During 2003 to 2005, all patients were treated
with surgical resection and adjuvant radiotherapy;
TMZ was only used in patients with recurrent
disease. Ability to pay for chemotherapy was the
key determinant of its application and utility. In our
hospital, TMZ was prescribed at a dose of 200 mg/m2
once per day for 5 days in a 28-day cycle.
With regard to the contouring methodology
of irradiation, either European Organisation for
Research and Treatment of Cancer or Radiation
Therapy Oncology Group protocol was chosen
according to the serial assessment of both pre- and
post-operative magnetic resonance imaging (MRI)
scans. A total dose of 60 Gy irradiation was delivered
to the tumour bed and its adjacent tissue in 30
fractions, with 2 Gy each.
Since 2009, neuroradiologists have been
responsible for assessing the extent of resection by
MRI on postoperative day 1. Total resection was
defined as no remaining contrast enhancement on
MRI T1-weighted and subtraction scans of T1 plain
with T1 plus contrast. For patients in whom the
enhancing lesion was still noticeable, the resection
was categorised as debulking.
In the 2010-2012 cohorts, TMZ was
recommended to all patients. The dosage was 75
mg/m2/day concomitant with radiotherapy, then
150-200 mg/m2/day on the first 5 days every 4
weeks for 6 cycles, in accordance with the regimen
described by Stupp et al.2 Methylation status of the
MGMT was detected using methylation-specific
polymerase chain reaction at our institution. The
method has been explained in detail in one of our
earlier studies.7 Survival was calculated from the
date of surgery for brain tumour to death. Kaplan-Meier survival curves were used to compare
different groups of biopsy versus surgical resection
and chemoradiotherapy versus radiotherapy alone.
This audit review was done in accordance
with the principles outlined in the Declaration of
Helsinki.
Results
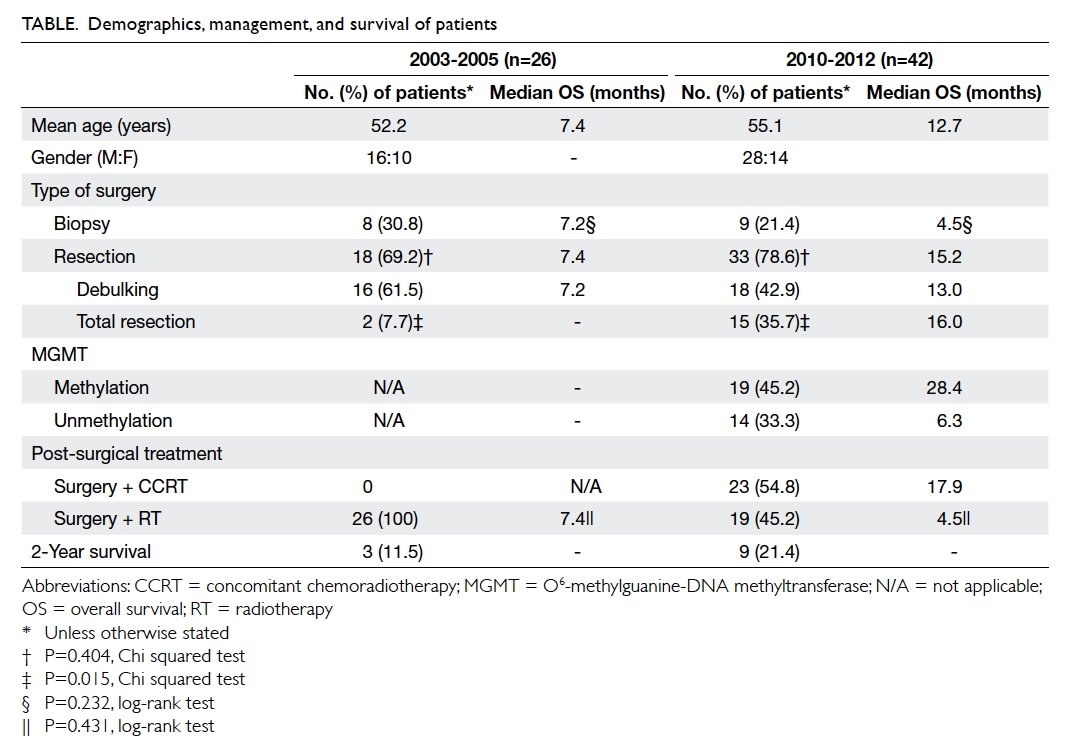
Demographics, management, and survival of
patients are shown in the Table.
