Hong Kong Med J 2016 Dec;22(6):538–45 | Epub 24 Oct 2016
DOI: 10.12809/hkmj154799
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Effectiveness of proximal intra-operative salvage Palmaz stent placement for endoleak during endovascular aneurysm repair
Y Law, MB, BS, FRCS (Edin);
YC Chan, MB, BS, FRCS (Eng);
Stephen WK Cheng, MB, BS, FRCS (Edin)
Division of Vascular and Endovascular Surgery, Department of Surgery, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
This paper was presented in abstract form at the 15th Congress of Asian
Society for Vascular Surgery (ASVS) Hong Kong, 5-7 September 2014,
Hong Kong.
Corresponding author: Dr Y Law (lawyuksimpson@gmail.com)
Abstract
Introduction: The use of a proximal Palmaz stent
is a well-recognised technique to treat proximal
endoleak in endovascular aortic repair. This study
aimed to report the effectiveness and safety of an
intra-operative Palmaz stent for immediate type 1a
endoleak in Hong Kong patients.
Methods: This case series was conducted at a
tertiary hospital in Hong Kong. In a cohort of 494
patients who underwent infrarenal endovascular
aortic repair from July 1999 to September 2015,
12 (2.4%) received an intra-operative proximal
Palmaz stent for type 1a endoleak. Immediate and
subsequent proximal endoleak on follow-up image
was documented.
Results: Morphological review of the pre-repair
aneurysm neck showed five conical, one funnel, five
cylindrical and one undetermined short neck, with
a median neck angle of 61 degrees (range, 19-109
degrees). Stent grafts used included seven Cook
Zenith, one Cook Aorto-Uni-Iliac device, three
Metronic Endurant, and one TriVascular Ovation.
Eleven Palmaz stents were placed successfully
as intended, but one of them was accidentally
placed too low. Of the 12 type 1a endoleaks, postoperative imaging revealed immediate resolution of eight whilst four had improved. After a median follow-up
of 16 (range, 1-59) months, none of the subsequent
imaging showed a type 1a endoleak. The mean size
of the aneurysm sac reduced from 7.4 cm preoperatively to 7.3 cm at 1 month, 6.9 cm at 6 months, 7.1 cm at 1 year, and 6.1 cm at 2 years postoperatively. None of these patients required aortic
reintervention or had device-related early- or mid-term
mortality. One patient required delayed iliac
re-interventions for an occluded limb at 10 days post-surgery.
Conclusion: In our cohort, Palmaz stenting was
effective and safe in securing proximal sealing and
fixation.
New knowledge added by this study
- Palmaz stenting is effective and safe as a salvage treatment of proximal endoleak during endovascular aortic repair (EVAR).
- Appropriate patient selection, meticulous preoperative planning, and diligent follow-up ensured the ultimate success of EVAR.
- A Palmaz stent should always be readily available during EVAR especially for aneurysms with a hostile aortic neck.
Introduction
Hostile proximal infrarenal aortic neck is often
considered one of the relative contra-indications
for infrarenal endovascular aortic repair (EVAR),
but large multicentre registries have shown that
more EVARs have been performed worldwide
beyond manufacturers’ indications.1 2 3 Data from
the EUROSTAR Registry more than a decade ago
looking at aneurysm morphology and procedural
details of 2272 patients showed that perioperative
type 1a endoleak was significantly associated with
a larger angulated infrarenal neck with thrombus.4
Other morphological features included short aortic
neck length, large sac diameter, severe angulation,
and conical neck configuration.5 Endoleak is defined
as the persistence of blood flow outside the lumen of
an endovascular graft but within an aneurysm sac.
Type 1a endoleak is a leak around the proximal graft
attachment site. They are clinically important since
they can lead to continual aneurysm enlargement
and eventual rupture.6
Although the development of renal and
mesenteric fenestration or branched stent graft
technology to extend the landing zone more
proximally may overcome type 1a endoleaks, these
devices may not be ideal because of regulations,
complexity, and manufacturing delays.7 In addition,
contemporary published literature does not provide
sufficiently strong evidence to warrant a change in
treatment guidelines for juxtarenal or short-neck
aneurysm.8
The use of a proximal giant Palmaz stent
(Cordis Corp, Fremont [CA], US) is a well-recognised
technique to manage a type 1a endoleak
intra-operatively.9 10 11 12 A prophylactic Palmaz stent
inserted for hostile neck has also been reported.13 14 15
The aim of this study was to report the effectiveness
and safety of an intra-operative Palmaz stent for an
immediate type 1a endoleak.
