DOI: 10.12809/hkmj166027
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
An update of the Hong Kong Epilepsy Guideline:
consensus statement on the use of antiepileptic
drugs in Hong Kong
Jason KY Fong, FHKCP, FHKAM (Medicine)1;
Eric LY Chan, FHKCP, FHKAM (Medicine)2;
Howan Leung, FHKCP, FHKAM (Medicine)3;
Iris Chan, PhD4;
Richard SK Chang, FHKCP, FHKAM (Medicine)5;
Gardian CY Fong, FHKCP, FHKAM (Medicine)1;
Eva LW Fung, FHKCP, FHKAM (Paediatrics)6;
Colin HT Lui, FHKCP, FHKAM (Medicine)7;
Ben BH Fung, FHKCP, FHKAM (Medicine)8;
TL Poon, FCSHK, FHKAM (Surgery)9;
Deyond Siu, FHKCR, FHKAM (Radiology)10;
HT Wong, FCSHK, FHKAM (Surgery)11;
Eric Yeung, FHKCP, FHKAM (Medicine)12;
Ada WY Yung, FHKCP, FHKAM (Paediatrics)13;
Cannon XL Zhu, FRCS, FHKAM (Surgery)14;
Subcommittee on the Consensus Statement of The Hong Kong Epilepsy Society
1 Private practice, Hong Kong
2 Department of Medicine and Geriatrics, Tuen Mun Hospital, Tuen Mun,
Hong Kong
3 Department of Medicine and Therapeutics, Prince of Wales Hospital,
Shatin, Hong Kong
4 Department of Medicine, Queen Elizabeth Hospital, Jordan, Hong Kong
5 Department of Medicine, Queen Mary Hospital, Pokfulam, Hong Kong
6 Department of Paediatrics, Prince of Wales Hospital, Shatin, Hong Kong
7 Department of Medicine, Tseung Kwan O Hospital, Tseung Kwan O,
Hong Kong
8 Department of Medicine, United Christian Hospital, Kwun Tong, Hong Kong
9 Department of Neurosurgery, Queen Elizabeth Hospital, Jordan, Hong Kong
10 Department of Radiology, Kwong Wah Hospital, Yaumatei, Hong Kong
11 Department of Neurosurgery, Kwong Wah Hospital, Yaumatei, Hong Kong
12 Department of Medicine, Pamela Youde Nethersole Eastern Hospital,
Chai Wan, Hong Kong
13 Department of Paediatrics, Queen Mary Hospital, Pokfulam, Hong Kong
14 Department of Surgery, Prince of Wales Hospital, Shatin, Hong Kong
Corresponding authors: Dr Howan Leung (howanleung@cuhk.edu.hk)
Abstract
Objective: New information about antiepileptic
drugs has arisen since the publication of the Hong
Kong Epilepsy Guideline in 2009. This article set out
to fill the knowledge gap between 2007 and 2016 on
the use of antiepileptic drugs in Hong Kong.
Participants: Between May 2014 and April 2016, four
consensus meetings were held in Hong Kong, where
a group comprising 15 professionals (neurologists,
paediatricians, neurosurgeons, radiologists, and
clinical psychologists) from both public and private
sectors aimed to review the best available evidence
and update all practising physicians on a range of
clinical issues including drug-related matters. All
participants were council members of The Hong
Kong Epilepsy Society.
Evidence: A literature review of the clinical use of
antiepileptic drugs as monotherapy suggested Level
A evidence for levetiracetam and Level B evidence
for lacosamide. No change in the level of evidence
was found for oxcarbazepine (Level A evidence) or
pregabalin (undesignated), and no evidence was found
for perampanel. A literature review on the clinical use
of antiepileptic drugs as adjunctive therapy suggested
Level A evidence for both lacosamide and perampanel.
No change to the level of evidence was found for
levetiracetam (Level A evidence), oxcarbazepine
(Level A evidence), or pregabalin (Level A evidence).
A literature search on the use of generic antiepileptic
drugs suggested Level A evidence for the use of
lamotrigine in generic substitution.
Consensus process: Three lead authors of the
Subcommittee drafted the manuscript that
consisted of two parts—part A: evidence on new
antiepileptic drugs, and part B: generic drugs. The
recommendations on monotherapy/adjunctive
therapy were presented during the meetings. The
pros and cons for our health care system of generic
substitution were discussed. The recommendations
represent the ‘general consensus’ of the participants
in keeping with the evidence found in the literature.
Conclusions: Recommendations for the use
of levetiracetam, lacosamide, oxcarbazepine,
pregabalin, and perampanel were made. The
consensus statements may provide a reference to
physicians in their daily practice. Controversy exists
over the use of generic products among patients
who are currently taking brand medications. In this
regard, approvals from prescriber and patient are
pivotal. Good communication between doctors and
patients is essential, as well as enlisting the assistance
of doctors, nurses, and pharmacists, therapeutic
blood monitoring if available, and the option of
brand antiepileptic drug as a self-financed item.
The physical appearance of generic drugs should
be considered as it may hamper drug compliance.
Support from medical services is recommended.
In the longer term, the benefit of flexibility and the
options to have a balance between the generic and
brand drug market may need to be addressed by
institutions and regulatory bodies.
Introduction
Epilepsy is a chronic neurological condition that
places a high economic burden on patients from
childhood to senescence. In Hong Kong alone,
more than 70 000 patients have seizures as a chronic
condition and many more have developed seizures as
a result of an acute symptomatic medical condition;
both of which may require the use of antiepileptic
drugs (AEDs). There are currently 155 registered
pharmaceutical products in Hong Kong classed as
AEDs and approved by the Department of Health,
excluding drugs that are prescribed off-label. The
general guiding principles for physicians in the
selection of AEDs are derived from evidence-based
medicine and the last version of The Hong Kong
Epilepsy Guideline already provides ample advice.1
As the number of published papers and meta-analysis
is fast-growing, The Hong Kong Epilepsy
Society (HKES) considers it important to review the
best available evidence and to update all practising
physicians with regard to their position on a range
of clinical issues including drug-related matters.
As such, HKES prepared a series of consensus
statements to supplement The Hong Kong Epilepsy
Guideline of 2009.
Four consensus meetings were convened
between May 2014 and April 2016 during which
time a group of 15 professionals consisting of
neurologists, paediatricians, neurosurgeons,
radiologists, and clinical psychologists participated
in structured discussions in four major areas: AEDs,
status epilepticus, refractory epilepsy, and women
and epilepsy. The participants represented both the
public and private sectors. They were all council
members of HKES. The current paper addresses the
topic of AEDs.
In part A of this consensus statement, we have
compiled all the papers and studies published in
2007 or later, using the citation index from PubMed,
Ovid and Google Scholar, that are concerned with
the clinical use of AEDs as either monotherapy or
adjunctive therapy. The research papers must be
written in English with seizure outcome as their
primary endpoint. Only AEDs licensed in Hong
Kong after 2001 are included in this review. Studies
pertaining to benzodiazepine and intravenous
preparations only of any AED were not reviewed,
nor were those that focused exclusively on
subgroups of patients in which prognosis may be
affected by parameters other than drug treatment
(eg neurosurgical cohorts).
The research papers were rated as randomised
controlled trial, cohort study (including retrospective
study), meta-analysis or review, and where possible,
graded as class I, II, or III level of evidence, in
line with the previous version of The Hong Kong
Epilepsy Guideline.1 Level A evidence is defined as
the availability of one Class I study or more, or meta-analysis
suggesting a similar rating. Level B evidence
is defined as the availability of one Class II study or
more, or meta-analysis suggesting a similar rating.
Level C evidence is defined as the presence of more
than two Class III studies.
In part B of this consensus statement, we
have compiled all the studies published in 2007 or
later, using the citation index from PubMed, Ovid
and Google Scholar, that are related to human
studies of generic preparations of AEDs. The same
classification of evidence is employed. The analyses
in both parts A and B are of particular importance
to local health care providers, because Hong Kong
has a special health-financing situation in which
the majority of patients are treated under the public
hospital system. As a result, hospital-based practice
is likely to influence the standard of care delivered
to the majority of chronic epilepsy patients and the
health care costs of medical treatment.
Part A: evidence on new antiepileptic drugs
A total of 95 eligible papers were submitted for the
purpose of writing this consensus statement. Articles
that focused on zonisamide, eslicarbazepine, and
brivaracetam were not reviewed because these agents
were not registered with Department of Health at
the time of writing. Papers pertaining to topiramate
were not reviewed as the drug was registered in
Hong Kong before 2001. Papers on retigabine were
not reviewed as this drug has currently limited usage
in Hong Kong following an alert from the Food and
Drug Administration (FDA) of the United States.
The remaining drugs of interest were collated based
on their indications.
Monotherapy
Levetiracetam
Two Class I studies, 10 Class II studies, and 16
Class III studies were found under this indication
for levetiracetam (LEV). One Class I study that
randomised patients to LEV or carbamazepine
found non-inferiority of LEV.2 Another Class I study
randomised paediatric patients with juvenile absence
epilepsy to LEV or placebo and reported a non-significant
superiority in terms of seizure response.3
One Class II study compared LEV with lamotrigine
(LTG) and another Class II study compared LEV
with carbamazepine or sodium valproate. Both
studies demonstrated that LEV was as efficacious as
the other standard regimens.4 5
The evidence in the paediatric population was
generally positive.3 4 6 7 At the opposite end of the
spectrum, geriatric patients were also shown in a
Class II study to benefit from LEV monotherapy.8 One
Class II study detailed the conversion of treatment
in patients with existing partial-onset epilepsy to
extended-release LEV monotherapy.9 In the Chinese
population, one Class III study demonstrated the
usefulness of LEV monotherapy.10 The overall level
of conclusion is supported by an expedited review
from the International League Against Epilepsy
(ILAE).11
Statement 1: The level of evidence for LEV
monotherapy reaches Level A.