During the period 1 January 2003 to 31
December 2005, 26 patients with a mean age of 52.2
years were eligible for study. Two patients below
the age of 18 years were excluded from the registry.
The median overall survival for this cohort was 7.4
months (Fig 1). Eight (30.7%) patients underwent biopsy only, with a non-inferior median overall
survival compared with the remaining 18 patients
who underwent resection (7.2 months vs 7.4
months, P=0.988, log-rank test; Fig 2). Total removal could be achieved in only two patients, with overall
survival of 14.7 and 28.8 months, respectively. For the remaining
16 patients who underwent debulking surgery, the
median overall survival was 7.2 months.
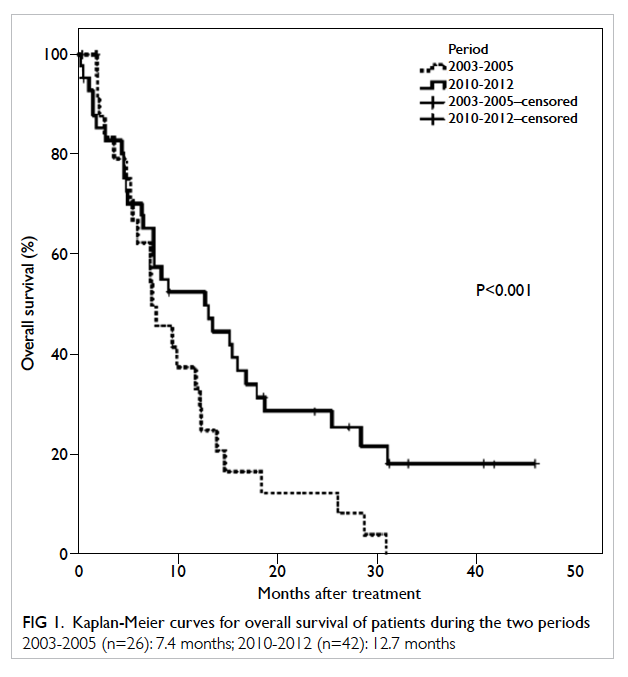
Figure 1. Kaplan-Meier curves for overall survival of patients during the two periods
2003-2005 (n=26): 7.4 months; 2010-2012 (n=42): 12.7 months
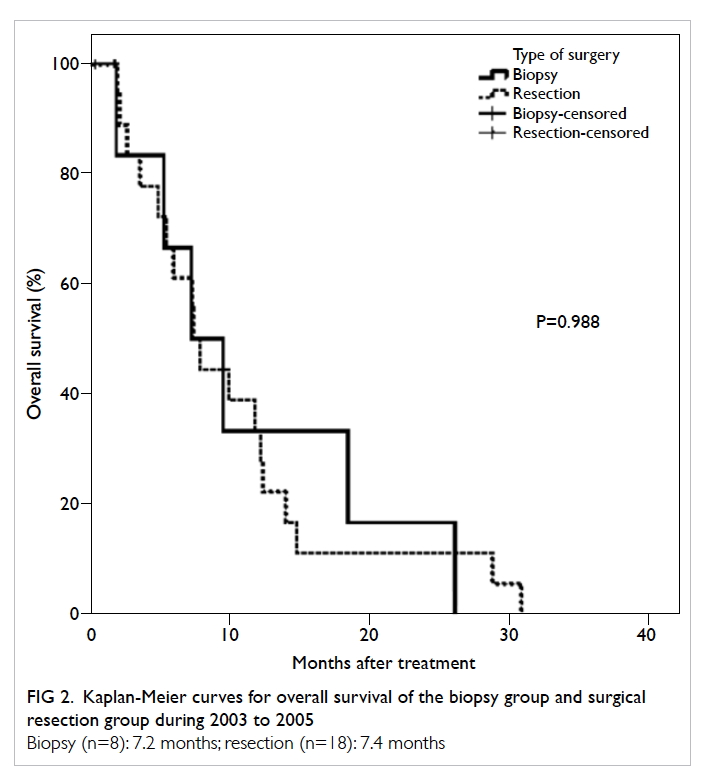
Figure 2. Kaplan-Meier curves for overall survival of the biopsy group and surgical resection group during 2003 to 2005
Biopsy (n=8): 7.2 months; resection (n=18): 7.4 months
During the period 1 January 2010 to 31
December 2012, 42 patients with a mean age of 55.1
years were identified. One patient was excluded
because he declined CCRT after surgery and
opted for alternative medicine. The median overall
survival was markedly prolonged to 12.7 months
(P<0.001, log-rank test; Fig 1). The proportion of patients who had biopsy (9/42, 21.4%) during 2010
to 2012 remained similar to 10 years ago (8/26,
30.8%). Patients with resection performed was not
significantly different between the two periods
