Hong Kong Med J 2016 Oct;22(5):428–34 | Epub 15 Jul 2016
DOI: 10.12809/hkmj154769
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Multimodal analgesia model to achieve low postoperative opioid requirement following
bariatric surgery
Katherine KY Lam, FHKCA, FHKAM (Anaesthesiology);
Wilfred LM Mui, FCSHK, FHKAM (Surgery)
Hong Kong Bariatric and Metabolic Institute and Evangel Hospital Weight
Management Centre, Room 610, Champion Building, 301-309 Nathan
Road, Jordan, Hong Kong
Corresponding author: Dr Katherine KY Lam (katherinelamky@gmail.com)
Abstract
Objective: To investigate whether a new anaesthesia
protocol can reduce opioid use in obese patients
following laparoscopic sleeve gastrectomy.
Methods: This prospective observational case series
was conducted in a private hospital in Hong Kong
that has been accredited as a Centre of Excellence
for Bariatric Surgery. Thirty consecutive patients
scheduled for laparoscopic sleeve gastrectomy from
1 January 2015 to 31 March 2015 were reviewed.
Results: Of the 30 patients, 14 (46.7%) did not
require any opioids for rescue analgesia during the
entire postoperative period; six (20.0%) required
rescue opioids only in the post-anaesthetic care unit,
but not in the surgical ward. The mean postoperative
total opioid requirement per patient was 32 mg of pethidine.
Conclusion: With combination of multimodal
analgesia with local anaesthetic infiltration, it is
possible to avoid giving potent long-acting opioids
in anaesthesia for bariatric surgery.
New knowledge added by this study
- It is possible to avoid giving potent long-acting opioids in anaesthesia for bariatric surgery, by using multimodal analgesia with a combination of paracetamol, pregabalin, COX-2 inhibitors, tramadol, ketamine, dexmedetomidine, and local anaesthetic wound infiltration.
- The use of this opioid-sparing anaesthetic technique can potentially reduce the adverse effects and morbidity associated with the use of opioids in obese patients. The technique can be extended to other types of surgery in obese patients.
Introduction
Obese patients are particularly sensitive to the
sedative and respiratory depressive effects of long-acting
opioids. Many obese patients also have
obstructive sleep apnoea syndrome (OSAS) and will
be prone to airway obstruction and desaturation in
the postoperative period, especially if opioids have
been given.1 2 Given this background, multimodal
analgesia is advocated for bariatric surgery with
the aim of reducing opioid use.3 4 At the time of writing, no studies were able to demonstrate a
technique that can consistently remove the need
for any postoperative opioid analgesia. In this study,
we report the use of an anaesthesia protocol that
allowed a significant proportion of our patients
undergoing laparoscopic sleeve gastrectomy to be
completely free from any long-acting potent opioids
in the intra-operative and postoperative period.
Methods
Patient selection
This was a prospective observational study. The
study was conducted in a private hospital in
Hong Kong that has been accredited as a Centre
of Excellence for Bariatric Surgery. All patients
scheduled for laparoscopic sleeve gastrectomy for
management of obesity or type 2 diabetes from 1
January 2015 onwards were anaesthetised using
the same protocol. We analysed 30 consecutive
cases between 1 January 2015 and 31 March 2015
to investigate the postoperative opioid requirements
using this anaesthesia protocol. Patients were
excluded from the case series if they had contra-indications
or allergy to any of the anaesthetic or
analgesic drugs, or if anaesthesia deviated from the
standard protocol for any reason. Three patients
were excluded—one was taking serotonin-specific
reuptake inhibitor antidepressants and pethidine
was avoided to prevent serotonin syndrome
(morphine given instead); one was allergic to non-steroidal
anti-inflammatory drugs (NSAIDs), so
intravenous parecoxib and oral etoricoxib were not
given; one accidentally had a larger dose of ketamine
given intra-operatively than allowed by the protocol.
Concomitant laparoscopic cholecystectomy was
performed with laparoscopic sleeve gastrectomy in
three patients who were included in the study.
