Hong Kong Med J 2016 Dec;22(6):534–7 | Epub 9 Sep 2016
DOI: 10.12809/hkmj154694
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Nephrolithiasis among male patients with newly diagnosed gout
KS Wan, MD, PhD1,2;
CK Liu, MD, MPH3,4;
MC Ko, MD3;
WK Lee, MD3;
CS Huang, MD2
1 Department of Immunology and Rheumatology, Taipei City Hospital-Zhongxing Branch, Taiwan
2 Department of Pediatrics, Taipei City Hospital-Renai Branch, Taiwan
3 Department of Urology, Taipei City Hospital-Zhongxing Branch, Taiwan
4 Fu Jen Catholic University School of Medicine, Taiwan
Corresponding author: Dr KS Wan (gwan1998@gmail.com)
Abstract
Introduction: An elevated serum urate level is
recognised as a cause of gouty arthritis and uric acid
stone. The level of serum uric acid that accelerates
kidney stone formation, however, has not yet been
clarified. This study aimed to find out if a high serum
urate level is associated with nephrolithiasis.
Methods: Patients were recruited from the
rheumatology clinic of Taipei City Hospital (Renai
and Zhongxing branches) in Taiwan from March
2015 to February 2016. A total of 120 Chinese male
patients with newly diagnosed gout and serum urate
concentration of >7 mg/dL and no history
of kidney stones were divided into two groups
according to their serum urate level: <10 mg/dL
(group 1, n=80) and ≥10 mg/dL (group 2, n=40).
The mean body mass index, blood urea nitrogen
level, creatinine level, urinary pH, and kidney
ultrasonography were compared between the two
groups.
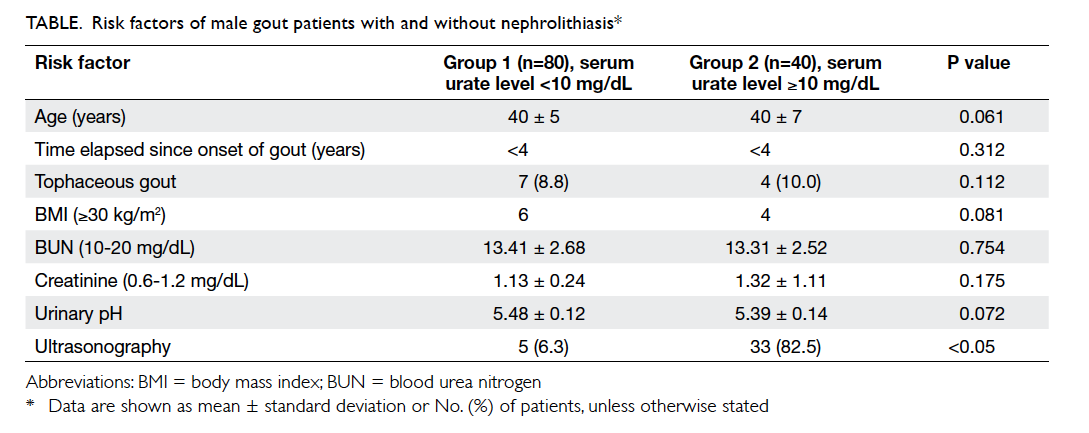
Results: There were no significant differences in
blood urea nitrogen or creatinine level between
the two groups. The urine pH in both groups was
similar and not statistically significant. Kidney stone
formation was detected via ultrasonography in 6.3%
(5/80) and 82.5% (33/40) of patients in groups 1 and
2, respectively (P<0.05).
Conclusion: A serum urate level of ≥10 mg/dL
may precipitate nephrolithiasis. Further studies are
warranted to substantiate the relationship between
serum urate level and kidney stone formation.
New knowledge added by this study
- Hyperuricaemia is a risk factor for renal stone formation, which is associated with a substantially higher prevalence of nephrolithiasis on ultrasonography.
- Patients with gouty arthritis and serum urate level of ≥10 mg/dL should be advised to have renal ultrasonography.
