Hong Kong Med J 2017 Feb;23(1):13–8 | Epub 2 Dec 2016
DOI: 10.12809/hkmj154617
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Comparison of green pit viper and Agkistrodon
halys antivenom in inhibition of coagulopathy
due to Trimeresurus albolabris venom: an in-vitro
study using human plasma
SK Lam, FHKAM (Emergency Medicine)1;
SF Yip, FHKAM (Pathology), FHKAM (Medicine)2;
Paul Crow, BSc (Zoology)3;
HT Fung, FHKAM (Emergency Medicine)1;
Jeff MH Cheng, MSc (Biomedical Science)4;
KS Tan, BSc3;
OF Wong, FHKAM (Emergency Medicine), FHKAM (Anaesthesiology)5;
Daisy YT Yeung, BSc (Biomedical Science)4;
YK Wong, BSc3;
KM Poon, FHKAM (Emergency Medicine)1;
Gary Ades, PhD3
1 Department of Accident and Emergency, Tuen Mun Hospital, Tuen Mun, Hong Kong
2 Department of Pathology and Department of Medicine and Geriatrics, Tuen Mun Hospital, Tuen Mun, Hong Kong
3 Fauna Conservation Department, Kadoorie Farm and Botanic Garden, Tai Po, Hong Kong
4 Department of Pathology, Tuen Mun Hospital, Tuen Mun, Hong Kong
5 Department of Accident and Emergency, North Lantau Hospital, Lantau, Hong Kong
Corresponding authors: Dr SK Lam (tommylam@yahoo.com)
Abstract
Introduction: There are two antivenoms that may
be administered in Hong Kong following a bite
by Trimeresurus albolabris: the green pit viper
antivenom from the Thai Red Cross Society in
Thailand and the Agkistrodon halys antivenom from
the Shanghai Institute of Biological Products in China.
Both are recommended by the Central Coordinating
Committee of Accident and Emergency Services of
the Hospital Authority for treating patients with a
bite by Trimeresurus albolabris. The choice of which
antivenom to use is based on physician preference.
This study aimed to compare the relative efficacy of
the two antivenoms.
Methods: This in-vitro experimental study was
carried out by a wildlife conservation organisation
and a regional hospital in Hong Kong. Human
plasma from 40 adult health care worker volunteers
was collected. The Trimeresurus albolabris venom
was added to human plasma and the mixture was
assayed after incubation with each antivenom (green
pit viper and Agkistrodon halys) using saline as a
control. Fibrinogen level and clotting time in both
antivenom groups were studied.
Results: The mean fibrinogen level was elevated from
0 g/L to 2.86 g/L and 1.11 g/L after the addition of
green pit viper antivenom and Agkistrodon halys
antivenom, respectively. When mean clotting time was
measured, the value was 6.70 minutes in the control,
prolonged to more than 360 minutes by green pit
viper antivenom and to 19.06 minutes by Agkistrodon halys antivenom.
Conclusions: Green pit viper antivenom was superior
to Agkistrodon halys antivenom in neutralisation
of the thrombin-like and hypofibrinogenaemic
activities of Trimeresurus albolabris venom.
New knowledge added by this study
- In human plasma, both green pit viper antivenom (GPVA) and Agkistrodon halys antivenom (AHA) can antagonise the haemotoxicity in terms of fibrinogen and clotting time derangement induced by Trimeresurus albolabris venom.
- In contrast to a lower protection against mortality in mice in a previous study, the species-specific GPVA is more potent than AHA on a volume basis in neutralisation of the haemotoxic effects in humans.
- GPVA is preferable to AHA in reversing the haemotoxicity in T albolabris envenomation.
- Evaluation of other haemotoxicity parameters such as platelet count may give a more comprehensive understanding of the relative efficacy of the two antivenoms.
- A clinical trial in human snakebite victims should be conducted to validate the clinical applicability of our study results and provide information about appropriate antivenom dosage.