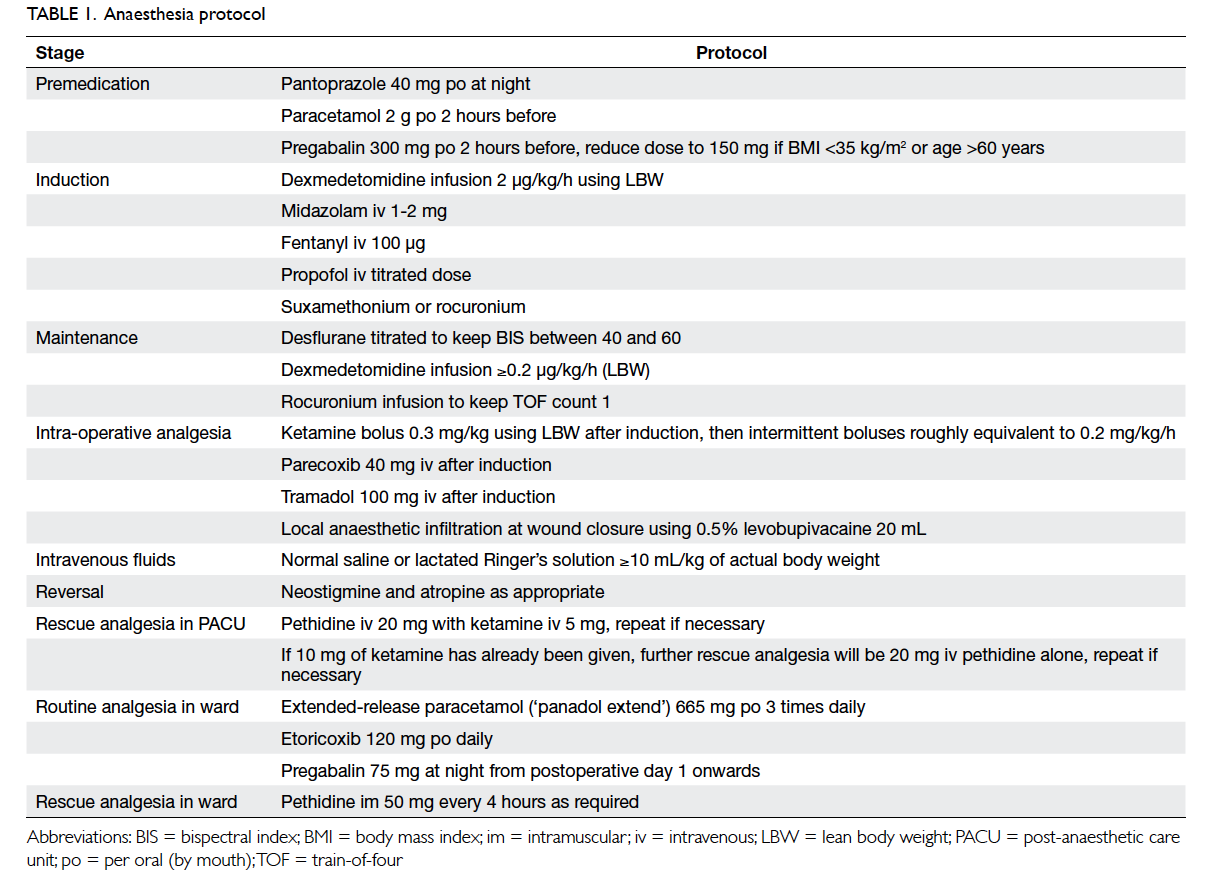
The anaesthesia protocol
All patients were fasted from midnight on the night
before surgery. All operations were scheduled in
the morning. Patients were premedicated with oral
pantoprazole 40 mg on the night before surgery,
and 2 g of oral paracetamol and 150 mg or 300 mg
of oral pregabalin (for patients of body mass index
<35 kg/m2 or ≥35 kg/m2, respectively) 2 hours before
surgery.