Methods
Patient selection
Patients with an infrarenal abdominal aortic
aneurysm (AAA) who had undergone EVAR were
retrospectively reviewed for the period 1 July 1999
to 30 September 2015. Data were extracted from
prospectively collected computerised departmental
database, supplemented by patient records. This study was done in accordance with the principles outlined in the Declaration of Helsinki. All patients had undergone a preoperative fine-cut
computed tomographic (CT) scan and careful
preoperative planning with a conclusion that EVAR
was feasible. All our patients who underwent EVAR
had preoperative three-dimensional aortic
anatomy examined using the stationary TeraRecon
Aquarius workstation (TeraRecon, San Mateo [CA],
US) that rapidly manipulates all preoperative data
sets in the Digital Imaging and Communications
in Medicine, and examines the aortic morphology.
These details would be difficult to appreciate on
two-dimensional planar CT imaging, especially in those with
inadequate short hostile neck. Those patients who
were deemed unsuitable for infrarenal sealing
would either undergo open repair or, more recently,
fenestrated or adjunctive EVAR (such as chimney
technique).
Cook Zenith (Cook Medical, Bloomington
[IN], US), Medtronic Endurant (Medtronic Inc,
Minneapolis [MN], US), and TriVascular Ovation
(TriVascular Inc, Santa Rosa [CA], US) stent
grafts were most commonly used. We sometimes
extended the manufacturers’ indications of use to
include difficult aortic necks. All operations were
performed in our institution, a tertiary vascular
referral centre, by experienced vascular specialists
in a hybrid endovascular suite (Siemens Artis zee
Multipurpose System; Siemens, Erlangen, Germany).
Post-deployment balloon moldings with Coda
balloon (Cook Medical, Bloomington [IN], US) and
completion angiograms with power injection were
performed in all patients.
Proximal Palmaz stent placement
In the event of proximal endoleak on completion
angiogram, further balloon molding would be
attempted. Persistent endoleak was managed with
balloon expandable giant Palmaz stent P4014 (Cordis
Corp). Our preference was for the Palmaz stent
to be positioned at the infrarenal region over the
fabric of the stent graft, so as not to jeopardise any
future chance of proximal extension with a renal or
mesenteric fenestrated cuff. They would be crimped
on a Coda balloon (maximum inflated diameter, 40
mm) and railed through a stiff guidewire to the top
neck.
Preoperative aneurysm neck morphological analysis
Cause of proximal endoleak was usually hostile neck.
We focused on aortic infrarenal neck anatomy where
the Palmaz stent was placed and exerted its effect. All
preoperative CT scans were analysed. Measurement
was automatically calculated by computer software
Aquarius iNtuition Client version 4.4 (TeraRecon
Inc), after a median centre line path (MCLP) was
drawn. We used definitions of neck measurement as
defined by Filis et al16:
(1) Neck length is calculated between the point of
MCLP at the orifice of the lower renal artery
and MCLP at the start of the aneurysm.
(2) Neck diameter is measured on the orthogonal
cross-section of the neck, from the outer wall to
the outer wall. It is measured at the lower renal
artery level, 1 cm and 2 cm below.
(3) Neck angle is the angle between the axis of the
neck (straight line between the MCLP of the
aorta at the level of the orifice of the lower renal
artery and the MCLP of the aorta at the start of
the aneurysm) and the axis of the lumen of the
aneurysm (straight line between the MCLP at
the start of the aneurysm and the MCLP at the
end of the aneurysm).
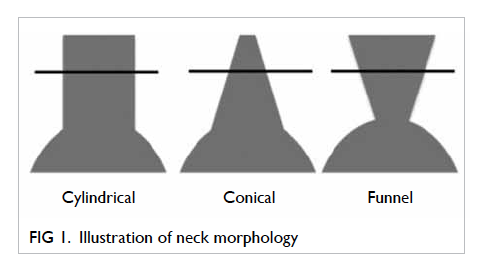
(4) Neck morphology (Fig 1):
(a) Funnel shape is defined as 20% decrease in neck area between the level of the lower renal artery and 2 cm below.
(b) Conical shape is defined as 20% increase in neck area between the level of the renal artery and 2 cm below.
(c) Cylindrical shape is defined between the above two.
(d) Shape is undetermined if neck length is less than 2 cm.
(a) Funnel shape is defined as 20% decrease in neck area between the level of the lower renal artery and 2 cm below.
(b) Conical shape is defined as 20% increase in neck area between the level of the renal artery and 2 cm below.
(c) Cylindrical shape is defined between the above two.
(d) Shape is undetermined if neck length is less than 2 cm.
Clinical and radiological outcomes
Immediate radiological result following Palmaz stent
placement was reported. Any procedural mortality
or morbidity was recorded. Our departmental
protocol recommends postoperative imaging, either
CT scan or duplex scan, at 1 to 3 months, then
every 6 months for the first 2 years, and annually
thereafter. These were supplemented by X-ray to
detect any stent fracture or migration. Any endoleak
detected during follow-up was reported as well as
any alteration in sac size. Follow-up was dated to the
most recent objective imaging available.