(P=0.404, Chi squared test). Overall survival of the
surgical resection group was distinctly longer than
that for the biopsy group (15.2 months and 4.5
months, respectively; P=0.026, log-rank test; Fig 3). A higher proportion of patients achieved total
surgical removal in 2010-2012 than in 2003-2005,
being 35.7% (15/42) and 7.7% (2/26), respectively
(P=0.015, Chi squared test). The difference between
debulking and total resection remained undefined
in the 2010-2012 arm (13.0 months vs 16.0 months;
P=0.966, log-rank test) by the time of analysis.
Figure 3. Kaplan-Meier curves for overall survival of the biopsy group and surgical resection group during 2010 to 2012
Biopsy (n=9): 4.5 months; resection (n=33): 15.2 months
Of the 42 patients with GBM during 2010-2012, CCRT was initiated in 23, accompanied by a
meaningful longer median survival of 17.9 months
compared with only 4.5 months for those given
radiotherapy only (P=0.001, log-rank test; Fig 4). Data for MGMT were available in 33 patients. The
overall survival of 19 patients with methylated
MGMT promoter was longer than that of 14 patients
with unmethylated MGMT promoter, being 28.4
months and 6.3 months, respectively (P<0.001, log-rank
test).
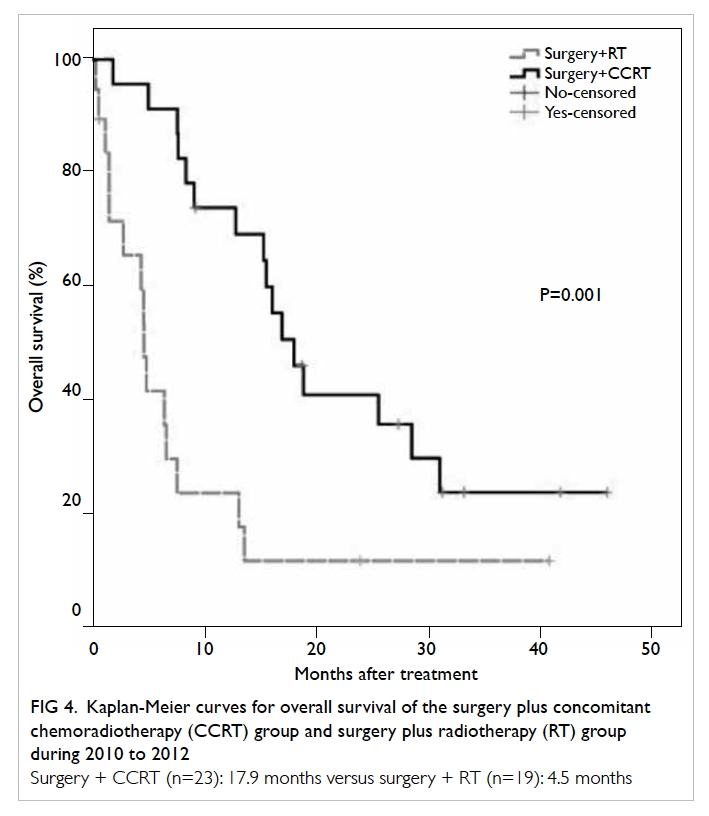
Figure 4. Kaplan-Meier curves for overall survival of the surgery plus concomitant chemoradiotherapy (CCRT) group and surgery plus radiotherapy (RT) group during 2010 to 2012
Surgery + CCRT (n=23): 17.9 months versus surgery + RT (n=19): 4.5 months
Improvement in 2-year survival was also
evident, from 11.5% in the earlier cohort, to 21.4% in
the later one.