Upon arrival in the operating theatre,
intravenous access was established and 1 to 2 mg of
intravenous midazolam was administered followed
by an infusion of dexmedetomidine. The dose of
dexmedetomidine was titrated according to the
calculated lean body weight (LBW) using the Hume
formula.5 The starting dose of the dexmedetomidine
was 0.2 µg/kg/h using LBW.6 No loading dose was
given.
Standard monitoring was applied to the patient
together with a bispectral index (BIS) monitor and
peripheral nerve stimulation monitor. Graduated
compression stockings and sequential compression
devices were used for all patients. Induction
of anaesthesia was accomplished with fentanyl
100 µg, a titrated dose of propofol, and either
suxamethonium or rocuronium as appropriate. The
trachea was intubated and patients were ventilated
with a mixture of air, oxygen, and desflurane.
Intra-operatively, desflurane was titrated to
maintain BIS value between 40 and 60. Muscle
relaxation was maintained with a rocuronium
infusion to keep a train-of-four count of 1.
Dexmedetomidine infusion continued at 0.2 µg/kg/h
or higher if necessary. Shortly after induction, the
various supplementary analgesic drugs were given.
A loading dose of ketamine 0.3 mg/kg LBW was
given followed by intermittent boluses roughly
equivalent to 0.2 to 0.3 mg/kg/h of LBW. Intravenous
parecoxib 40 mg and tramadol 100 mg were given.
Dexamethasone 8 mg and tropisetron 5 mg were
given intravenously for prophylaxis of postoperative
nausea and vomiting (PONV).
For intravenous fluids, patients were given 10
mL/kg actual body weight of either lactated Ringer’s
solution or normal saline, then more were given as
appropriate. Hypotension was treated with either
ephedrine or phenylephrine.
When the surgeon started to close the
wounds, rocuronium infusion was stopped and
dexmedetomidine infusion rate was reduced to
0.1 µg/kg/h. Wounds were infiltrated with 20 mL
of 0.5% levobupivacaine. When all wounds were
closed, dexmedetomidine infusion was stopped
and desflurane switched off, muscle relaxation
reversed by neostigmine and atropine. Patients were
extubated when awake and able to obey command.
After extubation, patients were transferred
to the post-anaesthetic care unit (PACU) for
observation for 30 minutes, or longer if appropriate.
If a patient required rescue analgesia, intravenous
pethidine 20 mg with intravenous ketamine 5 mg
was given, and the dose repeated if necessary.
When 10 mg of intravenous ketamine had been
given, further rescue analgesia was intravenous
pethidine 20 mg without any more ketamine. This
avoided administration of too much ketamine in an
awake patient causing dizziness or hallucinations.
When patients had good pain control and stable
vital signs, they were transferred back to the ward.
The standard postoperative protocol was initiated:
if patients requested analgesics, an intramuscular
injection of pethidine 50 mg was given, and repeated
after 4 hours if necessary. By early evening, when
vital signs were stable, patients were allowed sips of
water followed by a fluid diet of 60 mL/h. Regular
oral paracetamol and etoricoxib were given, and oral
pregabalin was added to the protocol the next day.
Opioid requirements were reviewed for 24 hours
after surgery.
As part of the standard postoperative protocol,
patients were asked to get off the bed and walk
around the ward with the assistance of nursing
or physiotherapy staff by the evening of the day of
surgery. Provided there were no complications,
patients were discharged on the second postoperative
day. The anaesthesia protocol is summarised in
Table 1.