Introduction
Snakebite is an important medical emergency in
Hong Kong. The consequences are potentially
serious, especially if not treated quickly and
appropriately. In 2014, 121 cases were recorded by
the Clinical Data Analysis and Reporting System of
the Hospital Authority in Hong Kong. Trimeresurus
albolabris, also known locally as the white-lipped
pit viper or bamboo snake, accounts for 95% of all
human envenomation cases.1 Its bite can cause
potentially life-threatening bleeding.2 In Hong
Kong, death following a T albolabris is, fortunately,
rare. The last reported case occurred in 1986 when
an aged woman died of cerebral haemorrhage.3
Nonetheless non-lethal coagulopathy is common.
In a local case series (n=21), laboratory coagulation
abnormalities were frequent (hypofibrinogenaemia
in 48% of cases, prolonged prothrombin time [PT] in
19%, and prolonged activated partial thromboplastin
time [aPTT] in 14%) and sometimes accompanied
by bleeding (skin bruising in one patient, both
gastrointestinal haemorrhage and haematuria in
another).4
Trimeresurus albolabris venom has a thrombin-like
effect in vitro but causes a defibrination
syndrome in vivo. The snake venom’s thrombin-like
enzymes are responsible for the formation of friable
and loose fibrin clots, hypofibrinogenaemia, and
defibrination syndrome.5 We studied the thrombin-like
effect and defibrinating activity of T albolabris
venom by assessing the clotting time and fibrinogen
level, respectively, in human plasma.
There are two antivenoms available in Hong
Kong for T albolabris bite, the green pit viper
antivenom (GPVA; raised against T albolabris)
from the Thai Red Cross Society in Thailand and the
Agkistrodon halys antivenom (AHA; raised against
A halys) from the Shanghai Institute of Biological
Products in China. Both are recommended by the
Central Coordinating Committee of Accident and
Emergency Services of the Hospital Authority in
treating patients with T albolabris bite.6 Reports on
their relative efficacy in reversing coagulopathy in
humans are scarce. A case report described prompt
reversal of coagulopathy that was refractory to two
ampoules of AHA given 3 days apart by five vials
of GPVA.7 Conclusions can hardly be drawn in this
case, however, about whether the failure of AHA was
due to the species mismatch or simply inadequate
dosage. The choice of antivenom to use in a clinical
setting is determined by physician preference.8 In
this study, we compared the potency of GPVA and
AHA against the haemotoxicity from T albolabris
envenoming using an in-vitro human plasma model.
Methods
This study was approved by the ethics committees
of the New Territories West Cluster of Hospital
Authority and Kadoorie Farm and Botanic Garden
(KFBG), a non-governmental organisation actively
participating in the conservation of Hong Kong
wildlife.
Venom
From August to November 2013, herpetologists
from KFBG identified T albolabris for venom
extraction from locally captured stray snakes.
Venom was extracted by allowing the snakes to bite
into a paraffin sheet over a plastic collection pot (Fig 1). The venom was extracted and stored in sterilised bottles at -70°C.
Antivenom
The GPVA (batch number TA00512) and AHA
(batch number 20130401) [Fig 2] were purchased from the Thai Red Cross Society in Thailand and the
Shanghai Institute of Biological Products in China,
respectively. Both were F(ab’)2, in powder form, and
reconstituted in 10 mL of sterile water in another
vial in the same package before clinical use.

Figure 2. The green pit viper antivenom (left) from the Thai Red Cross Society in Thailand and the Agkistrodon halys antivenom (right) from the Shanghai Institute of Biological Products in China
Plasma preparation
Blood was collected from 40 adult health care
workers who had no history of snake bite. They had
no history of any coagulopathy problems and were
not prescribed any anticoagulant. The samples were
sodium citrate anticoagulated, centrifuged, and
stored at -70°C before use. In the following assays,
each blood sample was individually tested.
Fibrinogen assay
For green pit viper envenoming, the manufacturer
recommends a first dose of three vials (30 mL)
of GPVA. According to the clinical guidelines of
our emergency department, three vials were the
appropriate dose for both GPVA and AHA.9 As a
typical adult has a blood volume of approximately
5 L or plasma of 3 L, the dilution of 30 mL antivenom
to 3 L of plasma by intravenous infusion route would
therefore be 1:100. The amount of venom yield per
bite was 8 to 15 mg for the T albolabris.10 Venom
yields are an average range for a ‘standard’ snake
of the species and the amount of venom injected
during a bite.10 If a maximum of 15 mg of venom
was injected into the circulation of an adult, the
maximum concentration of venom in the circulating
plasma would be around 5 µg/mL (lower in real
snakebites unless intravascular inoculation occurs).