Results
Patient population
During the study period 1 July 1999 to 30 September
2015, a total of 842 AAA surgeries were performed,
of which 320 (38%) were open repair and 522 (62%)
were endovascular repair (28 fenestrated/branched
EVAR and 494 infrarenal EVAR). In a cohort of 494
patients with infrarenal EVAR, 12 (2.4%) received
an intra-operative proximal Palmaz stent for type 1a
endoleak that was noticed on completion angiogram.
No patients received prophylactic Palmaz stent for
difficult neck anatomy.
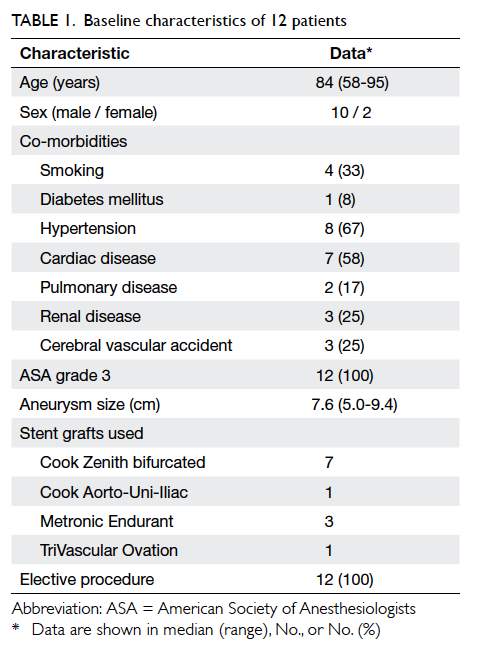
Patient demographics are summarised in Table 1. The median age was 84 (range, 58-95) years. All had undergone elective surgery for asymptomatic
AAA. The median AAA size was 7.6 cm (range,
5.0-9.4 cm). Seven patients received a Cook Zenith
stent graft, one patient a Cook Aorto-Uni-Iliac
device, three had Metronic Endurant stent grafts,
and one had TriVascular Ovation stent graft. The
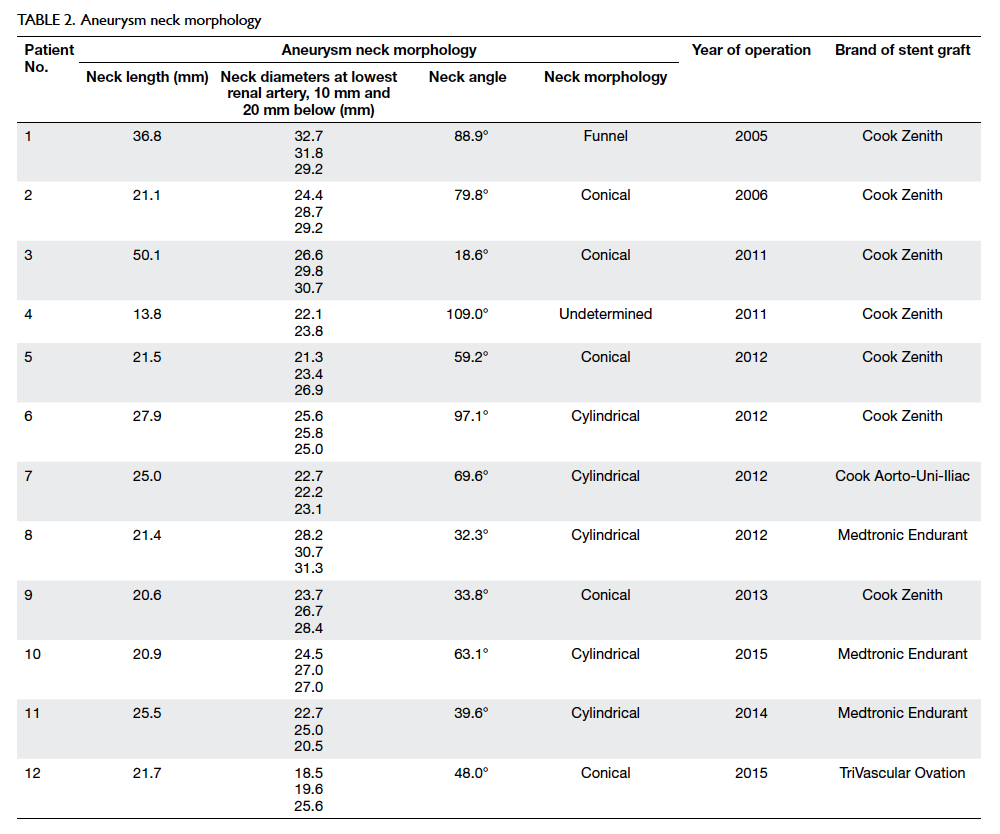
occurrence of type 1a endoleak was more common in recent years (Table 2).