Results
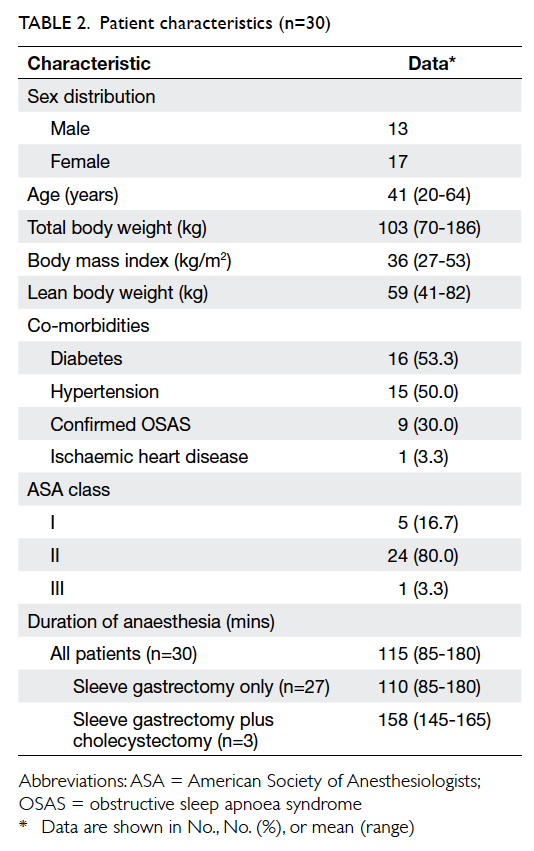
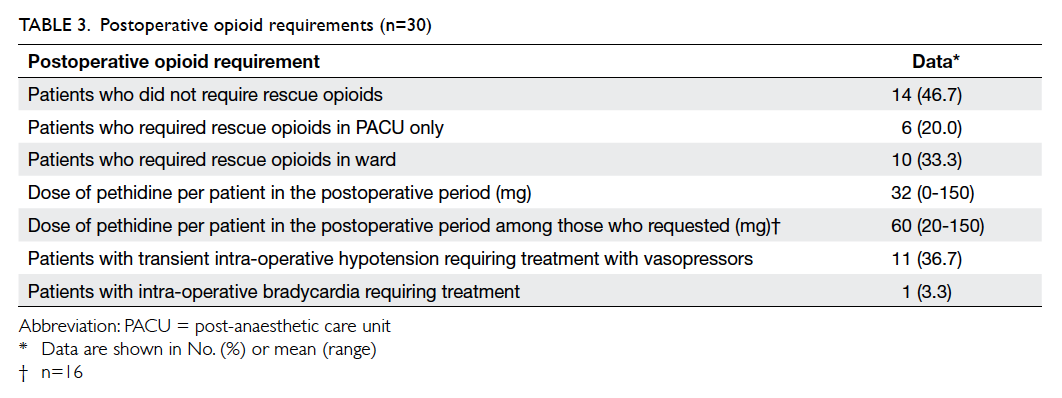
Patient characteristics are shown in Table 2, and
postoperative opioid requirements are listed in
Table 3.
Of the 30 patients, no opioid rescue analgesia
was required in 14 (46.7%) throughout the
postoperative period; six (20%) required intravenous
pethidine for rescue analgesia in the PACU, but not
after their return to the ward. The remaining 10
(33.3%) patients were given intramuscular pethidine
injections in the ward on request.
The mean postoperative opioid requirement
per patient in the whole case series was 32 mg of
pethidine. Among the 16 patients who required
rescue analgesia in the ward or in the PACU, their
mean opioid requirement was 60 mg of pethidine,
with a range of 20 to 150 mg.
This anaesthetic protocol included a
dexmedetomidine infusion that might cause
hypotension and bradycardia due to its alpha-2
adrenoceptor blocking action. In our case series,
11 (36.7%) patients developed transient
hypotension despite intravenous fluid loading
and required either intravenous ephedrine or
phenylephrine. One patient had transient intra-operative
bradycardia requiring atropine, probably
due to preoperative use of a beta blocker and low
resting heart rate.
Discussion
Importance of reducing postoperative opioid
use in obese patients
Opioids are among the world’s oldest known drugs.