Oxcarbazepine
Four Class III studies and one meta-analysis were
found under this indication for oxcarbazepine
(OXC). Another three Class III studies recruited
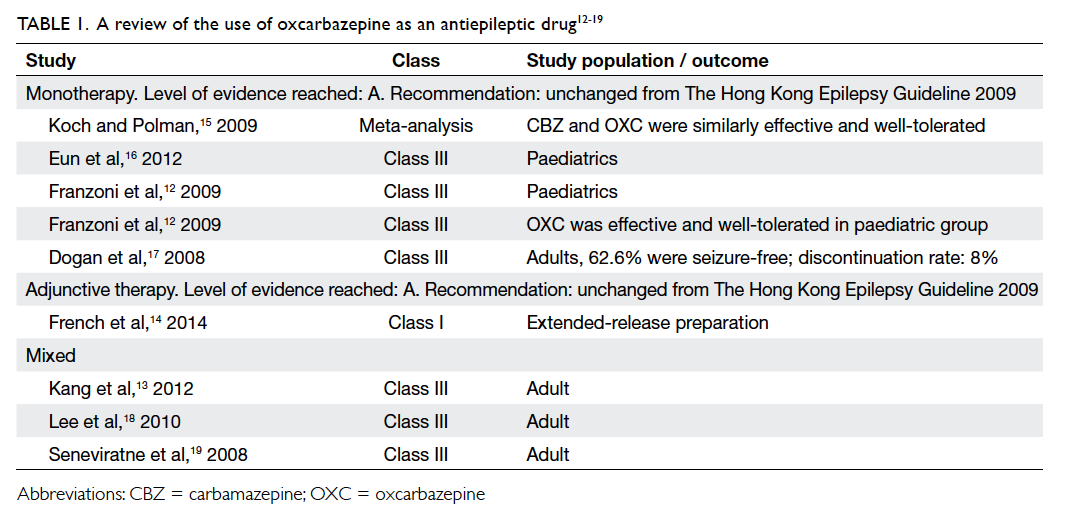
patients with mixed indications (Table 112 13 14 15 16 17 18 19). The evidence in the paediatric subgroup suggested
that OXC may be useful in children across a range
of conditions, from idiopathic to symptomatic and
cryptogenic epilepsy.12 Of interest, one study that
recruited Chinese patients for the purpose of both
mono- and adjunctive therapy showed that OXC
was as effective as LTG or topiramate.13
Oxcarbazepine is already indicated as monotherapy
in partial epilepsy. The recommendation for the use
of OXC remains unchanged.
Statement 2: The level of evidence for OXC
monotherapy remains unchanged (Level A).
Lacosamide
Lacosamide (LCS) produces slow inactivation of
neuronal sodium channels. We found one Class
II study and two Class III studies on the use of
LCS monotherapy and two Class III studies with
mixed indications (Table 220
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45). One conversion
study showed that 425 patients completed LCS
maintenance with a favourable safety profile at
a nominal dose of 400 mg per day.20 In another
study, the seizure-free rate was 72.3% at 1 year
and the withdrawal rate was 15%.21 In the study by
Lattanzi et al,22 58 patients were converted from
a background single AED to LCS with just over
half (55.2%) becoming seizure-free. Only 20.8% of
patients reported mild-to-moderate adverse events.
The FDA has approved use of LCS as monotherapy in
epilepsy since September 2014 and there was a plan
to seek its approval for use with the same indication
in Europe in 2016.
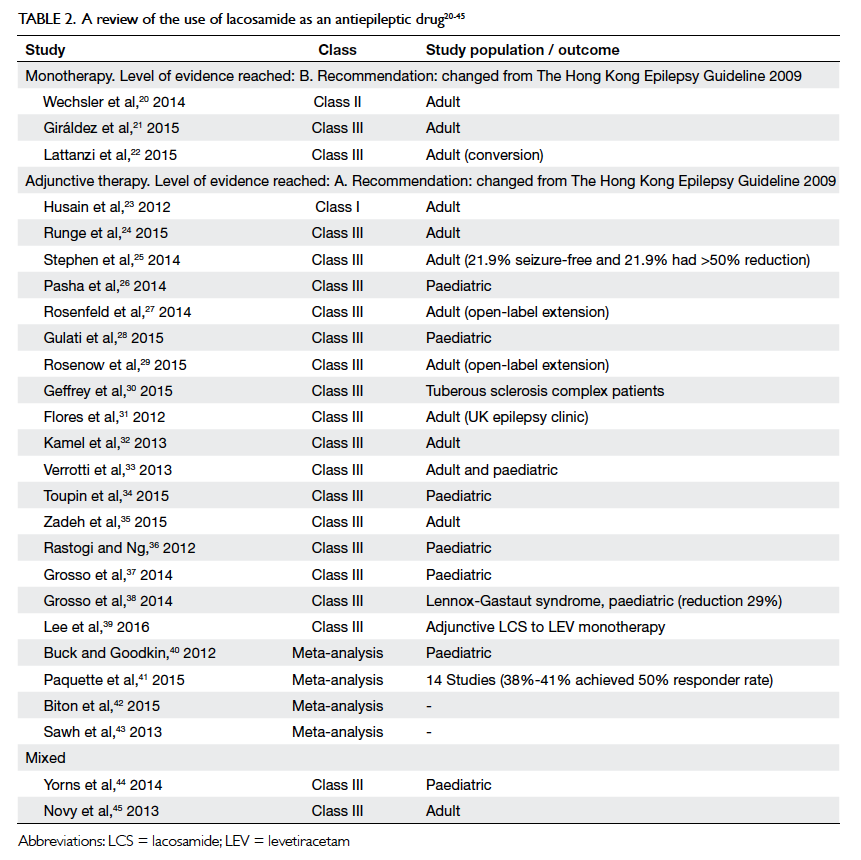
Table 2. A review of the use of lacosamide as an antiepileptic drug20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45
Statement 3: The level of evidence for LCS
monotherapy reaches Level B.
Pregabalin
Pregabalin (PGB) has binding properties to the
alpha-2-delta units of calcium channels. We found
one Class I study, one Class II study, and one meta-analysis
for PGB under this indication (Table 314
46
47
48
49
50
51
52
53
54
55
56
57).
Pregabalin was compared with LTG in a study
of 330 patients using a double-blind, non-inferiority
design with the primary efficacy endpoint being the
proportion of patients to achieve seizure freedom for
6 months. In the study, however, PGB was inferior
to LTG on both intention-to-treat and per-protocol
analyses.46 In the study by French et al,14 conversion from a first or second AED to PGB was
undertaken in 125 patients and the results showed
that PGB monotherapy was safe and efficacious in
partial epilepsy. No recommendation may be given
at this stage regarding the use of PGB monotherapy
in epilepsy.
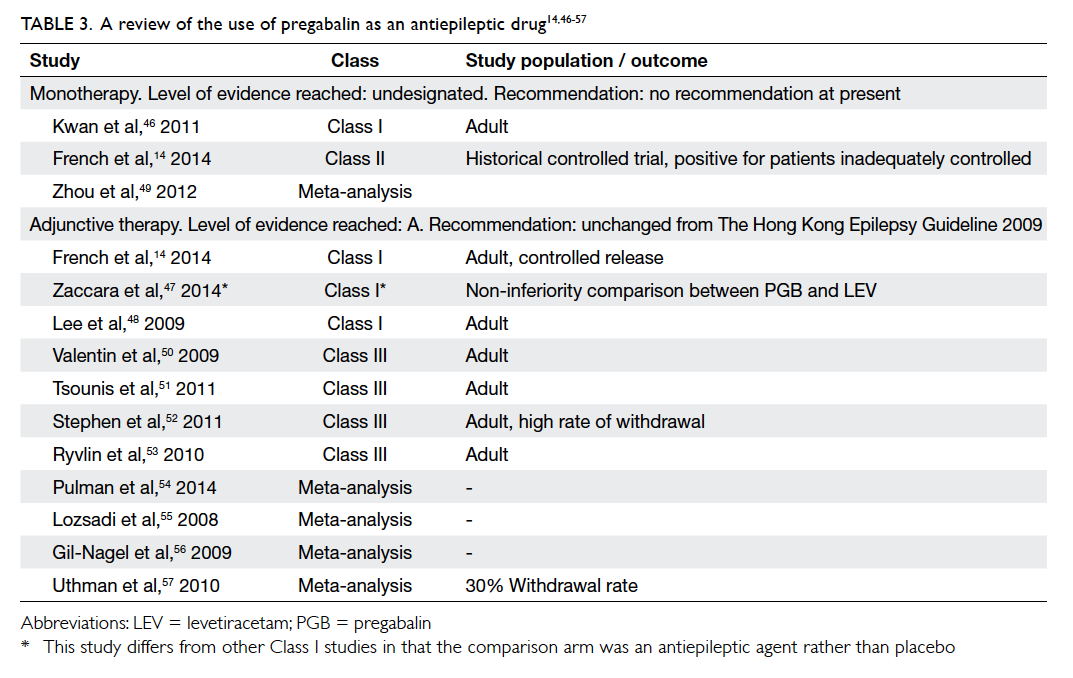
Table 3. A review of the use of pregabalin as an antiepileptic drug14 46 47 48 49 50 51 52 53 54 55 56 57
Statement 4: The level of evidence for
PGB monotherapy remains unchanged (not designated).