To simulate the in-vivo condition, plasma was
incubated with venom at a concentration of 5 µg/mL;
the antivenom-to-plasma ratio used was 1:100, that
is, 10 µL of GPVA or AHA to 1000 µL plasma.
This test was performed in duplicate and the
mean result was analysed. Venom was added at a
concentration of 50 µg/mL to homemade phosphate
buffered saline. Then 100 µL (5 µg venom) of this
solution was added to 1000 µL of human plasma
in plain glass test tubes and mixed for 30 seconds.
The final concentration of the testing mixtures was
5 µg venom per mL plasma. Then 10 µL of GPVA
or AHA was mixed with the venom/plasma mixture
and incubated at 37°C for 45 minutes. The same
procedures were performed in controls using 10 µL
of saline instead of antivenom. The fibrinogen level
was measured after 45 minutes using a Sysmex
CA-7000 analyser (Siemens, Germany) with
Thrombin Reagent (Clauss assay, Dade; Siemens,
Germany).
Clotting time assay
The working venom was added at a concentration of
50 µg/mL to homemade phosphate buffered saline.
An amount of 100 µL antivenom (GPVA or AHA)
was added to 1000 µL of working venom solution.
The samples were mixed and incubated at 37°C for
45 minutes. After incubation, one tenth or 110 µL of
the antivenom/venom mixture was withdrawn and
added to 1000-µL plasma. A final concentration of
5-µg venom per mL plasma mixture was added to a
glass test tube and clotting time was measured. The
same procedures were performed in controls with
100 µL of saline used instead of antivenom. Fibrin
formation (precipitation) was carefully observed and
clotting time was recorded. No fibrin clot observed
after 360 minutes was recorded as no clot formation
(NCF). Theoretically, NCF would indicate that all the
clotting activity (thrombin-like effect) of the venom
in the plasma had been completely neutralised by
the neutralising antibodies in the antivenom.
Data analysis and statistics
Continuous variables such as fibrinogen level and
clotting time were expressed as means and standard
deviations. Analysis of variance (ANOVA) test and
post-hoc Tukey’s honest significant difference (HSD)
test were used to compare three means. All statistical
analysis was performed with the Statistical Package
for the Social Sciences (Windows version 22.0; SPSS
Inc, Chicago [IL], US).
Results
Venom was harvested from a total of 46 snakes and
pooled together for subsequent testing. There were
two bottles containing no venom, that is, dry bite.
The total weight and total volume of venom collected
was 2.3791 g and 2170 µL, respectively.
Fibrinogen assay
As illustrated in Table 1, GPVA showed a higher
neutralising capacity against venom than AHA.
The measured fibrinogen in the GPVA group
(mean ± standard deviation, 2.86 ± 0.52 g/L) was
higher than that in the AHA group (1.11 ± 0.23
g/L), and undetectable in the control group, ie 0 g/L. The ANOVA test yielded significant variation
between them. Post-hoc Tukey’s HSD test showed
that differences in all pairwise comparisons were
statistically significant (Table 2).