Discussion
Glioma has attracted international research interest
over the last 20 years in both clinical and laboratory
setting. The determination to fight the disease yielded
with proven survival benefit of TMZ in recurrent
high-grade glioma in 2000.8 A 6-month event-free
survival of 21% in TMZ compared favourably with
9% for procarbazine.8 The full effect of TMZ was
reported in a randomised trial as primary treatment
for GBM in 2005.2 The regimen included two phases
of TMZ, starting with a concomitant phase of daily
low-dose TMZ during the course of radiotherapy,
followed by the adjuvant phase of a high-dose TMZ
for 5 days during each 28-day cycle for 6 cycles.2
The results of the study benchmarked a standard for
chemotherapy in the treatment of GBM. The median
survival of 14.6 months in the CCRT arm compared
favourably with the 12.1 months of the control
radiotherapy-alone arm.2
In Hong Kong, TMZ was first introduced
in 2001. Its safety and effect had been tested and
reported in a small series of recurrent high-grade
glioma.4 The use of CCRT in Hong Kong was also
reported with favourable results.9 The overall survival
was much improved in these 10 years from 7.4
months in 2003-2005 to 12.7 months in 2010-2012.
Among the cohorts in 2010-2012, however, only 54.8%
(23/42) received CCRT. This can be attributed to the
fact that in 2010, TMZ, whilst already incorporated
into the Hospital Authority Drug Formulary, was
listed as a self-financed item only. The financial
burden on patients was the major cause of low usage
during the time.
In 2011, TMZ was granted conditional funding
through the Samaritan Fund scheme. Approval of
funding was based on the financial situation of the
patient and the tumour’s MGMT methylation status,
with approval only granted to patients with tumours
with MGMT methylation. This may have been a
cost-effectiveness consideration because the largest
survival benefit would be in MGMT-methylated
GBM. Local data showed that only 43% of local GBM
were methylated in MGMT status,9 thus essentially
limiting the possibility of funding for less than half
of the patients with GBM. Thus, within the 2010-2012 cohort, only patients diagnosed from 2011
onwards with tumours of methylated MGMT status
(accounting for roughly a further half of the patient
population) would have benefited from the scheme.
This may account for the relatively low number of
patients treated with CCRT. Then the policy of
restricting funding based on MGMT status was re-addressed
and such criterion was removed in 2013.
Currently, support of Samaritan Fund for TMZ is
available for eligible patients with GBM based on
their financial situation, and regardless of their
tumour MGMT status.
The treatment of CCRT had made an impact
not only on clinical outcomes, but also on the
working dynamics between different professional
disciplines involved in the management of patients
with GBM. The need for timely arrangement and
administration of radiotherapy and chemotherapy
within a short postoperative window has encouraged
a multidisciplinary team approach. This continues
to be the current treatment delivery model for
patients with GBM in many hospitals in Hong Kong.
Better clinical outcomes encouraged professional
enthusiasm. In this atmosphere, a local group of
clinicians got together and founded the Hong Kong
Neuro-Oncology Society in 2011.