Analysis of aneurysm neck morphology
Table 2 summarises the neck morphologies of our
cohort. Morphological review of the pre-EVAR
aneurysm neck showed five conical, one funnel, five
cylindrical, and one undetermined short necks. The
median neck angle was 61 degrees (range, 19-109
degrees). Use of the stent graft was outside of the
manufacturer’s guidelines in six (50%) patients. Most
patients had one or more features of hostile neck,
rendering them at high risk of proximal endoleak.
Radiological outcomes
All 12 patients had persistent proximal type 1a
endoleak after stent graft placement with standard
balloon molding. Placement of a giant Palmaz stent
in the infrarenal position was technically successful
in all cases, although one Palmaz stent was placed
too low and lodged in one of the iliac limbs.
Nonetheless, it served its function well in correcting
the proximal endoleak (Figs 2 and 3). Immediate
resolution of the endoleak was achieved in eight
(67%); whilst four (33%) had improved but persistent
leak at completion of the procedure.
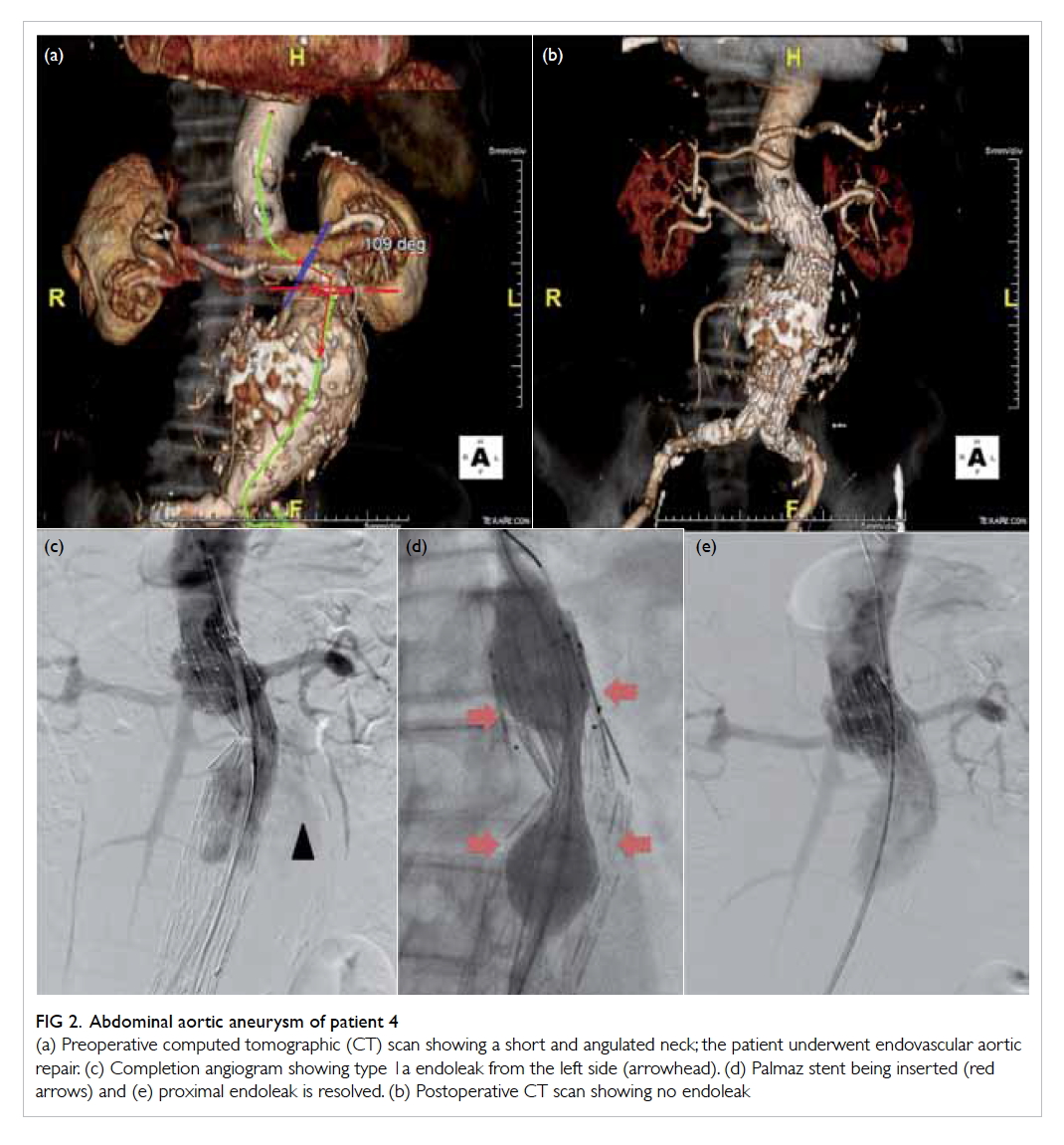
Figure 2. Abdominal aortic aneurysm of patient 4
(a) Preoperative computed tomographic (CT) scan showing a short and angulated neck; the patient underwent endovascular aortic repair. (c) Completion angiogram showing type 1a endoleak from the left side (arrowhead). (d) Palmaz stent being inserted (red arrows) and (e) proximal endoleak is resolved. (b) Postoperative CT scan showing no endoleak
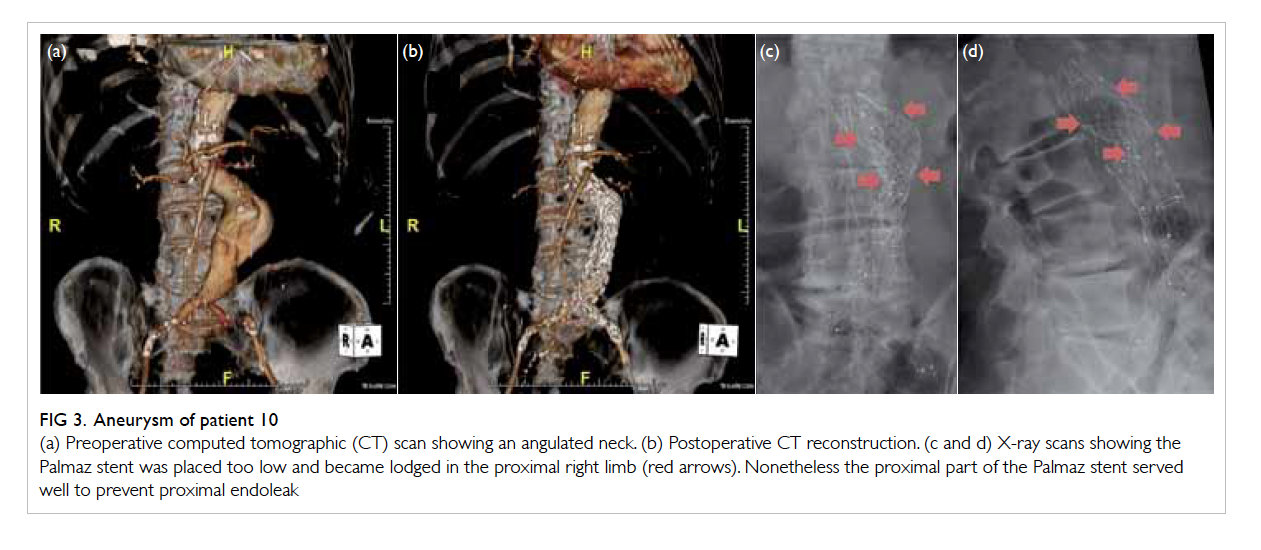
Figure 3. Aneurysm of patient 10