They have been used in anaesthesia traditionally as
part of a balanced anaesthesia, to provide hypnosis
and analgesia, to blunt the sympathetic response to
surgery, and are the mainstay of postoperative analgesia
in many situations. Morbidly obese patients,
however, are particularly sensitive to the respiratory
depressant effects of opioids. Taylor et al2 found that
the use of opioids per se is a risk factor for respiratory
events in the first 24 hours after surgery. Ahmad et al1 demonstrated in their study of 40 morbidly obese
patients who presented for laparoscopic bariatric
surgery, that in using desflurane and remifentanil-morphine–based anaesthesia, hypoxaemic episodes
in the first 24 hours were common, and 14 of their
40 patients had more than five hypoxic episodes per
hour despite supplementary oxygen.
Another concern with use of opioids in
bariatric patients is the high incidence (>70%) of
OSAS.7 In our study, 30% (n=9) of patients had OSAS
confirmed by an overnight sleep study. The remaining
patients were not tested although many had varying
symptoms of OSAS. These untested patients were
assumed to have OSAS unless proven otherwise. The
American Society of Anesthesiologists recommends
that in patients with OSAS, methods should be used
to reduce or eliminate the requirement for systemic
opioids.8 Hence, reducing perioperative opioid
use by these obese patients can potentially reduce
morbidity.
How can the anaesthetist avoid or reduce
the use of perioperative opioids, and yet still
provide balanced anaesthesia with hypnosis,
analgesia, haemodynamic stability, and satisfactory
postoperative analgesia? The first method is to
combine general anaesthesia with regional analgesia
techniques, such that anaesthetic agents will provide
hypnosis while the regional blocks will provide
analgesia and block sympathetic responses to surgery.
Any form of major regional block in a morbidly obese
patient can be technically challenging, however.
Furthermore, with respect to bariatric surgery, most
procedures are now performed laparoscopically,
so that thoracic epidural analgesia techniques have
become largely unnecessary.
Putting aside the use of regional analgesia, the
second method to reduce perioperative opioid use
is to use a combination of non-opioid agents with
volatile agents or propofol to achieve analgesia and
haemodynamic control.3 A point to note here is that
as acute tolerance to the analgesic effects of opioids
can rapidly develop (such as after 90 minutes of
remifentanil infusion),9 any attempts to reduce
postoperative opioid requirement must include an
effort to either eliminate or reduce the use of intra-operative
opioids. These techniques are now often
described as opioid-free anaesthesia or non-opioid techniques.
Paracetamol, NSAID, or COX-2 inhibitors,
gabapentinoids, ketamine and alpha-2 agonists,
when used individually, have all been shown to reduce
postoperative opioid requirement and improve pain
relief.10 11 12 13 14 Different combinations of these agents, together with local anaesthetic infiltration of the
wounds, have been reported for bariatric surgery, as
discussed below.
Development of the study protocol based on
previous studies
In 2003, Feld et al15 described a technique of using
sevoflurane combined with ketorolac, clonidine,
ketamine, lignocaine, and magnesium for patients
undergoing open gastric bypass. Compared with
the control group where sevoflurane was used
with fentanyl, they found the non-opioid group to
be less sedated, with less morphine use in PACU
although the total morphine use at 16 hours was not
significantly different to the opioid group.
In 2006 Feld et al16 again described using
desflurane combined with dexmedetomidine
infusion, and compared it with a control group using
desflurane and fentanyl, for patients undergoing
open gastric bypass. In the dexmedetomidine group,
there were lower pain scores and less morphine use
in the PACU.
In 2005, Hofer et al17 described a case report of a
super-obese patient weighing 433 kg who underwent
open gastric bypass. No opioids were used but
instead replaced with a high-dose dexmedetomidine
infusion together with isoflurane.
As laparoscopic techniques have become
more common in bariatric surgery, more studies
have been carried out of non-opioid anaesthetic
techniques for laparoscopic bariatric surgery.
Tufanogullari et al18 described a technique in which
either fentanyl or varying doses of dexmedetomidine
were used with desflurane for laparoscopic bariatric
surgery. All patients were also given celecoxib.
Postoperatively, patients were given fentanyl boluses
in PACU, then intravenous morphine via a patient-controlled
analgesia system. The only statistical
difference was decreased PACU fentanyl use in the
dexmedetomidine groups.