The reasons for longer survival of GBM in
recent years are likely to be multifactorial. The
extent of surgical resection has been intensely
studied over the last two decades. Nonetheless,
since a prospective randomised surgical study
would be unethical, evidence to support maximum
safe resection must be gleaned retrospectively.
Despite this, neurosurgical professionals were
convinced that surgical resection was the first and
major treatment for GBM. Surgical conservatism
was abandoned and the demand for maximum safe
resection was set out by neurosurgeons. This change
was reflected in the decrease in surgical biopsy rate
from 30.8% in 2003-2005 to 21.4% in 2010-2012.
The ability of total surgical removal of the contrast-enhancing
tumour was also increased from 7.7% in
2003-2005 to 35.7% in 2010-2012. Local neurosurgeons
have been equipped with two surgical techniques
to achieve maximum safe resection in the last 10 years—the technique of cortical mapping and awake
surgery was brought to all local neurosurgeons in
two workshops of commissioned training organised
by the Hospital Authority in 2003 and 2010. This
technique allows safer resection of the tumour at
or near the eloquent area of the brain. A tumour
fluorescent technique (5-ALA) was introduced in
2009 that facilitated detection of residual tumour
for maximum resection. In 2003-2005, the survival
of the surgical resection group and biopsy group
was similar but in 2010-2012, those in the surgical resection
group survived longer. The difference was
probably due to both aggressive surgical resection
and CCRT in the latter group.
The major limitation of this study was the
presence of potential confounding factors during
the 10-year study period. Such factors included
incomplete data of MGMT methylation status
and extent of resection in the 2003-2005 group.
There was no MGMT methylation testing or
day-1 MRI scan after resection in 2003-2005. The
interval between surgery and commencement of
radiotherapy has been controlled to within 4 weeks
since 2009 but this was not the case in 2003-2005. All
these confounding factors made valid comparison
of the effect of surgical resection or chemotherapy
during these two time periods difficult. Moreover,
the registry included only surgical patients who had
undergone biopsy or resection, and excluded a small
group of patients, who were usually elderly (age >70
years) or with poor co-morbidities, who may have
received radiotherapy or chemotherapy alone.
Conclusions
Hong Kong has made substantial improvements in
the management of GBM with improved survival
over the last decade. The combination of aggressive
surgical strategy and CCRT are probably the driving
force for the improvement.
Declaration
None of the authors has disclosed any conflicts of interest.
References
1. Pu JK, Ng GK, Leung GK, Wong CK. One-year review of
the incidence of brain tumours in Hong Kong Chinese
patients as part of the Hong Kong Brain and Spinal
Tumours Registry. Surg Pract 2012;16:133-6. Crossref
2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy
plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med 2005;352:987-96. Crossref
3. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene
silencing and benefit from temozolomide in glioblastoma.
N Engl J Med 2005;352:997-1003. Crossref
4. Chan DT, Poon WS, Chan YL, Ng HK. Temozolomide in
the treatment of recurrent malignant glioma in Chinese
patients. Hong Kong Med J 2005;11:452-6.
5. Chan DT, Kan PK, Lam JM, et al. Cerebral motor cortical
mapping: awake procedure is preferable to general
anaesthesia. Surg Pract 2010;14:12-8. Crossref
6. Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided
surgery with 5-aminolevulinic acid for resection
of malignant glioma: a randomised controlled multicentre
phase III trial. Lancet Oncol 2006;7:392-401. Crossref
7. Dong SM, Pang JC, Poon WS, et al. Concurrent
hypermethylation of multiple genes is associated with
grade of oligodendroglial tumors. J Neuropathol Exp
Neurol 2001;60:808-16. Crossref
8. Yung WK, Albright RE, Olson J, et al. A phase II study of
temozolomide vs. procarbazine in patients with glioblastoma
multiforme at first relapse. Br J Cancer 2000;83:588-93. Crossref
9. Chan DT, Kam MK, Ma BB, et al. Association of molecular
marker O6Methylguanine DNA methyltransferase
and concomitant chemoradiotherapy with survival in
Southern Chinese glioblastoma patients. Hong Kong Med
J 2011;17:184-8.