(a) Preoperative computed tomographic (CT) scan showing an angulated neck. (b) Postoperative CT reconstruction. (c and d) X-ray scans showing the Palmaz stent was placed too low and became lodged in the proximal right limb (red arrows). Nonetheless the proximal part of the Palmaz stent served well to prevent proximal endoleak
Clinical outcomes
After a median follow-up of 16 (range, 1-59) months,
no patient had a type 1a endoleak on subsequent
imaging, either CT scan or duplex ultrasound scan.
All patients had at least one postoperative CT scan.
Patient 2 had a type 1c endoleak from an
embolised left internal iliac artery that was managed
conservatively. Patients 9 and 11 had a
type 2 endoleak, also managed conservatively. All
had shrinkage of sac size. Sac size of the aneurysms
decreased from a mean of 7.4 cm pre-EVAR to 7.3
cm, 6.9 cm, 7.1 cm, and 6.1 cm at 1 month, 6 months,
1 year, and 2 years post-EVAR, respectively (Fig 4).
Routine X-ray surveillance did not reveal any Palmaz
stent fracture or migration.
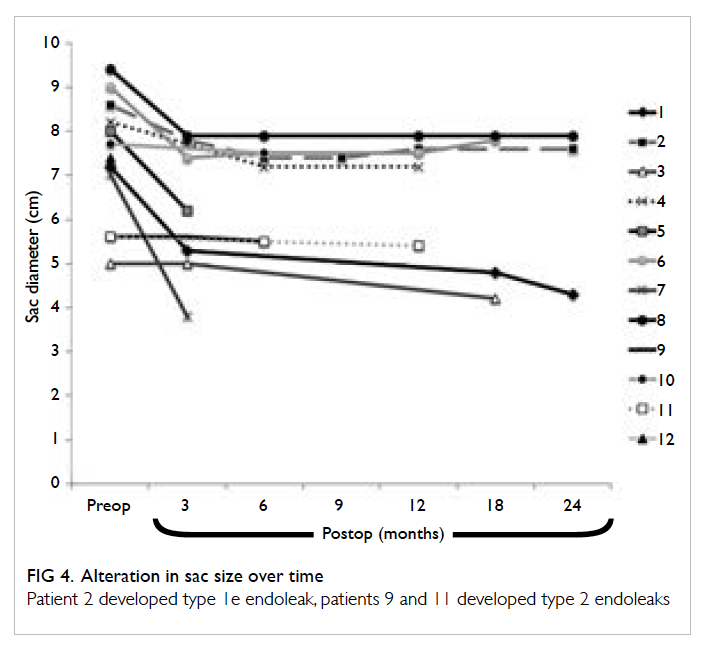
Figure 4. Alteration in sac size over time
Patient 2 developed type 1e endoleak, patients 9 and 11 developed type 2 endoleaks
One patient required secondary endovascular
re-intervention for occluded left iliac limb at day 10
postoperatively. It was due to a tortuous iliac system
causing an acute bend to the stent graft limb. There
was no other reported secondary intervention. Seven
patients have since died (at 6, 9, 16, 16, 20, 24, and 59
postoperative months) from non-aneurysm–related
causes. Five remain alive at the time of writing.