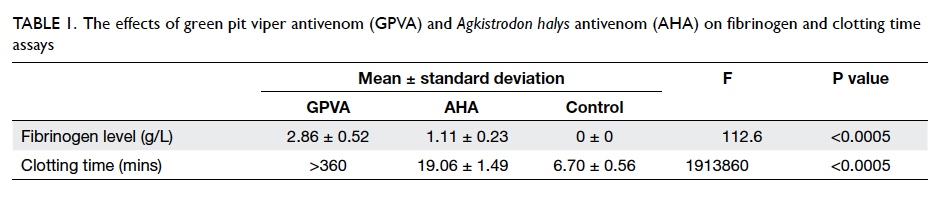
Table 1. The effects of green pit viper antivenom (GPVA) and Agkistrodon halys antivenom (AHA) on fibrinogen and clotting time assays
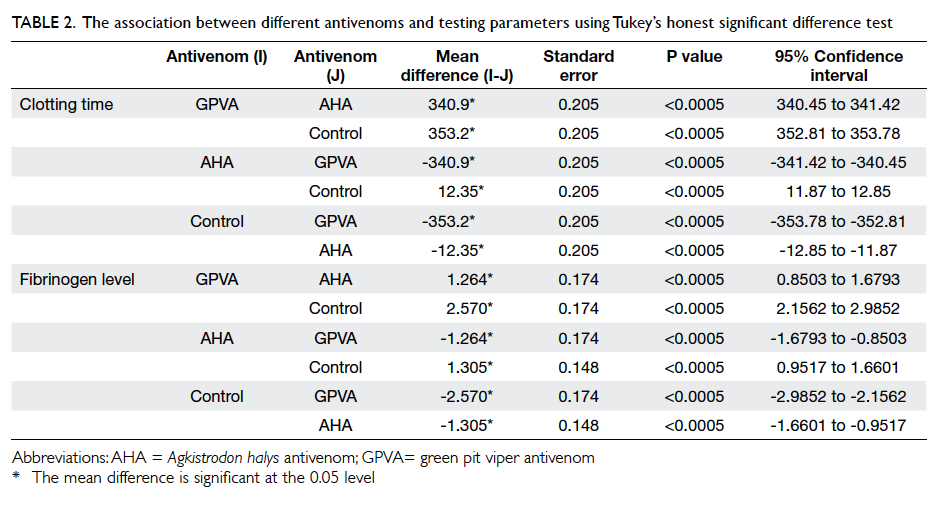
Table 2. The association between different antivenoms and testing parameters using Tukey’s honest significant difference test
Clotting time assay
The ANOVA was performed for the clotting time of
the three groups and yielded significant variation.
Post-hoc Tukey’s HSD test showed that all pairwise
comparisons were significantly different (Table 2).
The mean clotting time in the AHA group was 19.06
minutes, which was significantly longer than the 6.70
minutes in the control group (Table 1). This indicated that venom in the plasma was partly neutralised by
the neutralising antibodies in AHA.
The mean clotting time in the GPVA group was
>360 minutes, which was significantly longer than that
in the AHA group (Table 1). The fulfilment of NCF definition implied that venom in the plasma was
completely neutralised by the neutralising antibodies
in GPVA.
Discussion
Although both belong to the family Viperidae
and subfamily Crotalinae, T albolabris and A
(synonym Gloydius) halys differ with respect to
genus, geographic range, venom composition, and
envenoming features. The species T albolabris
is endemic to South-East Asia encompassing
Thailand, Vietnam, and southern China, including
Hong Kong. Its toxins encompass jerdonitin
(a metalloproteinase), stejnobin (a fibrinogen
clotting enzyme),11 and alboaggregins (the platelet
agglutinants).12 They give rise to local swelling and
coagulopathy. The species A halys ranges from Russia
to northern and central China. Its venom contains
metalloproteinase, haemotoxins, and neurotoxins.13 A bite may produce local swelling, ecchymosis, and
neurotoxicity, mostly in the form of ptosis, blurred
vision, and diplopia.14
Despite the differences in zoology and
toxicology between T albolabris and A halys, AHA
has been shown to be more effective than GPVA on
a volume basis in the reduction of mouse mortality
arising from T albolabris envenoming. In an in-vivo
study, the intraperitoneal lethal dose 50 (LD50) of T albolabris (called Cryptelytrops albolabris in the
study but T albolabris is the latest name for the same
species) was elevated from 0.14 µL to 0.36 µL and
0.52 µL by GPVA and AHA, respectively; and the
effective dose 50 was 32.02 µL for GPVA and 6.98 µL
for AHA.8 Nonetheless these favourable results for
AHA may not be applicable to humans for several
reasons. Firstly, haemotoxicity rather than death
is the primary concern in T albolabris bite. In the
above paper, the authors also pointed out the need
for further study of clinically relevant toxicities
other than mortality.8 Second, studies in animals
revealed that the mortality and haemotoxicity
outcomes might not correlate with each other. Of
the six Trimeresurus species including T albolabris
in Thailand, there was an inconsistent ratio between
the LD50 and minimum haemorrhagic dose (MHD).15
An animal study on T albolabris venom revealed
that GPVA antivenom could neutralise a greater
LD50 than Habu antivenom (200 by GPVA, 106 by
Habu) and likewise a greater MHD (2000 by GPVA,
750 by Habu).16 Sánchez et al17 tested the efficacy of two antivenoms against LD50 and MHD of different
snakes of North America. Within a single species, the
relative superiority of one antivenom might apply to
only one outcome, ie LD50 or MHD, but not both.