Introduction
Over the past century, kidney stones have become
increasingly prevalent, particularly in more
developed countries. The incidence of urolithiasis in
a given population is dependent on the geographic
area, racial distribution, socio-economic status, and
dietary habits.1 In general, patients with a history of
gout are at greater risk of forming uric acid stones,
as are patients with obesity, diabetes, or complete
metabolic syndrome.2 Moreover, elevated serum
urate levels are known to lead to gouty arthritis,
tophi formation, and uric acid kidney stones.3
The incidence of uric acid stones varies between
countries and accounts for 5% to 40% of all urinary
calculi.4 Certain risk factors may be involved in the
pathogenesis of uric acid nephrolithiasis, including
low urinary volume and persistently low urinary pH.5
Calcium oxalate stones may form in some
patients with gouty diathesis due to increased
urinary excretion of calcium and reduced excretion
of citrate. In addition, relative hypercalciuria in
gouty diathesis with calcium oxalate stones may be
due to intestinal hyperabsorption of calcium.6 Most
urinary uric acid calculi are not pure in composition
and complex urates, sodium, potassium, and calcium
have been found together in various proportions.7 An
analysis of stones in gout patients in Japan showed
that the incidence of common calcium salt stones
was over 60%, while that of uric acid stones was only
30%.8 This implies that the disruption of uric acid
metabolism promotes not only uric acid stones, but
also calcium salt stones. Therefore, a high serum
urate level might be associated with nephrolithiasis
and this provided the rationale for this study.
Methods
Overall, 120 male gouty arthritis patients with newly diagnosed gout and serum urate concentration of >7 mg/dL, and without
previous kidney stone disease were allocated to one
of the two groups according to their serum uric acid
level: <10 mg/dL (group 1, n=80) and ≥10 mg/dL
(group 2, n=40). Patients were recruited from the
rheumatology clinic of Taipei City Hospital (Renai
and Zhongxing branches), a tertiary community
hospital in Taiwan, from March 2015 to February
2016. They had been newly diagnosed with gout
but had no clinical suggestions of renal stone
disease. The exclusion criteria included previously
treated gouty arthritis and current prescription of
urate reabsorption inhibitors. The patient’s age,
duration of gout arthritis, presence of tophi, body
mass index (BMI), blood urea nitrogen (BUN),
creatinine, urinary pH, and kidney ultrasonography
were all measured and analysed. This study has
been approved by the hospital’s Institutional Review
Board with informed consent waived.
Results for continuous variables were given as
means ± standard deviations. Student’s t test was
used to compare the physical characteristics that
were continuous in nature among the different
subject groups and the Chi squared test was used
to compare the difference in the stone detection
rate between the two groups. A P value of <0.05 was
regarded as statistically significant for two-sided
tests. The Statistical Package for the Social Sciences
(Windows version 12.0; SPSS Inc, Chicago [IL], US)
was used for all statistical analyses.
Results
The mean age of the two study groups was similar
(40 years). Family history of gout was present in
67.5% and 90% of groups 1 and 2, respectively. The
time elapsed since onset of gout was less than 4 years
in both groups. Tophaceous gout was found in 8.8%
in group 1 and 10.0% in group 2. The prevalence of
patients with a BMI of ≥30 kg/m2 was not statistically
significant between the two groups. Only 6% of
group 2 patients with kidney stones had a BMI of
>95th percentile. In most cases, urinary pH was less
than 5.5 in both groups and there were no abnormal
changes to BUN or creatinine levels. Interestingly,
the prevalence of kidney stones detected by
ultrasonography was 6.3% in group 1 and 82.5% in
group 2 (P<0.05). The sensitivity and specificity of
high serum urate level (>10 mg/dL) in predicting
kidney stones was 87% and 91%, respectively (Table).
Discussion
Gout is a common metabolic disorder characterised
by chronic hyperuricaemia, and serum urate level of
>6.8 mg/dL that exceeds the physiological threshold
of saturation. Urolithiasis is one of the well-known
complications of gout. We hypothesise that serum
urate level can be used as a predictive marker for
urolithiasis. Uric acid, a weak organic acid, has very
low pH-dependent solubility in aqueous solution.