Discussion
Hostile aortic neck anatomy often precludes
endovascular treatment of AAA. It has been shown
in large clinical cohorts that up to 42% of EVARs
are performed outside the instructions for use for
commercially available stent grafts.3 17 18 19 Multiple
measures have been developed to include more of
these difficult necks for endovascular treatment.
Evolution of stent graft design including suprarenal
fixation20 and renal and mesenteric fenestration21 are examples. Adjunctive neck measures, eg
endostapling,22 23 proximal fibrin glue embolisation,24
open aortic neck banding,25 and proximal covered cuff
extension10 may be used in cases of perioperative
type 1a endoleak following EVAR. Endostapling and
glue embolisation, though minimally invasive, are
not always feasible and risk major aortic injury. Open
aortic neck banding requires laparotomy. Proximal
cuff extension is only feasible if there is an additional
sealing zone to the most caudal renal artery. Since
we routinely landed our stent graft at the level of the
lowest renal artery, this technique was not usually
practical. The simplest and most well-recognised
manoeuvre remains placement of a proximal Palmaz
stent.
The morphology of the infrarenal aortic neck
is important in securing the proximal landing zone.
Three-dimensional workstation planning has been considered
useful by many26 27; for example, Sobocinski et al28
showed that it reduced the rate of type 1 endoleaks
and Velazquez et al29 indicated that it decreased
the rate of extra iliac extension. We emphasise the
importance of proper pre-EVAR planning, as this
is one of the obvious factors that may compromise
long-term durability and outcome. The fact that half
of stent graft usage in our series was outside the
instructions for use and most patients had one or
more hostile neck feature rendered them at high risk
of proximal endoleak. Under these circumstances,
a Palmaz stent should always be readily available
during EVAR.
Multiple series have reported their experience
in its successful use. Early study by Dias et al9
reported nine patients who received a Palmaz
stent and in whom aneurysm remained excluded
at a median follow-up of 13 months (range, 6-24
months). Rajani et al10 reported successful treatment
of intra-operative type 1 endoleak with Palmaz stent
in 27 patients who had no recurrence at follow-up,
although length of follow-up was not mentioned.
Arthurs et al11 reported no type 1 endoleak in 31
patients after a median follow-up of 53 months
(interquartile range, 14-91 months). The Palmaz
stent was effective across a variety of available
devices with suprarenal fixation (eg Cook Zenith)
or infrarenal fixation (eg Gore Excluder; WL Gore
& Associates, Inc, Newark [DE], US). Our results are
in agreement with these findings.
Other series have shown controversial
results. Farley et al15 reported 18 cases of Palmaz
stent placement. Technical placement failed in one
patient, in whom attempts at passing the access
sheath to the proximal landing zone resulted in
proximal migration of the main body of the aortic
stent graft. An attempt at passage of the balloon-mounted
stent without sheath protection resulted
in slippage of the stent from the balloon. The stent
could not be retrieved and was deployed in the iliac
limb. With a mean follow-up period of 254 days
in the 17 successful Palmaz stent placements, one
patient had unresolved type 1 endoleak. Malposition
of the stent was not an unusual complication.30 31 Kim et al32 described a deployment technique to ensure
accuracy. Palmaz stent was asymmetrically hand-crimped
on an appropriately sized valvuloplasty
balloon that assured the proximal aspect would
deploy first.
Some series have advocated prophylactic
Palmaz stent placement in hostile necks, including
15 patients reported by Cox et al.13 One (7%) patient
had secondary endoleak with intervention after
a mean follow-up of 12 months. Qu and Raithel14
reported 117 cases of difficult neck treated with
unibody Powerlink device (Endologix Inc, Irvine
[CA], US). In this series, 83 (72.8%) had proximal
Palmaz stent as an adjunctive procedure. Proximal
cuff extension was also used. The mean follow-up
was 2.6 years (range, 4 months-5 years). Results were
satisfactory with an overall re-intervention rate of
5.3%, and no device migration, conversion, or post-EVAR rupture.