Perampanel
No study on the use of perampanel (PER)
monotherapy could be found using the current
search criteria. Other information pertaining to PER
is shown in Table 4.58
59
60
61
62
63
64
65
66
67
68
69
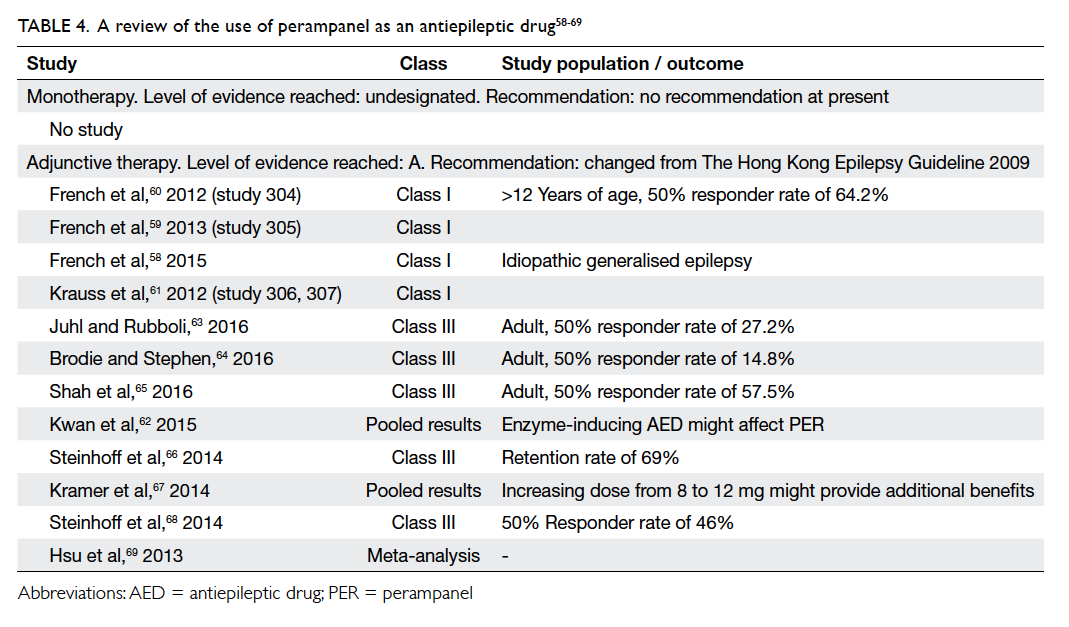
Table 4. A review of the use of perampanel as an antiepileptic drug58 59 60 61 62 63 64 65 66 67 68 69
Statement 5: The level of evidence for
PER monotherapy remains unchanged (no recommendation).
Adjunctive therapy
Levetiracetam
One Class I and two Class III studies were identified
using the search criteria. In addition, two Class
III studies reported mixed indications and two
meta-analyses were published (Table 52
3
4
5
6
7
8
9
10
13
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94). In the only Class I study available for this indication,
patients with idiopathic generalised epilepsy were
randomised to receive LEV 3000 mg per day or
placebo. The results suggested that a reduction by
≥50% of myoclonic seizures may be achieved in
58.3% of patients.70 One Class III study reported the
use of LEV among patients with rolandic epilepsy
or variants: a >50% reduction in seizure frequency
was achieved by 62.5% of patients.71 There is no new
recommended level of evidence for LEV under this
indication.
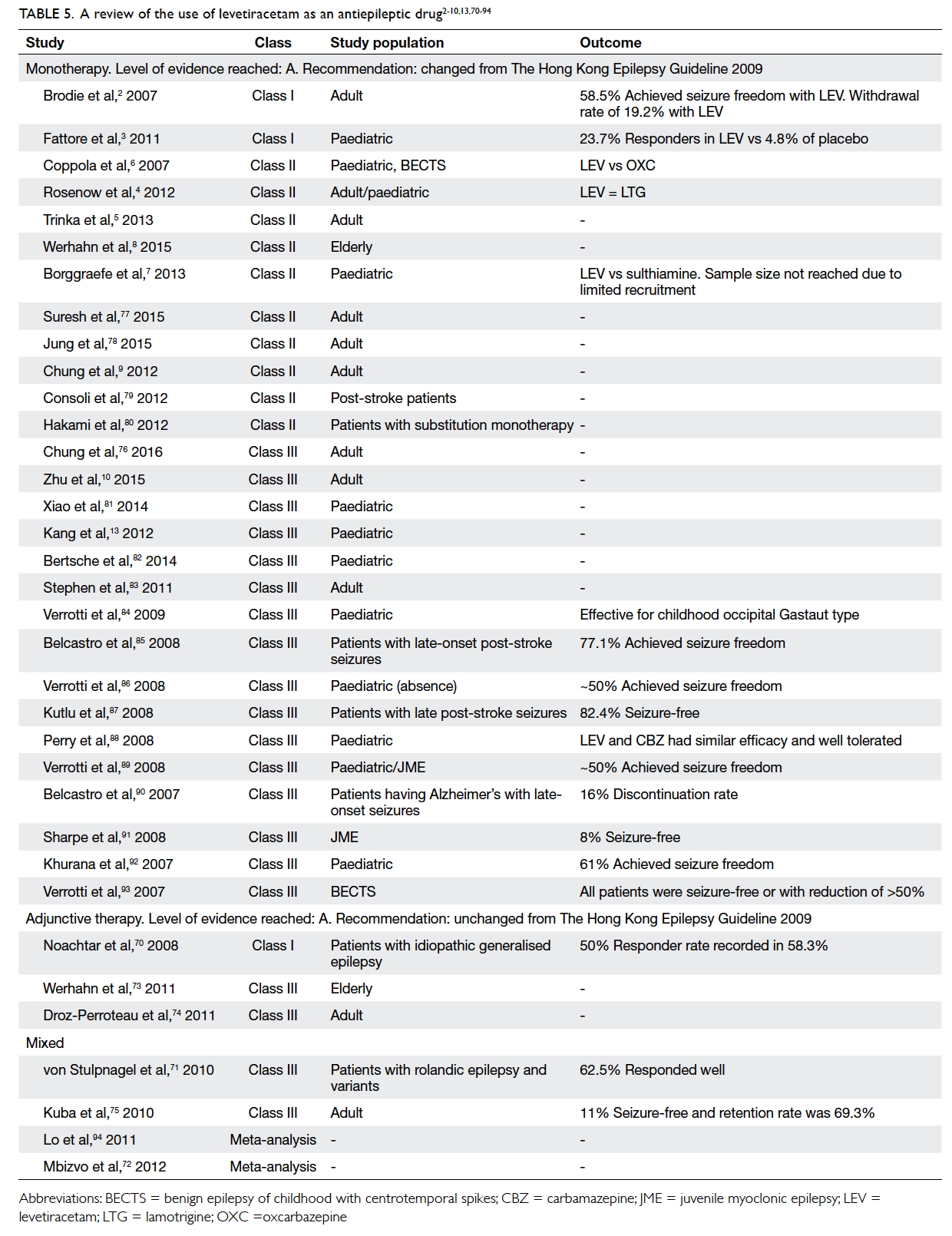
Table 5. A review of the use of levetiracetam as an antiepileptic drug2 3 4 5 6 7 8 9 10 13 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94
A review of the behavioural side-effects of LEV
revealed possible variation among paediatric and
adult subjects. Nervousness, aggression, and hostile
behaviour have been reported as putative behavioural
adverse events. In paediatric cohorts, the proportion
of such adverse events was 20% to 30%.70 71 72 94 By
comparison, the behavioural side-effects in adults
were less prominent.72 73 74 75 94
Statement 6: The level of evidence for LEV
adjunctive therapy remains unchanged (Level A).
Oxcarbazepine
One Class I study and three Class III studies (with
mixed indications) were identified (Table 112 13 14 15 16 17 18 19). In the study by the PROSPER Investigators Study Group,
adjunctive OXC reduced seizure magnitude by 38.2%
to 42.9%. Adverse event rates and safety profiles
suggested improved tolerability.95 Oxcarbazepine is
currently licensed for adjunctive therapy in epilepsy
and no change to the current recommended level of
evidence was made.
Statement 7: The level of evidence for OXC
adjunctive therapy remains unchanged (Level A).
Lacosamide
Three pivotal clinical studies outlined the clinical
usefulness of LCS in patients with refractory
epilepsy: one Phase II and two Phase III studies.76 96 97
These 12-week, randomised, double-blind, placebo-controlled,
multicentre trials enrolled subjects with
partial-onset seizures with or without secondary
generalisation who were not adequately controlled
with one to three concomitant AEDs. Study 1
compared doses of LCS 200, 400, and 600 mg/day
with placebo.96 Study 2 compared doses of LCS 400
and 600 mg/day with placebo.76 Study 3 compared
doses of LCS 200 and 400 mg/day with placebo.97
Following an 8-week phase to establish baseline
seizure frequency, subjects were titrated to the
randomised dose. During the titration phase in all
three trials, treatment was initiated at 100 mg/day
(50 mg given twice daily) and increased by weekly
increments of 100 mg/day to the target dose.