We evaluated the antivenoms on a volume
basis in order to simulate the way in which a
patient is treated. Evaluation based on molecular
weight and contents of proteins, all immunoglobulins
or specific immunoglobulins towards venom antigens are
alternative methods. Given that GPVA and AHA
are supplied in powder form without a dosage-based
weight and dissolved in liquid for administration, a
volume-based result is deemed more practical for
clinical dosing and drug reconstitution.
In human snakebite victims, venom is mostly
deposited subcutaneously, not intravascularly. We
employed a dose of venom assumed to be higher
than that achievable in the plasma of most human
snakebite victims for two reasons. First, the primary
aim of this study was to compare the relative potency
of two antivenoms, therefore a single dose of venom
in both antivenom groups was more important
than the dose quantity itself. Second, there were
inadequate data on the usual venom concentrations,
particularly the concentrations associated with
coagulopathy, in the circulation of humans bitten by
T albolabris.
In 1981, Visudhiphan et al18 reported the
effect of GPVA on clotting time and fibrinogen
level in human plasma exposed to green pit viper
(Trimeresurus) venom. The venom promoted
clotting and depleted fibrinogen level in a dose-dependent
fashion. After incubation of the venom
with plasma at a concentration of 5 µg/mL (the same
concentration employed in our study), clotting time
was 12 minutes at 1 hour and a drop in fibrinogen
level from that of normal plasma control occurred at
45 minutes. At the same venom plasma concentration
(5 µg/mL) and for the same incubation time, GPVA
added to plasma in a volume ratio of 1:5 prolonged
the clotting time to more than 3 times that of the
saline control, and there was failure to correct the
hypofibrinogenaemia in 1:20 samples.18 In contrast,
we observed a marked antidotal response to GPVA.
It is possible that the purity of the antivenom has
improved over the intervening years.
There are limitations to our study. First, in
addition to its procoagulant and fibrinolytic effects
on the coagulation pathway, T albolabris venom
also affects platelets. Of the patients in a local case
series, thrombocytopenia was detected in 29% of
cases, not necessarily associated with prolongation
of PT or aPTT.4 Future study may consider checking
for any thrombocytopenia. Second, laboratory and
clinical outcomes may be disparate. In contrast with
its inferior clinical performance, the Behringwerke
antivenom has been proven to be more effective
than the Pasteur antivenom in promoting mouse
survival.19 Third, potential inconsistencies in the
composition of antivenoms in various batches and
venoms derived from individual snakes may affect
the applicability of the results in another setting.
Nevertheless given the in-vitro benefits of GPVA
in antagonising coagulopathy in our study, future
trials, particularly in-vivo clinical trials, should be
conducted to determine its effect on other clotting
parameters and the required dosage. Furthermore,
fibrin formation (precipitation) and clotting time
were recorded by a single observer who was not
blinded to the treatment. This could have introduced
information bias.
Conclusions
We conducted in-vitro clotting and fibrinogen assays
on human plasma to assess the relative therapeutic
effects of GPVA and AHA on the haemotoxicity
produced by T albolabris envenomation. The
results indicated a higher potency of GPVA than
AHA in neutralisation of the thrombin-like and
hypofibrinogenaemic activities of T albolabris
venom.
Acknowledgements
The authors would like to thank Jeanie Sum-yin Mak,
Lucy Man-chi Lai, Shuk-han Tang, and Shuk-ching
Fan from the Department of Pathology of Tuen Mun
Hospital for their technical support.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Ng WS, Cheung WL. Snake bites in Hong Kong (T.
albolabris and other species): clinical features and
management. Hong Kong J Emerg Med 1998;5:71-6.