Approximately 70% of urate elimination occurs
in urine, and the kidney plays a dominant role in
determining plasma level.9 A serum urate level of
>7 mg/dL is recognised as leading to gouty arthritis
and uric acid stone formation. Moreover, recent
epidemiological studies have identified serum
urate elevation as an independent risk factor for
chronic kidney disease, cardiovascular disease, and
hypertension.3 Impaired renal uric acid excretion is
the major mechanism of hyperuricaemia in patients
with primary gout.10 The molecular mechanisms
of renal urate transport are still incompletely
understood. Urate transporter 1 is an
organic anion transporter with highly specific urate
transport activity, exchanging this anion with others
including most of the endogenous organic anions
and drug anions that are known to affect renal uric
acid transport.10 11
Uric acid stones account for 10% of all kidney
stones and are the second most common cause of
urinary stones after calcium oxalate and calcium
phosphate. The most important risk factor for uric
acid crystallisation and stone formation is a low urine
pH (<5.5) rather than an increased urinary uric acid
excretion.12 The proportion of uric acid stones varies
between countries and accounts for 5% to 40% of all
urinary calculi.4 Uric acid homeostasis is determined
by the balance between its production, intestinal
secretion, and renal excretion. The kidney is an
important regulator of circulating uric acid levels
by reabsorbing about 90% of filtered urate and being
responsible for 60% to 70% of total body uric acid
factor underpinning hyperuricaemia and gout.13 Pure
uric acid stones are radiolucent but well visualised on
renal ultrasound or non-contrast helical computed
tomographic scanning; the latter is especially good
for detection of stones which are <5 mm in size.14
Nonetheless the reason why most patients with gout
present with acidic urine, even though only 20%
have uric acid stones, remains unclear. In a US study,
the prevalence of kidney stone disease was almost
two-fold higher in men with a history of gout than
in those without (15% vs 8%).15 Higher adiposity
and weight gain are strong risk factors for gout in
men, while weight loss is protective.15 An analysis by
Shimizu8 of stones in gout patients revealed that the
proportion of common calcium salt stones was over
60%, while that of uric acid stones was only about
30%. Overweight/obesity and older age associated
with low urine pH were the principal characteristics
of ‘pure’ uric acid stone formers. Impaired urate
excretion associated with increased serum uric acid
is also another characteristic of uric acid stone
formers and resembles patients with primary gout.
Patients with pure calcium oxalate stones were
younger; they had a low proportion of obese subjects
and higher urinary calcium.16
Conventionally, BMI was stratified as normal
(<25 kg/m2), overweight (25-29.9 kg/m2), or obese
(≥30 kg/m2). In males, the proportion of uric acid
stones gradually increased with BMI, from 7.1% in
normal BMI to 28.7% in obese subjects.17 The same
was true in females, with the proportion of uric acid
stones rising from 6.1% in normal BMI to 17.1% in
obese subjects.17 Studies found that BMI is associated
with an increased risk of kidney stone disease,
but with a BMI of >30 kg/m2, further increases do not
appear to significantly increase the risk of stone
disease.17 18 An independent association between kidney stone disease and gout strongly suggests that
they share common underlying pathophysiological
mechanisms.19
Three major conditions control the potential
for uric acid stone formation: the quantity of uric
acid, the volume of urine as it affects the urinary
concentration of uric acid, and the urinary pH.20 Two
major abnormalities have been suggested to explain
overly acidic urine: increased net acid excretion
and impaired buffering caused by defective urinary
ammonium excretion, with the combination resulting
in abnormally acidic urine.21 Urinary alkalisation,
which involves maintaining a continuously high
urinary pH (pH 6-6.5), is considered by some or
many to be the treatment of choice for uric acid
stone dissolution and prevention.20 In general, gout
is caused by the deposition of monosodium urate
crystals in tissue that provokes a local inflammatory
reaction. The formation of monosodium urate
crystals is facilitated by hyperuricaemia. In a study
by Sakhaee and Maalouf,21 being overweight and of
older age were associated with low urine pH and one
of the principal characteristics of pure uric acid stone
formation. Impaired urate excretion associated with
increased serum uric acid was another characteristic
of uric acid stone formation that resembles patients
with primary gout.