The titration phase lasted 6 weeks in Study 1 and
Study 2 and 4 weeks in Study 3. In all three trials,
the titration phase was followed by a maintenance
phase for 12 weeks. The primary endpoint was
reduction in 28-day seizure frequency (baseline
to maintenance phase) compared with the placebo
group. A statistically significant effect was observed
with LCS treatment at doses of 200 mg/day (Study
3), 400 mg/day (Study 1, 2, and 3), and 600 mg/day
(Study 1 and 2).
An observational phase IV open-label study to
assess the efficacy, safety, tolerability, and additional
outcomes of LCS in Hong Kong patients aged ≥18
years showed that LCS had efficacy and adverse
effects similar to those described in the literature
from other parts of the world. In a cohort of 105
patients, the proportion who achieved a 50%
reduction in seizure frequency was 54.5 with a mean
titration time of 6.75 weeks and a mean maintenance
dose of 158.6 mg/day. The efficacy profile was
satisfactory whether or not LCS was combined with
concomitant sodium channel blockers (45.8% vs
46.5%). The side-effect profile included apprehension
and aggression, drowsiness and tiredness, headache,
memory problems, dizziness, numbness, and gait
disturbance (local data).
Statement 8: The level of evidence for LCS as
adjunctive therapy reaches Level A.
Pregabalin
Three Class I studies, four Class III studies, and four
meta-analyses were found pertaining to PGB under
this indication (Table 314
46
47
48
49
50
51
52
53
54
55
56
57). One study evaluated
the efficacy and tolerability of adjunctive PGB as a
controlled-release formulation. The 50% responder
rate (ie percentage of patients achieving 50%
reduction in seizure frequency) was 45.9% for a daily
dose of 330 mg.98 Another randomised study tested
PGB versus LEV in a head-to-head comparison
in 409 patients. The drug PGB was non-inferior to
LEV with a similar tolerability to LEV as adjunctive
therapy.47 In a multicentre, randomised study of PGB
versus placebo, PGB was effective and tolerable as
adjunctive therapy in the Asian population.48 This
drug is currently licensed for adjunctive therapy
in epilepsy and there is no change to the level of
evidence regarding its recommended use.
Statement 9: The level of evidence for PGB as
adjunctive therapy remains unchanged (Level A).
Perampanel
A total of four Class I clinical studies demonstrated
the efficacy of PER among patients with refractory
epilepsy.58 59 60 61 These were all double-blind studies and
all evaluated the 50% responder rate as a seizure
outcome. The corresponding risk ratio for 50%
responder rate for 4 mg, 8 mg, and 12 mg were 1.54,
1.8, and 1.72. The most common treatment-emergent
adverse effects were dizziness, drowsiness, headache,
fatigue, and nasopharyngitis. The pooled results
suggested that a higher dose was more efficacious if
the side-effects could be tolerated.62 There was one
ongoing study on the use of PER among patients
with secondary generalised seizures.
Statement 10: The level of evidence for PER as
adjunctive therapy reaches Level A.
Part B: Generic drugs
The last version of The Hong Kong Epilepsy Guideline gave advice
on the use of generic drugs, details of which can be
revisited in the original guideline of 2009.1 There
might be a perceived difference between pharmaceutical
equivalence, which is the requirement of the
exact product, and bioequivalence, which is the concept
of assigning no difference among products in
terms of drug absorption. There have been positional
statements that outline the possible risks involved
when switching antiepileptic agents from a brand to a
generic preparation.99 Clinicians are understandably
perturbed by the prospect of inadvertent seizures and
loss of quality of life for their patients. The criteria
applied by authorities to license generic products
give rise to various issues. For instance, the concept of
bioequivalence does not require the generic product
to demonstrate clinical efficacy among patients.
Most bioequivalence studies are performed among
healthy subjects rather than individual patients.
Antiepileptic drugs are placed in the same category
as immunosuppressants and psychotropic drugs,
in which generic substitution is necessarily given
consideration before implementation. The benefit of
generic AEDs is clear in countries where health care
financing is either state-run or public-funded, but
may still be important in terms of patient choice in
countries where private health care or an insurance-based
system is practised because patients may want
to lower their premium by using generic products.
It may be argued that the use of generic products
will increase the potential availability of drugs to a
broader population of patients including those who
are underprivileged or resident in communities
where the drug budget is restricted.
There is a growing need for review and update
of recommended guidelines on issues related to
generic products as the evidence for newer drugs
has become more eminent. The prescription of
and expenditure on newer agents has risen sharply
over the last 5 to 10 years. Clinicians now have a far
greater number of AEDs at their disposal compared
with a decade ago. There is divided opinion in the
professional community about the use of generic
products and when it will be considered optimal and
safe for epilepsy patients. In general, communities
that rely on a state-financed or government-funded
health care system are under greater pressure to
consider generic product prescription, compared
with private-funded or out-of-pocket payment
health care financing systems.
Our literature search identified 13 studies
published in or after 2007 that fulfilled the initial
inclusion criteria. Four studies were of the Class I
category, one of the Class II category, and eight of
the Class III category (Table 6100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116). Six studies had
LTG as the study AED.100 101 102 103 104 105 Two studies had
topiramate as the study focus106 115 and the remaining studies adopted multiple drug regimens.107 108 109 110 111 A good
level of evidence came from a randomised controlled
trial of ‘generic-brittle’ patients in a double-blind,
multiple-dose, steady-state, fully replicated crossover
bioequivalence study of LTG. The
study demonstrated that the generic product
was bioequivalent to the brand medication. Such
observations were supported by the secondary
outcomes of seizure control and tolerability—32 of
35 patients reported no deterioration of seizures,
and dose-related adverse events were experienced
by 14 patients while on the generic product and
15 patients while on the brand product. The study
highlighted the use of the therapeutic level as a guide
over a period of time while the patient is switched
from brand to generic or vice versa.100 Two Class
I studies with preliminary results disseminated
during the annual meeting of the American Epilepsy
Society in 2015 showed no deviation from FDA’s
bioequivalence standards in Cmax and area under the
curve when comparing two most disparate generic
products in a single dose and chronic disease model
respectively (methodology given in Diaz et al in
2013101). One well-designed study of 35 patients
randomised patients from six epilepsy centres
to receive LTG as one of two treatment
sequences that comprised four study periods of 14
days each, during which time balanced doses of an
oral generic LTG product were given every
12 hours. Disparate generic LTG in patients
with epilepsy demonstrated bioequivalence with no
detectable difference in clinical effects.102 A similar
result was found from the only Class II study from
our literature search.103 The best level of evidence in
epilepsy patients supported the switch of LTG
(sodium channel blocker) from brand to generic
preparation. It remains controversial whether these
findings can be extrapolated to other AEDs because
LTG is by far one of the most widely used
first-line AEDs.
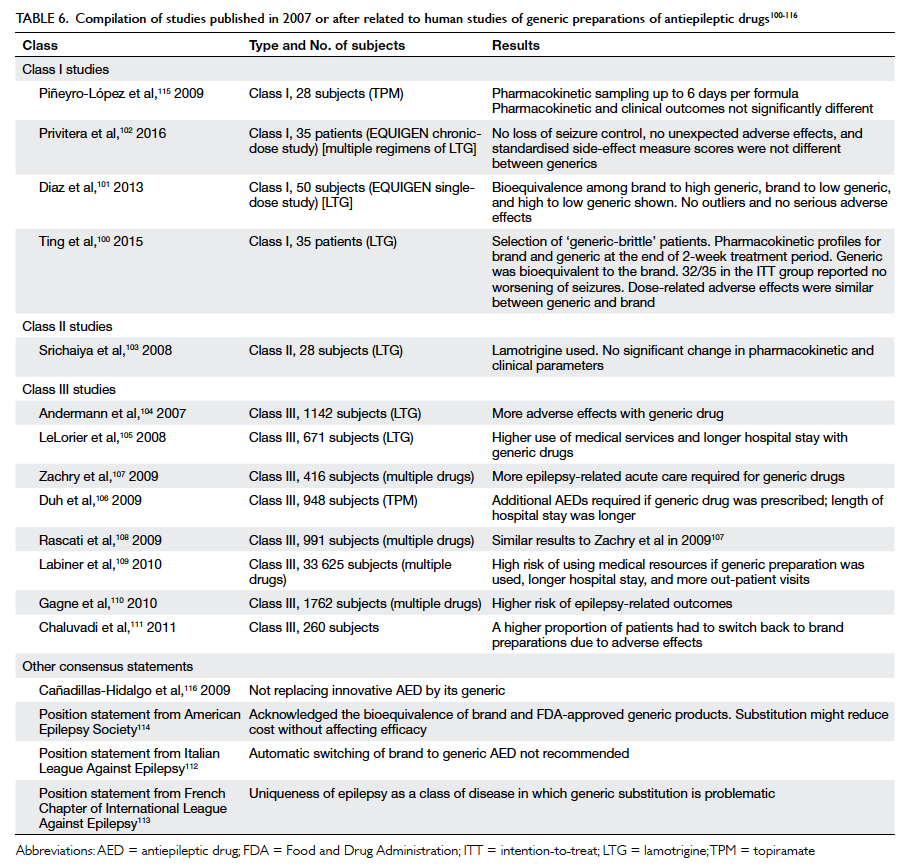
Table 6. Compilation of studies published in 2007 or after related to human studies of generic preparations of antiepileptic drugs100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116
Most Class III studies indicated an opposite
result compared with the Class I and II studies.