Palmaz stents were routinely placed at an
infrarenal position in our unit on the basis that
future extension with a fenestrated cuff is possible.
If a transrenal position is adopted, the strut of the
Palmaz stent, which is a very tight space, may hinder
catheterisation of renal or visceral arteries should
a fenestrated cuff be inserted. This is not absolute,
however. Oikonomou et al33 reported a case of post-treatment
with Powerlink stent graft and transrenal
Palmaz stent. Treatment of proximal endoleak at 3
years after operation was successful by means of a
proximal fenestrated graft. Selective catheterisation
of both renal arteries and dilation of the stent struts
prior to stent graft repair ensured that it would be
feasible to catheterise the renal arteries through the
fenestrated cuff.
There are limitations to this study. The
retrospective nature of our cohort may risk inaccurate
information. The efficacy of the Palmaz stent in
aneurysms with short infrarenal neck may not be
tested, as the majority were considered straight for
custom-made fenestrated or branched EVAR. In our
limited experience, a Palmaz stent is a valuable tool
to expand the boundary of endovascular treatment
for AAA.
Conclusion
Palmaz stent helps proximal sealing and fixation.
In our experience, Palmaz stenting is effective
and safe as a salvage treatment of immediate
proximal endoleak during EVAR. We emphasise the
importance of appropriate patient selection, pre-EVAR planning, and diligent follow-up.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Bachoo P, Verhoeven EL, Larzon T. Early outcome of
endovascular aneurysm repair in challenging aortic neck
morphology based on experience from the GREAT C3
registry. J Cardiovasc Surg (Torino) 2013;54:573-80.
2. Matsumoto T, Tanaka S, Okadome J, et al. Midterm
outcomes of endovascular repair for abdominal aortic
aneurysms with the on-label use compared with the off-label
use of an endoprosthesis. Surg Today 2015;45:880-5. Crossref
3. Hoshina K, Hashimoto T, Kato M, Ohkubo N, Shigematsu
K, Miyata T. Feasibility of endovascular abdominal aortic
aneurysm repair outside of the instructions for use and
morphological changes at 3 years after the procedure. Ann
Vasc Dis 2014;7:34-9. Crossref
4. Buth J, Harris PL, van Marrewijk C, Fransen G. The
significance and management of different types of
endoleaks. Semin Vasc Surg 2003;16:95-102. Crossref
5. Stanley BM, Semmens JB, Mai Q, et al. Evaluation of
patient selection guidelines for endoluminal AAA repair
with the Zenith Stent-Graft: the Australasian experience.