Ziemann-Gimmel et al19 looked at 181
patients undergoing laparoscopic gastric bypass.
In the treatment group, volatile anaesthetics were
used together with intravenous paracetamol and
ketorolac. Postoperatively patients were given
regular paracetamol and ketorolac. If there was
breakthrough pain, intermittent oral oxycodone
or intravenous hydromorphone was given. A small
number of patients in this treatment group (3/89)
were able to remain opioid-free throughout, and
15 patients did not require opioid medications
when they were back to the ward.
In another study where the primary outcome
was the incidence of PONV, Ziemann-Gimmel et al20 evaluated 119 patients undergoing laparoscopic bariatric surgery. The treatment group was managed
with propofol infusion, dexmedetomidine infusion,
paracetamol, ketorolac, and ketamine. The other
group was managed with volatile anaesthetic and
opioids. Postoperative analgesia regimen was the
same as the previous study.19 They reported a large
reduction in PONV in their treatment group.
While most studies reported decreased
requirement of opioids for postoperative analgesia
in their non-opioid groups, very few studies
could achieve zero postoperative opioid use. Only Ziemann-Gimmel et al19 could achieve total opioid sparing in a small proportion (3 out of 92 patients) of the treatment group by using intra-operative and postoperative
intravenous paracetamol and ketorolac.
Most of these earlier studies used a
combination of only a few of the available non-opioid
adjuncts. Dexmedetomidine remains a mainstay of non-opioid adjunct in most of these studies. We hence proposed the use of a wider mix of non-opioid adjuncts, using a combination of
paracetamol, COX-2 inhibitor, pregabalin, ketamine,
dexmedetomidine, and local anaesthesia infiltration.
In contrast to the earlier studies, in our study we
were able to achieve zero postoperative opioid use in
a significant percentage of patients (46.7%).
In our protocol, the only opioid given during
anaesthesia was fentanyl 100 µg for intubation,
and tramadol 100 mg, a weak opioid, shortly after
induction. All other opioid analgesics, if required,
were given after the patient was awake. This avoided
having to blindly give intra-operative long-acting
opioids during anaesthesia, and allowed better
titration of the drug by giving small boluses each
time with the patient awake.
Dexmedetomidine
Dexmedetomidine was a useful agent in our protocol.
Before the addition of this agent to our protocol,
total opioid sparing was very difficult to achieve.
Dexmedetomidine is a highly selective alpha-2
adrenoceptor blocker, with analgesic and sedative
properties.21 Previous study of its use in bariatric
anaesthesia has failed to show any reduction in
opioid requirements.18 In our protocol, we used
more non-opioid adjuncts, and since we calculated
the infusion dose using LBW instead of total body
weight (TBW), overall we administered a much
lower dose of dexmedetomidine.
Infusion of dexmedetomidine may cause initial
hypertension and tachycardia (especially during
a loading dose infusion), followed by hypotension
and bradycardia. In our study, no loading dose was
given. Of the 30 patients, 11 (36.7%) developed
transient hypotension despite intravenous fluid
loading and required either intravenous ephedrine
or phenylephrine. This transient hypotension was
also aggravated by putting the patient in a steep
reverse trendelenburg position to facilitate surgical
exposure, which decreases the venous return. When
using dexmedetomidine in bariatric surgery, care
must be taken to ensure the patient is euvolaemic.
Ketamine
Ketamine was another useful adjunct in our
protocol. Ketamine is an N-methyl-D-aspartate
receptor antagonist with strong analgesic properties
when given at subanaesthetic doses.22 The use of
ketamine has advantages in morbidly obese patients
as it causes little respiratory depression compared
with opioids. In our protocol, we used LBW to
calculate the ketamine dose, and used relatively
low ketamine doses (0.3 mg/kg bolus followed by
0.2-0.3 mg/kg/h with intermittent boluses). This
resulted in a low total ketamine dose, with a mean
of 31 mg ketamine per patient (range, 25-50 mg).