These studies showed that generic substitution may
result in increased acute seizure–related events and
higher use of medical services. The switch-back rates
for AEDs from generic to brand were higher in these
studies. Of note, these studies had larger sample
sizes but all the studies were retrospective in nature.
These studies might also have involved a wide range
of prescribing practices and some patient factors
might not have been taken into account.
Overall, most studies suggested bioequivalence
of brand and generic AEDs. This result was also in
keeping with a meta-analysis which concluded that
if only the highest level of evidence is considered,
there is no significant difference in terms of seizure
control, whether or not the patient is taking brand or
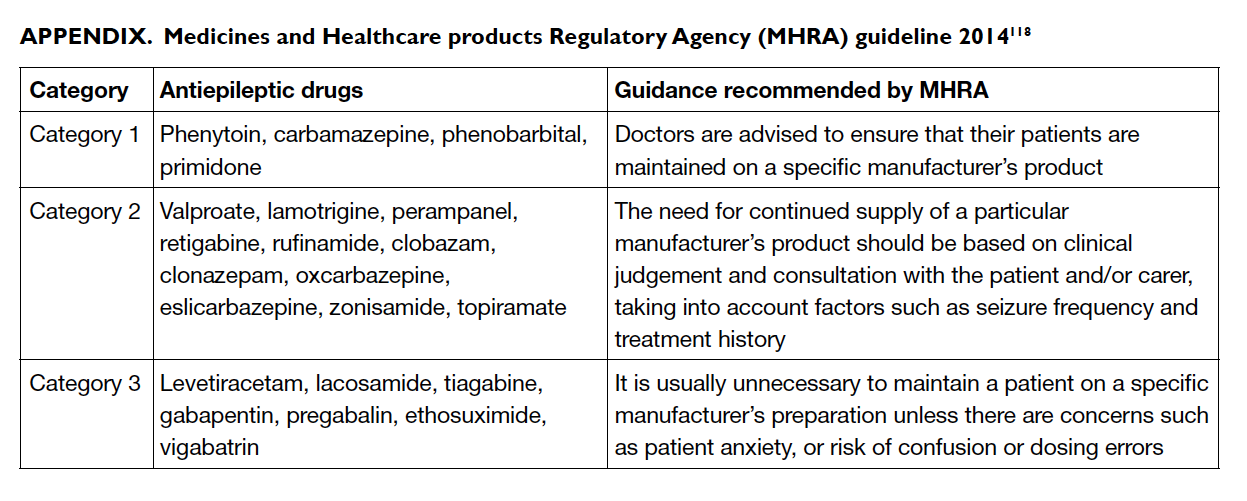
generic products.117 A UK pharmacovigilance body,
the Medicine and Healthcare products Regulatory
Agency, issued guidelines regarding the use of
generic products in 2013 and specifically divided
AEDs into three categories, each of which had
specific recommendations regarding the switching
of brand to generic products (Appendix).118
Category 1 relates to products among which a
specific manufacturer’s product should be ensured
(eg phenytoin, carbamazepine, phenobarbital, and
primidone). Category 2 relates to products for which
generic switching is considered neutral, but clinical
judgement should be exercised in so doing (eg
sodium valproate, LTG, OXC, topiramate).
Category 3 relates to products for which generic
substitution is considered safe (eg LEV, gabapentin)
[Table 6100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116]. The UK National Institute for Health
and Care Excellence guideline119 recommended that
a consistent supply should be made available to the
epilepsy patient unless the prescriber, in consultation
with the patient, considers that this is not a concern.
We acknowledge the controversy about
switching from a brand to a generic product. There
appears to be a divide in the positional statements
and guidelines between countries with public-funded
health care and those with private health care. Many
associations, including the Italian League Against
Epilepsy,112 American Academy of Neurology,114 and the French Chapter of ILAE113 have expressed
concerns about generic substitution of AEDs,
emphasising the uniqueness of epilepsy as a class of
disease in which generic substitution is problematic
when carried out for this indication. The latest
position statement from the American Epilepsy
Society acknowledges the bioequivalence of brand
and FDA-approved generic products and the fact that
substitution may reduce cost without compromising
efficacy. The Society advises the importance of
using either immediate-release or extended-release
preparations uniformly throughout the switching
process. They acknowledge that tablet or capsule
colour or shape may impact drug compliance. They
also state that the counselling of switching should
include an indication of bioequivalence and not
inferiority when the information is conveyed to the
patient(s) and their family members.114
A pilot study pioneered by the Hospital
Authority Head Office on the switching of phenytoin
from a generic back to a brand product due to supplier
issues suggested that proper counselling and follow-up
logistics in conjunction with a pre- and post-drug
level at 2 weeks may be adequate for the exercise.
In 40 patients recruited from the Prince of Wales
Hospital and Queen Mary Hospital, no patients
developed a toxic level of plasma phenytoin during
the switching process (four patients had a toxic-level
pre-switching that remained post-switching).
Plasma phenytoin concentration increased in 23
patients and decreased in 17. The conclusion was
that there was no consistent trend in the change of
plasma drug level (personal communication). Apart
from isolated cases of reported dizziness, no serious
adverse event occurred. The rate of hospitalisation as
a result of the switch in that study was not available
to us at the time of writing this review.
Statement 11: There is Level A evidence
for generic substitution of LTG (a sodium
channel blocker), taking into account the drug’s
pharmacodynamics and pharmacokinetics.
The HKES upholds the safety of patients
above all else. Following a review of the current
evidence, the HKES has made the following
revisions for the reference of physicians. Doctors
can initiate treatment in patients with epilepsy
with either a brand or generic product. Switching
from a brand to a generic product or between generic
products requires great care by clinicians and
health care administrators. Automatic substitution at a
pharmacy level is not recommended. If switching
takes place as a result of cost considerations,
prescriber and patient approval must be sought, in
liaison with the pharmacist. Prescriber approval is
not equivalent to a medical decision. The course
of treatment, including choice of drug and dosage,
is determined by the doctor and forms part of a
medical decision. When the use of generic drugs is
based on cost-effective analyses, prescriber approval
is a logistic and economic decision. Depending on
the type of health care setting, a request for generic
substitution may begin with the patient or the health
administrator, in liaison with the attending doctor/pharmacist. Patient approval may not be equivalent
to medical consent. This can be a requirement of
the health care system to which the patient belongs
or a self-initiated step from the patient who has
subscribed to insurance plans with affordable
premiums. The physician should discuss any switch
with the patient from both a medical and layman’s
perspective. Good communication is considered
fundamental to the provision of care.120 Therefore, in
a private health care system, the choice for generic
drugs may begin with a patient’s request, followed
by prescriber approval. In a public health care
system, the choice for generic drugs may begin with
prescriber’s request, followed by patient approval.
Follow-up and monitoring logistics should be
mutually agreed to ensure patient safety. A change in
the physical appearance of medications may hinder
compliance. This facet of the switch must be taken
into account by all parties. In the special situation
where switching from a brand to a generic product takes
place among patients who have achieved remission
while on antiepileptic therapy, clinicians must take
into account the drug’s pharmacokinetics and the
support of medical services. Assistance from nursing
staff, enlisting therapeutic blood monitoring, and the
option to use the AED as a self-financed item (both
public and private setting) should be made available.
Statement 12: Controversy exists over the
use of generic products among patients who are
currently taking brand medications. Prescriber
and patient approval is pivotal. There should
be good communication between doctors and
patients; enlisting assistance from doctors, nurses,
and pharmacists; therapeutic blood monitoring if
available; and the option of brand AED as a self-financed
item. The physical appearance of generic
drugs may hamper drug compliance. Support from
medical services is recommended. In the longer
term, the benefit of flexibility and the option to have
balanced use of generic and brand drugs may need to
be addressed by institutions and regulatory bodies.
Conclusions
New evidence on AEDs has arisen since the
publication of the Hong Kong Epilepsy Guideline in
2009. There is Level A evidence for LEV monotherapy
and Level B evidence for LCS monotherapy. There
is Level A evidence for LCS and PER adjunctive
therapy. No change to the level of evidence is
evident for LEV, OXC, and PGB. The use of generic
preparations of AEDs should be considered following
prescriber and patient approval, with support from
medical services (doctors, nurses, pharmacists). It is
important to emphasise that a generic preparation is
not inferior, that shape and colour of tablets may be
different, there may be therapeutic blood monitoring
(if available), and patients may have the option of
self-financing items.
Appendix
Additional material related to this article can be
found on the HKMJ website. Please go to <http://www.hkmj.org>, and search for the article.
Acknowledgement
This project was supported in part by an unrestricted
grant from the Hong Kong Epilepsy Society.
Disclaimer
This consensus statement is designed to assist
clinicians by providing an analytical framework for
the drug treatment of epilepsy. It is not intended to
establish a community standard of care, replace a
clinician’s medical judgement, or establish a protocol
for all patients.