J Endovasc Ther 2001;8:457-64. Crossref
6. Fransen GA, Vallabhaneni SR Sr, van Marrewijk CJ, et al.
Rupture of infra-renal aortic aneurysm after endovascular
repair: a series from EUROSTAR registry. Eur J Vasc
Endovasc Surg 2003;26:487-93. Crossref
7. Chuter T, Greenberg RK. Standardized off-the-shelf
components for multibranched endovascular repair of
thoracoabdominal aortic aneurysms. Perspect Vasc Surg
Endovasc Ther 2011;23:195-201. Crossref
8. Ou J, Chan YC, Cheng SW. A systematic review of
fenestrated endovascular repair for juxtarenal and short-neck
aortic aneurysm: evidence so far. Ann Vasc Surg
2015;29:1680-8. Crossref
9. Dias NV, Resch T, Malina M, Lindblad B, Ivancev K.
Intraoperative proximal endoleaks during AAA stent-graft
repair: evaluation of risk factors and treatment with
Palmaz stents. J Endovasc Ther 2001;8:268-73. Crossref
10. Rajani RR, Arthurs ZM, Srivastava SD, Lyden SP, Clair DG,
Eagleton MJ. Repairing immediate proximal endoleaks
during abdominal aortic aneurysm repair. J Vasc Surg
2011;53:1174-7. Crossref
11. Arthurs ZM, Lyden SP, Rajani RR, Eagleton MJ, Clair
DG. Long-term outcomes of Palmaz stent placement
for intraoperative type Ia endoleak during endovascular
aneurysm repair. Ann Vasc Surg 2011;25:120-6. Crossref
12. Chung J, Corriere MA, Milner R, et al. Midterm results
of adjunctive neck therapies performed during elective
infrarenal aortic aneurysm repair. J Vasc Surg 2010;52:1435-41. Crossref
13. Cox DE, Jacobs DL, Motaganahalli RL, Wittgen CM,
Peterson GJ. Outcomes of endovascular AAA repair
in patients with hostile neck anatomy using adjunctive
balloon-expandable stents. Vasc Endovascular Surg
2006;40:35-40. Crossref
14. Qu L, Raithel D. Experience with the Endologix Powerlink
endograft in endovascular repair of abdominal aortic
aneurysms with short and angulated necks. Perspect Vasc
Surg Endovasc Ther 2008;20:158-66. Crossref
15. Farley SM, Rigberg D, Jimenez JC, Moore W, Quinones-Baldrich W. A retrospective review of Palmaz stenting of
the aortic neck for endovascular aneurysm repair. Ann
Vasc Surg 2011;25:735-9. Crossref
16. Filis KA, Arko FR, Rubin GD, Zarins CK. Three-dimensional
CT evaluation for endovascular abdominal
aortic aneurysm repair. Quantitative assessment of the
infrarenal aortic neck. Acta Chir Belg 2003;103:81-6. Crossref
17. Walker J, Tucker LY, Goodney P, et al. Adherence to
endovascular aortic aneurysm repair device instructions
for use guidelines has no impact on outcomes. J Vasc Surg
2015;61:1151-9. Crossref
18. Igari K, Kudo T, Toyofuku T, Jibiki M, Inoue Y. Outcomes
following endovascular abdominal aortic aneurysm repair
both within and outside of the instructions for use. Ann
Thorac Cardiovasc Surg 2014;20:61-6. Crossref
19. Lee JT, Ullery BW, Zarins CK, Olcott C 4th, Harris
EJ Jr, Dalman RL. EVAR deployment in anatomically
challenging necks outside the IFU. Eur J Vasc Endovasc
Surg 2013;46:65-73. Crossref
20. Robbins M, Kritpracha B, Beebe HG, Criado FJ, Daoud Y,
Comerota AJ. Suprarenal endograft fixation avoids adverse
outcomes associated with aortic neck angulation. Ann
Vasc Surg 2005;19:172-7. Crossref
21. Verhoeven EL, Vourliotakis G, Bos WT, et al. Fenestrated
stent grafting for short-necked and juxtarenal abdominal
aortic aneurysm: an 8-year single-centre experience. Eur J
Vasc Endovasc Surg 2010;39:529-36. Crossref
22. Donas KP, Kafetzakis A, Umscheid T, Tessarek J, Torsello
G. Vascular endostapling: new concept for endovascular
fixation of aortic stent-grafts. J Endovasc Ther 2008;15:499-503. Crossref
23. Avci M, Vos JA, Kolvenbach RR, et al. The use of
endoanchors in repair EVAR cases to improve proximal
endograft fixation. J Cardiovasc Surg (Torino) 2012;53:419-26.
24. Feng JX, Lu QS, Jing ZP, et al. Fibrin glue embolization
treating intra-operative type I endoleak of endovascular
repair of abdominal aortic aneurysm: long-term result [in
Chinese]. Zhonghua Wai Ke Za Zhi 2011;49:883-7.
25. Scarcello E, Serra R, Morrone F, Tarsitano S, Triggiani
G, de Franciscis S. Aortic banding and endovascular
aneurysm repair in a case of juxtarenal aortic aneurysm
with unsuitable infrarenal neck. J Vasc Surg 2012;56:208-11. Crossref
26. Lee WA. Endovascular abdominal aortic aneurysm
sizing and case planning using the TeraRecon Aquarius
workstation. Vasc Endovascular Surg 2007;41:61-7. Crossref
27. Parker MV, O’Donnell SD, Chang AS, et al. What imaging
studies are necessary for abdominal aortic endograft sizing?
A prospective blinded study using conventional computed
tomography, aortography, and three-dimensional
computed tomography. J Vasc Surg 2005;41:199-205. Crossref
28. Sobocinski J, Chenorhokian H, Maurel B, et al. The benefits
of EVAR planning using a 3D workstation. Eur J Vasc
Endovasc Surg 2013;46:418-23. Crossref
29. Velazquez OC, Woo EY, Carpenter JP, Golden MA, Barker
CF, Fairman RM. Decreased use of iliac extensions and
reduced graft junctions with software-assisted centerline
measurements in selection of endograft components for
endovascular aneurysm repair. J Vasc Surg 2004;40:222-7. Crossref
30. Gabelmann A, Krämer SC, Tomczak R, Görich J.
Percutaneous techniques for managing maldeployed or
migrated stents. J Endovasc Ther 2001;8:291-302. Crossref
31. Slonim SM, Dake MD, Razavi MK, et al. Management of
misplaced or migrated endovascular stents. J Vasc Interv
Radiol 1999;10:851-9. Crossref
32. Kim JK, Noll RE Jr, Tonnessen BH, Sternbergh WC 3rd.
A technique for increased accuracy in the placement of
the “giant” Palmaz stent for treatment of type IA endoleak
after endovascular abdominal aneurysm repair. J Vasc Surg
2008;48:755-7. Crossref
33. Oikonomou K, Botos B, Bracale UM, Verhoeven EL.
Proximal type I endoleak after previous EVAR with
Palmaz stents crossing the renal arteries: treatment using a
fenestrated cuff. J Endovasc Ther 2012;19:672-6. Crossref