2. Pongpit J, Limpawittayakul P, Juntiang J, Akkawat B,
Rojnuckarin P. The role of prothrombin time (PT) in
evaluating green pit viper (Cryptelytrops sp) bitten
patients. Trans R Soc Trop Med Hyg 2012;106:415-8. Crossref
3. Cockram CS, Chan JC, Chow KY. Bites by the white-lipped
pit viper (Trimeresurus albolabris) and other species in
Hong Kong. A survey of 4 years’ experience at the Prince of
Wales Hospital. J Trop Med Hyg 1990;93:79-86.
4. Chan JC, Kwok MM, Cockram CS, Prematilleke MN,
Tomlinson B, Critchley JA. Blood coagulation abnormalities
associated with envenoming by Trimeresurus albolabris in
Hong Kong. Singapore Med J 1993;34:145-7.
5. Rojnuckarin P, Intragumtornchai T, Sattapiboon R, et al.
The effects of green pit viper (Trimeresurus albolabris and
Trimeresurus macrops) venom on the fibrinolytic system in
human. Toxicon 1999;37:743-55. Crossref
6. Management of snakebite. Accident and emergency clinical
guidelines number 9. Hong Kong: Central Coordinating
Committee of Accident and Emergency Services, Hospital
Authority; 2008.
7. Yang JY, Hui H, Lee AC. Severe coagulopathy associated
with white-lipped green pit viper bite. Hong Kong Med J
2007;13:392-5.
8. Fung HT, Yung WH, Crow P, et al. Green pit viper
antivenom from Thailand and Agkistrodon halys antivenom
from China compared in treating Cryptelytrops albolabris
envenomation of mice. Hong Kong Med J 2012;18:40-5.
9. Management guidelines for snakebite. Hong Kong:
Accident and Emergency Department, New Territories
West Cluster, Hospital Authority; 2014.
10. Thomas S. LD50 scores for various snakes. Available
from: http://www.seanthomas.net/oldsite/ld50tot.html.
Accessed 17 Nov 2014.
11. Soogarun S, Sangvanich P, Chowbumroongkait M, et
al. Analysis of green pit viper (Trimeresurus albolabris)
venom protein by LC/MS-MS. J Biochem Mol Toxicol
2008;22:225-9. Crossref
12. Asazuma N, Marshall SJ, Berlanga O, et al. The snake
venom toxin alboaggregin-A activates glycoprotein VI.
Blood 2001;97:3989-91. Crossref
13. Li S, Wang J, Zhang X, et al. Proteomic characterization of
two snake venoms: Naja naja atra and Agkistrodon halys.
Biochem J 2004;384:119-27. Crossref
14. Agkistrodon halys bite treated with specific antivenin.
Observation of 530 cases. Chin Med J (Engl) 1976;2:59-62.
15. Chanhome L, Khow O, Omori-Satoh T, Sitprija V. Capacity
of Thai green pit viper antivenom to neutralize the venoms
of Thai Trimeresurus snakes and comparison of biological
activities of these venoms. J Nat Toxins 2002;11:251-9.
16. Pakmanee N, Khow O, Wongtongkam N, Omori-Satoh T,
Sitprija V. Efficacy and cross reactivity of Thai green pit
viper antivenom among venoms of Trimeresurus species in
Thailand and Japan. J Nat Toxins 1998;7:173-83.
17. Sánchez EE, Galán JA, Perez JC, Rodríguez-Acosta A,
Chase PB, Pérez JC. The efficacy of two antivenoms
against the venom of North American snakes. Toxicon
2003;41:357-65. Crossref
18. Visudhiphan S, Dumavibhat B, Trishnananda M.
Prolonged defibrination syndrome after green pit viper
bite with persisting venom activity in patient’s blood. Am J
Clin Pathol 1981;75:65-9. Crossref
19. Warrell DA, Warrell MJ, Edgar W, Prentice CR, Mathison
J, Mathison J. Comparison of Pasteur and Behringwerke antivenoms in envenoming by the carpet viper (Echis carinatus). Br Med J 1980;280:607-9. Crossref