Midazolam 1 to 2 mg was also given at induction to
prevent any psychomimetic reactions caused by
ketamine. No patient developed any hallucinations
or dysphoria and there was no delay in emergence
noted in our patients.
The use of lean body weight as dosing scalar
In our protocol, we chose to use LBW to calculate
the dose for dexmedetomidine and ketamine.
The classic teaching is that for obese patients,
anaesthetic drugs can be dosed according to the
TBW versus ideal body weight or LBW according
to lipid solubility. Lipophilic drugs are better dosed
according to actual body weight due to an increase
in volume of distribution, whereas hydrophilic drugs
are better dosed according to LBW or ideal body
weight.23 Lean body weight is significantly correlated
with cardiac output, and drug clearance increases
proportionately with LBW.6
There is insufficient information regarding
the pharmacokinetics and pharmacodynamics of
dexmedetomidine and ketamine in the morbidly
obese patient. In the few previous studies regarding dexmedetomidine and bariatric
anaesthesia, TBW was used as dosing scalars. For
example, Feld et al16 used 0.5 µg/kg TBW loading
dose followed by 0.4 µg/kg/h infusion in their series
of 10 patients with open gastric bypass. Ziemann-Gimmel et al20 used 0.5 µg/kg TBW loading dose followed by 0.1 to 0.3 µg/kg/h infusion for their group
of 60 patients undergoing a variety of bariatric
procedures. Tufanogullari et al18 gave no loading
dose and infused from 0 to 0.8 µg/kg/h in their series
of 80 patients undergoing laparoscopic banding or
bypass. There were little data regarding ketamine
dose in bariatric surgery. We chose to dose these two
drugs using LBW to see how our results would differ
from the other published studies.
Limitations of the study
Our study has several limitations. It was a prospective
observational study with a relatively small number of
cases. We do not have data to compare this protocol
with our previous protocols, nor do we have data in
the form of a randomised controlled trial to look at the
isolated effect of any of the drugs used.
The opioid that we used for rescue analgesia
was pethidine, given intravenously in the recovery
room by the anaesthetist, or given intramuscularly
on the ward by the nurses upon standing order. One
can argue that the mean opioid dose per patient was
not accurate as some were given small intravenous
boluses and others were given intramuscular
injections of fixed dose. To accurately assess the
postoperative parenteral opioid requirements
in theory, all patients should be given a patient-controlled
analgesia system to deliver boluses of
parenteral opioids as required. This, however, is not
practical and not necessary for the patient, given that
two thirds of our patients did not require any opioids
at all. This would also represent a lot of drug wastage
when the whole cassette of drugs was unused.
We were able to demonstrate that a significant
proportion of patients did not require any opioids,
but we do not have data to demonstrate a reduction
in respiratory complications or an improvement in
time to ambulation or discharge. This could be the
basis for further studies.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Ahmad S, Nagle A, McCarthy RJ, et al. Postoperative
hypoxaemia in morbidly obese patients with and without
obstructive sleep apnea undergoing laparoscopic bariatric
surgery. Anesth Analg 2008;107:138-43. Crossref
2. Taylor S, Kirton OC, Staff I, Kozol RA. Postoperative day
one: a high risk period for respiratory events. Am J Surg
2005;190:752-6. Crossref
3. Mulier JP. Perioperative opioids aggravate obstructive
breathing in sleep apnea syndrome: mechanisms and
alternative anaesthesia strategies. Curr Opin Anaesthesiol
2016;29:129-33. Crossref
4. Alvarez A, Singh PM, Sinha AC. Postoperative analgesia in
morbid obesity. Obes Surg 2014;24:652-9. Crossref
5. Hume R. Prediction of lean body mass from height and
weight. J Clin Pathol 1966;19:389-91. Crossref
6. Ingrande J, Lemmens HJ. Dose adjustment of anaesthetics
in the morbidly obese. Br J Anaesth 2010;105 Suppl 1:i16-23. Crossref
7. Lopez PP, Stefan B, Schulman CI, Byers PM. Prevalence of
sleep apnea in morbidly obese patients who presented for
weight loss surgery evaluation: more evidence for routine
screening for obstructive sleep apnea before weight loss
surgery. Am Surg 2008;74:834-8.