References
1. Guideline Development Group, Hong Kong Epilepsy
Society. The Hong Kong Epilepsy Guideline 2009. Hong
Kong Med J 2009;15 Suppl 5:6S-28S.
2. Brodie MJ, Perucca E, Ryvlin P, et al. Comparison of
levetiracetam and controlled-release carbamazepine in
newly diagnosed epilepsy. Neurology 2007;68:402-8. Crossref
3. Fattore C, Boniver C, Capovilla G, et al. A multicenter,
randomized, placebo-controlled trial of levetiracetam in
children and adolescents with newly diagnosed absence
epilepsy. Epilepsia 2011;52:802-9. Crossref
4. Rosenow F, Schade-Brittinger C, Burchardi N, et al. The
LaLiMo Trial: lamotrigine compared with levetiracetam
in the initial 26 weeks of monotherapy for focal and
generalised epilepsy—an open label, prospective,
randomised controlled multicenter study. J Neurol
Neurosurg Psychiatry 2012;83:1093-8. Crossref
5. Trinka E, Marson AG, Van Paesschen W, et al. KOMET:
an unblinded, randomised, two parallel-group, stratified
trial comparing the effectiveness of levetiracetam with
controlled-release carbamazepine and extended-release
sodium valproate as monotherapy in patients with newly
diagnosed epilepsy. J Neurol Neurosurg Psychiatry
2013;84:1138-47. Crossref
6. Coppola G, Franzoni E, Verrotti A, et al. Levetiracetam
or oxcarbazepine as monotherapy in newly diagnosed
benign epilepsy of childhood with centrotemporal spikes
(BECTS): An open-label, parallel group trial. Brain Dev
2007;29:281-4. Crossref
7. Borggraefe I, Bonfert M, Bast T, et al. Levetiracetam vs.
sulthiame in benign epilepsy with centrotemporal spikes
in childhood: A double-blinded, randomized, controlled
trial (German HEAD study). Eur J Paediatr Neurol
2013;17:507-14. Crossref
8. Werhahn KJ, Trinka E, Dobesberger J, et al. A randomized,
double-blind comparison of antiepileptic drug treatment
in the elderly with new-onset focal epilepsy. Epilepsia
2015;56:450-9. Crossref
9. Chung S, Ceja H, Gawlowicz J, et al. Levetiracetam
extended release conversion to monotherapy for the
treatment of patients with partial-onset seizures: A
double-blind, randomised, multicentre, historical control
study. Epilepsy Res 2012;101:92-102. Crossref
10. Zhu F, Lang SY, Wang XQ, et al. Long-term effectiveness
of antiepileptic drug monotherapy in partial epileptic
patients: A 7-year study in an epilepsy center in China.
Chin Med J (Engl) 2015;128:3015-22. Crossref
11. Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated
ILAE evidence review of antiepileptic drug efficacy and
effectiveness as initial monotherapy for epileptic seizures
and syndromes. Epilepsia 2013;54:551-63. Crossref
12. Franzoni E, Gentile V, Pellicciari A, et al. Prospective
study on long-term treatment with oxcarbazepine in
pediatric epilepsy. J Neurol 2009;256:1527-32. Crossref
13. Kang HC, Hu Q, Liu XY, et al. A follow-up study on newer
anti-epileptic drugs as add-on and monotherapy for
partial epilepsy in China. Chin Med J (Engl) 2012;125:646-51.
14. French J, Kwan P, Fakhoury T, et al. Pregabalin
monotherapy in patients with partial-onset seizures: a
historical-controlled trial. Neurology 2014;82:590-7. Crossref
15. Koch MW, Polman SK. Oxcarbazepine versus
carbamazepine monotherapy for partial onset seizures.
Cochrane Database Syst Rev 2009;(4):CD006453. Crossref
16. Eun SH, Kim HD, Chung HJ, et al. A multicenter trial of
oxcarbazepine oral suspension monotherapy in children
newly diagnosed with partial seizures: A clinical and
cognitive evaluation. Seizure. 2012;21:679-84. Crossref
17. Dogan EA, Usta BE, Bilgen R, Senol Y, Aktekin B.
Efficacy, tolerability, and side effects of oxcarbazepine
monotherapy: A prospective study in adult and elderly
patients with newly diagnosed partial epilepsy. Epilepsy
Behav 2008;13:156-61. Crossref
18. Lee SA, Heo K, Kim WJ, et al. Clinical feasibility of
immediate overnight switching from slow-release
carbamazepine to oxcarbazepine in Korean patients with
refractory partial epilepsy. Seizure 2010;19:356-8. Crossref
19. Seneviratne U, D’Souza W, Cook M. Long-term
assessment of oxcarbazepine in a naturalistic setting: a
retrospective study. Acta Neurol Scand 2008;117:367-9. Crossref
20. Wechsler RT, Li G, French J, et al. Conversion to
lacosamide monotherapy in the treatment of focal
epilepsy: Results from a historical-controlled, multicenter,
double-blind study. Epilepsia 2014;55:1088-98. Crossref
21. Giráldez BG, Toledano R, García-Morales I, et al. Long-term
efficacy and safety of lacosamide monotherapy in
the treatment of partial-onset seizures: a multicenter
evaluation. Seizure 2015;29:119-22. Crossref
22. Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini
M. Lacosamide monotherapy for partial onset seizures.
Seizure 2015;27:71-4. Crossref
23. Husain A, Chung S, Faught E, Isojarvi J, McShea C,
Doty P. Long-term safety and efficacy in patients
with uncontrolled partial-onset seizures treated with
adjunctive lacosamide: results from a Phase III open-label
extension trial. Epilepsia 2012;53:521-8. Crossref
24. Runge U, Arnold S, Brandt C, et al. A noninterventional
study evaluating the effectiveness and safety of lacosamide
added to monotherapy in patients with epilepsy with
partial-onset seizures in daily clinical practice: The
VITOBA study. Epilepsia 2015;56:1921-30. Crossref
25. Stephen LJ, Kelly K, Parker P, Brodie MJ. Adjunctive
lacosamide—5 years’ clinical experience. Epilepsy Res
2014;108:1385-91. Crossref
26. Pasha I, Kamate M, Didagi SK. Efficacy and tolerability
of lacosamide as an adjunctive therapy in children with
refractory partial epilepsy. Pediatr Neurol 2014;51:509-14. Crossref
27. Rosenfeld W, Fountain NB, Kaubrys G, et al. Safety and
efficacy of adjunctive lacosamide among patients with
partial-onset seizures in a long-term open-label extension
trial of up to 8 years. Epilepsy Behav 2014;41:164-70. Crossref
28. Gulati P, Cannell P, Ghia T, et al. Lacosamide as adjunctive
therapy in treatment-resistant epilepsy in childhood. J
Paediatr Child Health 2015;51:794-7. Crossref
29. Rosenow F, Kelemen A, Ben-Menachem E, et al. Long-term
adjunctive lacosamide treatment in patients with
partial-onset seizures. Acta Neurol Scand 2015 Jul 2. Epub
ahead of print.
30. Geffrey AL, Belt OD, Paolini JL, Thiele EA. Lacosamide
use in the treatment of refractory epilepsy in tuberous
sclerosis complex. Epilepsy Res 2015;112:72-5. Crossref
31. Flores L, Kemp S, Colbeck K, et al. Clinical experience
with oral lacosamide as adjunctive therapy in adult
patients with uncontrolled epilepsy: a multicentre study
in epilepsy clinics in the United Kingdom (UK). Seizure
2012;21:512-7. Crossref
32. Kamel JT, DeGruyter MA, D’Souza WJ, Cook MJ. Clinical
experience with using lacosamide for the treatment
of epilepsy in a tertiary centre. Acta Neurol Scand
2013;127:149-53. Crossref
33. Verrotti A, Loiacono G, Pizzolorusso A, et al. Lacosamide
in pediatric and adult patients: comparison of efficacy and
safety. Seizure 2013;22:210-6. Crossref
34. Toupin JF, Lortie A, Major P, et al. Efficacy and safety of
lacosamide as an adjunctive therapy for refractory focal
epilepsy in paediatric patients: a retrospective single-centre
study. Epileptic Disord 2015;17:436-43.
35. Zadeh WW, Escartin A, Byrnes W, et al. Efficacy and
safety of lacosamide as first add-on or later adjunctive
treatment for uncontrolled partial-onset seizures: A
multicentre open-label trial. Seizure 2015;31:72-9. Crossref
36. Rastogi RG, Ng YT. Lacosamide in refractory mixed
pediatric epilepsy: a prospective add-on study. J Child
Neurol 2012;27:492-5. Crossref
37. Grosso S, Parisi P, Spalice A, Verrotti A, Balestri P. Efficacy
and safety of lacosamide in infants and young children
with refractory focal epilepsy. Eur J Paediatr Neurol
2014;18:55-9. Crossref
38. Grosso S, Coppola G, Cusmai R, et al. Efficacy and
tolerability of add-on lacosamide in children with Lennox-Gastaut syndrome. Acta Neurol Scand 2014;129:420-4. Crossref
39. Lee JW, Alam J, Llewellyn N, et al. Open label trial of
add on lacosamide versus high dose levetiracetam
monotherapy in patients with breakthrough seizures.