The limitations of this current study included
the lack of measurement of uric acid concentration
of urine in the participants, no further computed
tomographic scanning for kidney stones, no analysis
of stone composition, and limited representativeness
of the study subjects. For example, there were only
10 obese patients (BMI ≥30 kg/m2) in the analysis.
In this study, hyperuricaemia was a risk factor for
kidney stone formation. Patients with serum urate
level of >10 mg/dL should undergo ultrasound
examination to look for any nephrolithiasis.
Declaration
All authors have disclosed no conflicts of interest.
References
1. López M, Hoppe B. History, epidemiology and regional
diversities of urolithiasis. Pediatr Nephrol 2010;25:49-59. Crossref
2. Liebman SE, Taylor JG, Bushinsky DA. Uric acid
nephrolithiasis. Curr Rheumatol Rep 2007;9:251-7. Crossref
3. Edwards NL. The role of hyperuricemia and gout in kidney
and cardiovascular disease. Cleve Clin J Med 2008;75 Suppl 5:S13-6. Crossref
4. Shekarriz B, Stoller ML. Uric acid nephrolithiasis: current
concepts and controversies. J Urol 2002;168:1307-14. Crossref
5. Ngo TC, Assimos DG. Uric acid nephrolithiasis: recent
progress and future directions. Rev Urol 2007;9:17-27.
6. Pak CY, Moe OW, Sakhaee K, Peterson RD, Poindexter
JR. Physicochemical metabolic characteristics for calcium
oxalate stone formation in patients with gouty diathesis. J
Urol 2005;173:1606-9. Crossref
7. Bellanato J, Cifuentes JL, Salvador E, Medina JA. Urates in
uric acid renal calculi. Int J Urol 2009;16:318-21; discussion 322. Crossref
8. Shimizu T. Urolithiasis and nephropathy complicated with
gout [in Japanese]. Nihon Rinsho 2008;66:717-22.
9. Marangella M. Uric acid elimination in the urine.
Pathophysiological implications. Contrib Nephrol
2005;147:132-48.
10. Taniquchi A, Kamatani N. Control of renal uric acid
excretion and gout. Curr Opin Rheumatol 2008;20:192-7. Crossref
11. Yamauchi T, Ueda T. Primary hyperuricemia due to
decreased renal uric acid excretion [in Japanese]. Nihon
Rinsho 2008;66:679-81.
12. Ferrari P, Bonny O. Diagnosis and prevention of uric acid
stones [in German]. Ther Umsch 2004;61:571-4. Crossref
13. Bobulescu IA, Moe OW. Renal transport of uric acid:
evolving concepts and uncertainties. Adv Chronic Kidney
Dis 2012;19:358-71. Crossref
14. Wiederkehr MR, Moe OW. Uric acid nephrolithiasis: a
systemic metabolic disorder. Clin Rev Bone Miner Metab
2011;9:207-17. Crossref
15. Choi HK. Atkinson K, Karison EW, Curhan G. Obesity,
weight change, hypertension, diuretic use, and risk of gout
in men: the health professionals follow-up study. Arch
Intern Med 2005;165:742-8. Crossref
16. Negri AL, Spivacow R, Del Valle E, et al. Clinical and
biochemical profile of patients with “pure” uric acid
nephrolithiasis compared with “pure” calcium oxalate
stone formers. Urol Res 2007;35:247-51. Crossref
17. Daudon M, Lacour B, Jungers P. Influence of body size on
urinary stone composition in men and women. Urol Res
2006;34:193-9. Crossref
18. Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R,
Matlaga BR. The association of increasing body mass index
and kidney stone disease. J Urol 2010;183:571-5. Crossref
19. Kramer HM, Curhan G. The association between gout
and nephrolithiasis: the National Health and Nutrition
Examination Survey III, 1988-1994. Am J Kidney Dis
2002;40:37-42. Crossref
20. Cicerello E, Merlo F, Maccatrozzo L. Urinary alkalization
for the treatment of uric acid nephrolithiasis. Arch Ital
Urol Androl 2010;82:145-8.
21. Sakhaee K, Maalouf NM. Metabolic syndrome and uric
acid nephrolithiasis. Semin Nephrol 2008;28:174-80. Crossref