8. American Society of Anesthesiologists Task Force on
Perioperative Management of patients with obstructive
sleep apnea. Practice guidelines for the perioperative
management of patients with obstructive sleep apnea: an
updated report by the American Society of Anesthesiologists
Task Force on Perioperative Management of patients with
obstructive sleep apnea. Anesthesiology 2014;120:268-86. Crossref
9. Vinki HR, Kissin I. Rapid development of tolerance to
analgesia during remifentanil infusion in humans. Anesth
Analg 1998;86:1307-11. Crossref
10. Dahl JB, Nielsen RV, Wetterslev J, et al. Post-operative
analgesic effects of paracetamol, NSAIDs, glucocorticoids,
gabapentinoids and their combinations: a topical review.
Acta Anaesthesiol Scand 2014;58:1165-81. Crossref
11. Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect
of perioperative systemic α2 agonists on postoperative
morphine consumption and pain intensity: systematic
review and meta-analysis of randomized controlled trials.
Anesthesiology 2012;116:1312-22. Crossref
12. Cabrera Schulmeyer MC, de la Maza J, Ovalle C, Farias
C, Vives I. Analgesic effects of a single preoperative dose
of pregabalin after laparoscopic sleeve gastrectomy. Obes
Surg 2010;20:1678-81. Crossref
13. Weinbroum AA. Non-opioid IV adjuvants in the
perioperative period: pharmacological and clinical
aspects of ketamine and gabapentinoids. Pharmocol Res
2012;65:411-29. Crossref
14. Alimian M, Imani F, Faiz SH, Pournajafian A, Navadegi
SF, Safari S. Effect of oral pregabalin premedication on
post-operative pain in laparoscopic gastric bypass surgery.
Anesth Pain Med 2012;2:12-6. Crossref
15. Feld JM, Laurito CE, Beckerman M, Vincent J, Hoffman
WE. Non-opioid analgesia improves pain relief and
decreases sedation after gastric bypass surgery. Can J
Anaesth 2003;50:336-41. Crossref
16. Feld JM, Hoffam WE, Stechert MM, Hoffman IW, Anada
RC. Fentanyl or dexmedetomidine combined with
desflurane for bariatric surgery. J Clin Anesth 2006;18:24-8. Crossref
17. Hofer RE, Sprung J, Sarr MG, Wedel DJ. Anesthesia for
a patient with morbid obesity using dexmedetomidine
without narcotics. Can J Anaesth 2005;52:176-80. Crossref
18. Tufanogullari B, White PF, Peixoto MP, et al.
Dexmedetomidine infusion during laparoscopic bariatric
surgery: the effect on recovery outcome variables. Anesth
Analg 2008;106:1741-8. Crossref
19. Ziemann-Gimmel P, Hensel P, Koppman J, Marema R.
Multimodal analgesia reduces narcotic requirements and
antiemetic rescue medication in laparoscopic Roux-en-Y
gastric bypass surgery. Surg Obes Relat Dis 2013;9:975-80. Crossref
20. Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema
RT. Opioid-free total intravenous anaesthesia reduces
postoperative nausea and vomiting in bariatric surgery
beyond triple prophylaxis. Br J Anaesth 2014;112:906-11. Crossref
21. Carollo DS, Nossaman BD, Ramadhyani U.
Dexmedetomidine: a review of clinical applications. Curr
Opin Anaesthesiol 2008;21:457-61. Crossref
22. Gammon D, Bankhead B. Perioperative pain adjuncts.
In: Johnson KB, editor. Clinical pharmacology for
anesthesiology. McGraw-Hill Education; 2014: 157-78.
23. Sinha AC, Eckmann DM. Anesthesia for bariatric surgery.
In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish
JP, Young WL, editors. Miller’s anesthesia. 7th ed.
Philadelphia: Churchill Livingston; 2015: 2089-104.