Clin Neuropharmacol 2016;39:128-31. Crossref
40. Buck ML, Goodkin HP. Use of lacosamide in children
with refractory epilepsy. J Pediatr Pharmacol Ther
2012;17:211-9. Crossref
41. Paquette V, Culley C, Greanya ED, Ensom MH.
Lacosamide as adjunctive therapy in refractory epilepsy
in adults: a systematic review. Seizure 2015;25:1-17. Crossref
42. Biton V, Gil-Nagel A, Isojarvi J, et al. Safety and tolerability
of lacosamide as adjunctive therapy for adults with
partial-onset seizures: Analysis of data pooled from three
randomized, double-blind, placebo-controlled clinical
trials. Epilepsy Behav 2015;52:119-27. Crossref
43. Sawh SC, Newman JJ, Deshpande S, Jones PM.
Lacosamide adjunctive therapy for partial-onset seizures:
a meta-analysis. PeerJ 2013;1:e114. Crossref
44. Yorns WR Jr, Khurana DS, Carvalho KS, Hardison HH,
Legido A, Valencia I. Efficacy of lacosamide as adjunctive
therapy in children with refractory epilepsy. J Child
Neurol 2014;29:23-7. Crossref
45. Novy J, Bartolini E, Bell GS, Duncan JS, Sander JW.
Long-term retention of lacosamide in a large cohort of
people with medically refractory epilepsy: a single centre
evaluation. Epilepsy Res 2013;106:250-6. Crossref
46. Kwan P, Brodie MJ, Kälviäinen R, Yurkewicz L, Weaver
J, Knapp LE. Efficacy and safety of pregabalin versus
lamotrigine in patients with newly diagnosed partial
seizures: a phase 3, double-blind, randomised, parallel-group
trial. Lancet Neurol 2011;10:881-90. Crossref
47. Zaccara G, Almas M, Pitman V, Knapp L, Posner H.
Efficacy and safety of pregabalin versus levetiracetam
as adjunctive therapy in patients with partial seizures: a
randomized, double-blind, noninferiority trial. Epilepsia
2014;55:1048-57. Crossref
48. Lee BI, Yi S, Hong SB, et al. Pregabalin add-on therapy
using a flexible, optimized dose schedule in refractory
partial epilepsies: a double-blind, randomized, placebo-controlled,
multicenter trial. Epilepsia 2009;50:464-74. Crossref
49. Zhou Q, Zheng J, Yu L, Jia X. Pregabalin monotherapy for
epilepsy. Cochrane Database Syst Rev 2012;(10):CD009429. Crossref
50. Valentin A, Moran N, Hadden R, et al. Pregabalin as
adjunctive therapy for partial epilepsy: an audit study
in 96 patients from the South East of England. Seizure
2009;18:450-2. Crossref
51. Tsounis S, Kimiskidis VK, Kazis D, et al. An open-label,
add-on study of pregabalin in patients with partial seizures:
a multicenter trial in Greece. Seizure 2011;20:701-5. Crossref
52. Stephen LJ, Parker P, Kelly K, Wilson EA, Leach V, Brodie
MJ. Adjunctive pregabalin for uncontrolled partial-onset
seizures: findings from a prospective audit. Acta Neurol
Scand 2011;124:142-5. Crossref
53. Ryvlin P, Kälviäinen R, Von Raison F, Giordano S, Emir B,
Chatamra K. Pregabalin in partial seizures: a pragmatic
21-week, open-label study (PREPS). Eur J Neurol
2010;17:726-32. Crossref
54. Pulman J, Hemming K, Marson AG. Pregabalin add-on
for drug-resistant partial epilepsy. Cochrane Database
Syst Rev 2014;(3):CD005612. Crossref
55. Lozsadi D, Hemming K, Marson AG. Pregabalin add-on
for drug-resistant partial epilepsy. Cochrane Database
Syst Rev 2008;(1):CD005612.
56. Gil-Nagel A, Zaccara G, Baldinetti F, Leon T. Add-on
treatment with pregabalin for partial seizures with
or without generalisation: pooled data analysis of
four randomised placebo-controlled trials. Seizure
2009;18:184-92. Crossref
57. Uthman BM, Bazil CW, Beydoun A, et al. Long-term
add-on pregabalin treatment in patients with partial-onset
epilepsy: pooled analysis of open-label clinical
trials. Epilepsia 2010;51:968-78. Crossref
58. French JA, Krauss GL, Wechsler RT, et al. Perampanel for
tonic-clonic seizures in idiopathic generalized epilepsy. A
randomized trial. Neurology 2015;85:950-7. Crossref
59. French JA, Krauss GL, Steinhoff BJ, et al. Evaluation of
adjunctive perampanel in patients with refractory partial-onset
seizures: results of randomized global phase III
study 305. Epilepsia 2013;54:117-25. Crossref
60. French JA, Krauss GL, Biton V, et al. Adjunctive
perampanel for refractory partial-onset seizures. Randomized phase III study 304. Neurology 2012;79:589-96. Crossref
61. Krauss GL, Serratosa JM, Villanueva V, et al. Randomized
phase III study 306. Adjunctive perampanel for refractory
partial-onset seizures. Neurology 2012;78:1408-15. Crossref
62. Kwan P, Brodie MJ, Laurenza A, FitzGibbon H, Gidal
BE. Analysis of pooled phase III trials of adjunctive
perampanel for epilepsy: Impact of mechanism of action
and pharmacokinetics on clinical outcomes. Epilepsy Res
2015;117:117-24. Crossref
63. Juhl S, Rubboli G. Perampanel as add-on treatment in
refractory focal epilepsy. The Dianalund experience. Acta
Neurol Scand 2016;134:374-7. Crossref
64. Brodie MJ, Stephen LJ. Prospective audit with adjunctive
perampanel: Preliminary observations in focal epilepsy.
Epilepsy Behav 2016;54:100-3. Crossref
65. Shah E, Reuber M, Goulding P, Flynn C, Delanty N,
Kemp S. Clinical experience with adjunctive perampanel
in adult patients with uncontrolled epilepsy: A UK and
Ireland multicentre study. Seizure 2016;34:1-5. Crossref
66. Steinhoff BJ, Hamer H, Trinka E, et al. A multicenter
survey of clinical experiences with perampanel in real life
in Germany and Austria. Epilepsy Res 2014;108:986-8. Crossref
67. Kramer LD, Satlin A, Krauss GL, et al. Perampanel for
adjunctive treatment of partial-onset seizures: A pooled
dose-response analysis of phase III studies. Epilepsia
2014;55:423-31. Crossref
68. Steinhoff BJ, Bacher M, Bast T, et al. First clinical
experiences with perampanel—The Kork experience in 74
patients. Epilepsia 2014;55 Suppl 1:16-8. Crossref
69. Hsu WW, Sing CW, He Y, Worsley AJ, Wong IC, Chan
EW. Systematic review and meta-analysis of the efficacy
and safety of perampanel in the treatment of partial-onset
epilepsy. CNS Drugs 2013;27:817-27. Crossref
70. Noachtar S, Andermann E, Meyvisch P, et al. Levetiracetam
for the treatment of idiopathic generalized epilepsy with
myoclonic seizures. Neurology 2008;70:607-16. Crossref
71. von Stulpnagel C, Kluger G, Leiz S, Holthausen H.
Levetiracetam as add-on therapy in different subgroups of
“benign” idiopathic focal epilepsies in childhood. Epilepsy
Behav 2010;17:193-8. Crossref
72. Mbizvo GK, Dixon P, Hutton JL, Marson AG.
Levetiracetam add-on for drug-resistant focal epilepsy: an
updated Cochrane Review. Cochrane Database Syst Rev
2012;(9):CD001901. Crossref
73. Werhahn KJ, Klimpe S, Balkaya S, Trinka E, Krämer G.
The safety and efficacy of add-on levetiracetam in elderly
patients with focal epilepsy: A one-year observational
study. Seizure 2011;20:305-11. Crossref
74. Droz-Perroteau C, Dureau-Pournin C, Vespignani H, et al.
The EULEV cohort study: rates of and factors associated
with continuation of levetiracetam after 1 year. Br J Clin
Pharmacol 2011;71:121-7. Crossref
75. Kuba R, Novotná I, Brázdil M, et al. Long-term
levetiracetam treatment in patients with epilepsy: 3-year
follow up. Acta Neurol Scand 2010;121:83-8. Crossref
76. Chung S, Ceja H, Gawłowicz J, McShea C, Schiemann J,
Lu S. Levetiracetam extended release for the treatment of
patients with partial-onset seizures: A long-term, open-label
follow-up study. Epilepsy Res 2016;120:7-12. Crossref
77. Suresh SH, Chakraborty A, Virupakshaiah A, Kumar N.
Efficacy and safety of levetiracetam and carbamazepine
as monotherapy in partial seizures. Epilepsy Res Treat
2015;2015:415082. Crossref
78. Jung DE, Yu R, Yoon JR, et al. Neuropsychological effects
of levetiracetam and carbamazepine in children with focal
epilepsy. Neurology 2015;84:2312-9. Crossref
79. Consoli D, Bosco D, Postorino P, et al. Levetiracetam
versus carbamazepine in patients with late poststroke
seizures: a multicenter prospective randomized open-label
study (EpIC Project). Cerebrovasc Dis 2012;34:282-9. Crossref
80. Hakami T, Todaro M, Petrovski S, et al. Substitution
monotherapy with levetiracetam vs older antiepileptic
drugs: A randomized comparative trial. Arch Neurol
2012;69:1563-71. Crossref
81. Xiao F, An D, Deng H, Chen S, Ren J, Zhou D. Evaluation
of levetiracetam and valproic acid as low-dose
monotherapies for children with typical benign childhood
epilepsy with centrotemporal spikes (BECTS). Seizure
2014;23:756-61. Crossref
82. Bertsche A, Neininger MP, Dahse AJ, et al. Initial
anticonvulsant monotherapy in routine care of children
and adolescents: levetiracetam fails more frequently than
valproate and oxcarbazepine due to a lack of effectiveness.
Eur J Pediatr 2014;173:87-92. Crossref
83. Stephen LJ, Kelly K, Parker P, Brodie MJ. Levetiracetam
monotherapy—outcomes from an epilepsy clinic. Seizure
2011;20:554-7. Crossref
84. Verrotti A, Parisi P, Loiacono G, et al. Levetiracetam
monotherapy for childhood occipital epilepsy of gastaut.
Acta Neurol Scand 2009;120:342-6. Crossref
85. Belcastro V, Costa C, Galletti F, et al. Levetiracetam
in newly diagnosed late-onset post-stroke seizures:
A prospective observational study. Epilepsy Res
2008;82:223-6. Crossref
86. Verrotti A, Cerminara C, Domizio S, et al. Levetiracetam
in absence epilepsy. Dev Med Child Neurol 2008;50:850-3. Crossref
87. Kutlu G, Gomceli YB, Unal Y, Inan LE. Levetiracetam
monotherapy for late poststroke seizures in the elderly.
Epilepsy Behav 2008;13:542-4. Crossref
88. Perry S, Holt P, Benatar M. Levetiracetam versus
carbamazepine monotherapy for partial epilepsy in
children less than 16 years of age. J Child Neurol 2008;23:515-9. Crossref
89. Verrotti A, Cerminara C, Coppola G, et al. Levetiracetam
in juvenile myoclonic epilepsy: long-term efficacy in
newly diagnosed adolescents. Dev Med Child Neurol
2008;50:29-32. Crossref
90. Belcastro V, Costa C, Galletti F, et al. Levetiracetam
monotherapy in Alzheimer patients with late-onset
seizures: a prospective observational study. Eur J Neurol
2007;14:1176-8. Crossref
91. Sharpe DV, Patel AD, Abou-Khalil B, Fenichel GM.
Levetiracetam monotherapy in juvenile myoclonic
epilepsy. Seizure 2008;17:64-8. Crossref
92. Khurana DS, Kothare SV, Valencia I, Melvin JJ, Legido A. Levetiracetam monotherapy in children with epilepsy.
Pediatr Neurol 2007;36:227-30. Crossref
93. Verrotti A, Coppola G, Manco R, et al. Levetiracetam
monotherapy for children and adolescents with benign
rolandic seizures. Seizure 2007;16:271-5. Crossref
94. Lo BW, Kyu HH, Jichici D, Upton AM, Akl EA, Meade
MO. Meta-analysis of randomized trials on first line and
adjunctive levetiracetam. Can J Neurol Sci 2011;38:475-86. Crossref
95. French JA, Baroldi P, Brittain ST, Johnson JK; PROSPER
Investigators Study Group. Efficacy and safety of
extended-release oxcarbazepine (Oxtellar XR) as
adjunctive therapy in patients with refractory partial-onset
seizures: a randomized controlled trial. Acta Neurol
Scand 2014;129:143-53. Crossref
96. Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B,
Doty P, Rudd GD. Efficacy and safety of oral lacosamide as
adjunctive therapy in adults with partial-onset seizures.
Epilepsia 2007;48:1308-17. CrossRef
97. Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, et al. Adjunctive lacosamide for partial-onset seizures: efficacy
and safety results from a randomized controlled trial.
Epilepsia 2009;50:443-53. Crossref
98. French J, Brandt C, Friedman D, et al. Adjunctive use of
controlled-release pregabalin in adults with treatment-resistant
partial seizures: a double-blind, randomized,
placebo-controlled trial. Epilepsia 2014;55:1220-8. Crossref
99. Liow K, Barkley GL, Pollard JR, Harden CL, Bazil CW;
American Academy of Neurology. Position statement on
the coverage of anticonvulsant drugs for the treatment of
epilepsy. Neurology 2007;68:1249-50. Crossref
100. Ting TY, Jiang W, Lionberger R, et al. Generic lamotrigine
versus brand-name Lamictal bioequivalence in patients
with epilepsy: A field test of the FDA bioequivalence
standard. Epilepsia 2015;56:1415-24. Crossref
101. Diaz FJ, Berg MJ, Krebill R, et al. Random-effects linear
modeling and sample size tables for two special crossover
designs of average bioequivalence studies: the four-period,
two-sequence, two-formulation and six-period, three-sequence,
three-formulation designs. Clin Pharmacokinet
2013;52:1033-43. Crossref
102. Privitera MD, Welty TE, Gidal BE, et al. Generic-to-generic
lamotrigine switches in people with epilepsy: the
randomised controlled EQUIGEN trial. Lancet Neurol
2016;15:365-72. Crossref
103. Srichaiya A, Longchoopol C, Oo-Puthinan S, Sayasathid
J, Sripalakit P, Viyoch J. Bioequivalence of generic
lamotrigine 100-mg tablets in healthy Thai male
volunteers: A randomized, single-dose, two-period, two-sequence
crossover study. Clin Ther 2008;30:1844-51. Crossref
104. Andermann F, Duh MS, Gosselin A, Paradis PE.
Compulsory generic switching of antiepileptic drugs: High
switchback rates to branded compounds compared with
other drug classes. Epilepsia 2007;48:464-9. Crossref
105. LeLorier J, Duh MS, Paradis PE, et al. Clinical
consequences of generic substitution of lamotrigine for
patients with epilepsy. Neurology 2008;70(22 Pt 2):2179-86. Crossref
106. Duh MS, Paradis PE, Latrémouille-Viau D, et al. The risks
and costs of multiple-generic substitution of topiramate.
Neurology 2009;72:2122-9. Crossref
107. Zachry WM 3rd, Doan QD, Clewell JD, Smith BJ. Case-control
analysis of ambulance, emergency room, or
inpatient hospital events for epilepsy and antiepileptic
drug formulation changes. Epilepsia 2009;50:493-500. Crossref
108. Rascati KL, Richards KM, Johnsrud MT, Mann TA. Effects
of antiepileptic drug substitutions on epileptic events
requiring acute care. Pharmacotherapy 2009;29:769-74. Crossref
109. Labiner DM, Paradis PE, Manjunath R, et al. Generic
antiepileptic drugs and associated medical resource
utilization in the United States. Neurology 2010;74:1566-74. Crossref
110. Gagne JJ, Avorn J, Shrank WH, Schneeweiss S. Refilling
and switching of antiepileptic drugs and seizure-related
events. Clin Pharmacol Ther 2010;88:347-53. Crossref
111. Chaluvadi S, Chiang S, Tran L, Goldsmith CE, Friedman
DE. Clinical experience with generic levetiracetam in
people with epilepsy. Epilepsia 2011;52:810-5. Crossref
112. Perucca E, Albani F, Capovilla G, Bernardina BD,
Michelucci R, Zaccara G. Recommendations of the
Italian League Against Epilepsy working group on generic
products of antiepileptic drugs. Epilepsia 2006;47 Suppl
5:16-20. Crossref
113. French Chapter of the International League Against
Epilepsy (LFCE): Recommendations on the use of generics
for the treatment of epilepsy. Available from: http://www.ilae.org/visitors/MeetingProceedings/documents/PRESSRELEASEONGENERICAEDsFRENCHCHAPTER
OFTHEILAE_000.pdf. Accessed Mar 2016.
114. American Epilepsy
Society. Substitution of different formulations of antiepileptic
drugs for the treatment of epilepsy. Available from: https://www.aesnet.org/about_aes/generic-position-statement. Accessed Mar 2016.
115. Piñeyro-López A, Piñeyro-Garza E, Gómez-Silva M, et al. Bioequivalence of single 100-mg doses of two oral
formulations of topiramate: An open-label, randomized-sequence,
two-period crossover study in healthy adult
male Mexican volunteers. Clin Ther 2009;31:411-7. Crossref
116. Cañadillas-Hidalgo FM, Sánchez-Alvarez JC, Serrano-Castro PJ, Mercadé-Cerdá JM; en representación de
la Sociedad Andaluza de Epilepsia. Consensus clinical
practice guidelines of the Andalusian Epilepsy Society on
prescribing generic antiepileptic drugs [in Spanish]. Rev
Neurol 2009;49:41-7.
117. Yamada M, Welty TE. Generic substitution of antiepileptic
drugs: a systematic review of prospective and retrospective
studies. Ann Pharmacother 2011;45:1406-15. Crossref
118. Medicines & Healthcare products Regulatory Agency.
Available from: https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency. Accessed Mar 2016.
119. NICE guidance CG137. Epilepsies: diagnosis and
management. Available from: https://www.nice.org.uk/guidance/cg137. Accessed Mar 2016.
120. The Medical Council of Hong Kong. Available from:
http://www.mchk.org.hk/code.htm. Accessed Mar 2016.